A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Products
2.2. Ethics
2.3. Participants and Eligibility Criteria
2.4. Experimental Design
2.5. Primary Efficacy Assessment
2.6. Secondary Efficacy Assessments
2.6.1. K-VAS and Korean Medical Outcome Study 36-Item Short Form (KSF-36)
2.6.2. Korean Western Ontario and McMaster Universities Osteoarthritis Index™ (K-WOMAC)
2.6.3. Serum C-Reactive Protein (CRP)
2.6.4. Urinary C-Telopeptide of Type II Collagen (CTX-II)
2.6.5. Improvement Assessment
2.7. Safety Evaluations
2.8. Statistics
2.8.1. Sample Size
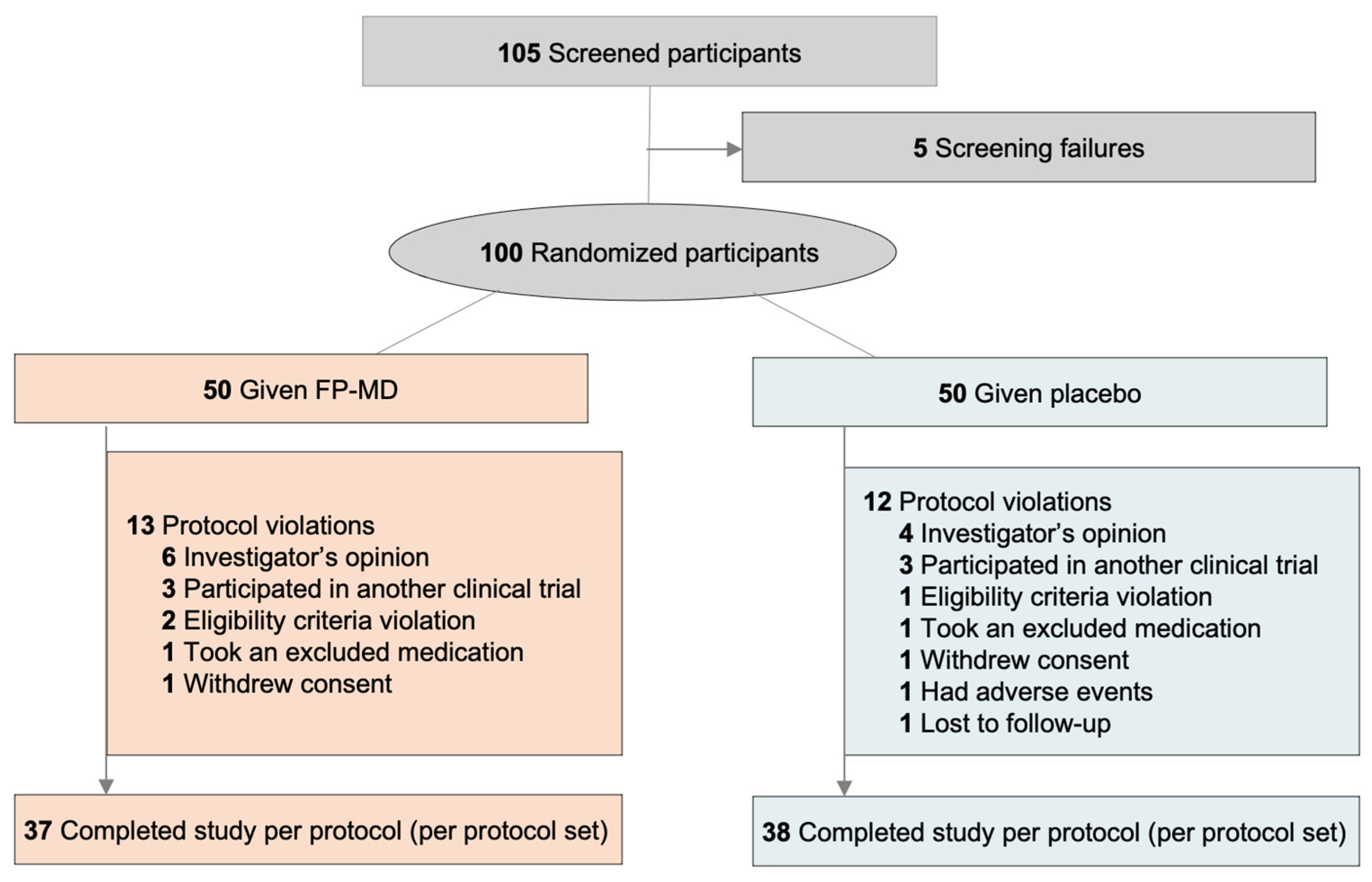
2.8.2. Analysis Sets
2.8.3. Baseline Characteristics Analyses
2.8.4. Primary Efficacy Analysis
2.8.5. Secondary Efficacy Analyses
2.8.6. Safety Analyses
3. Results
3.1. Participant Characteristics
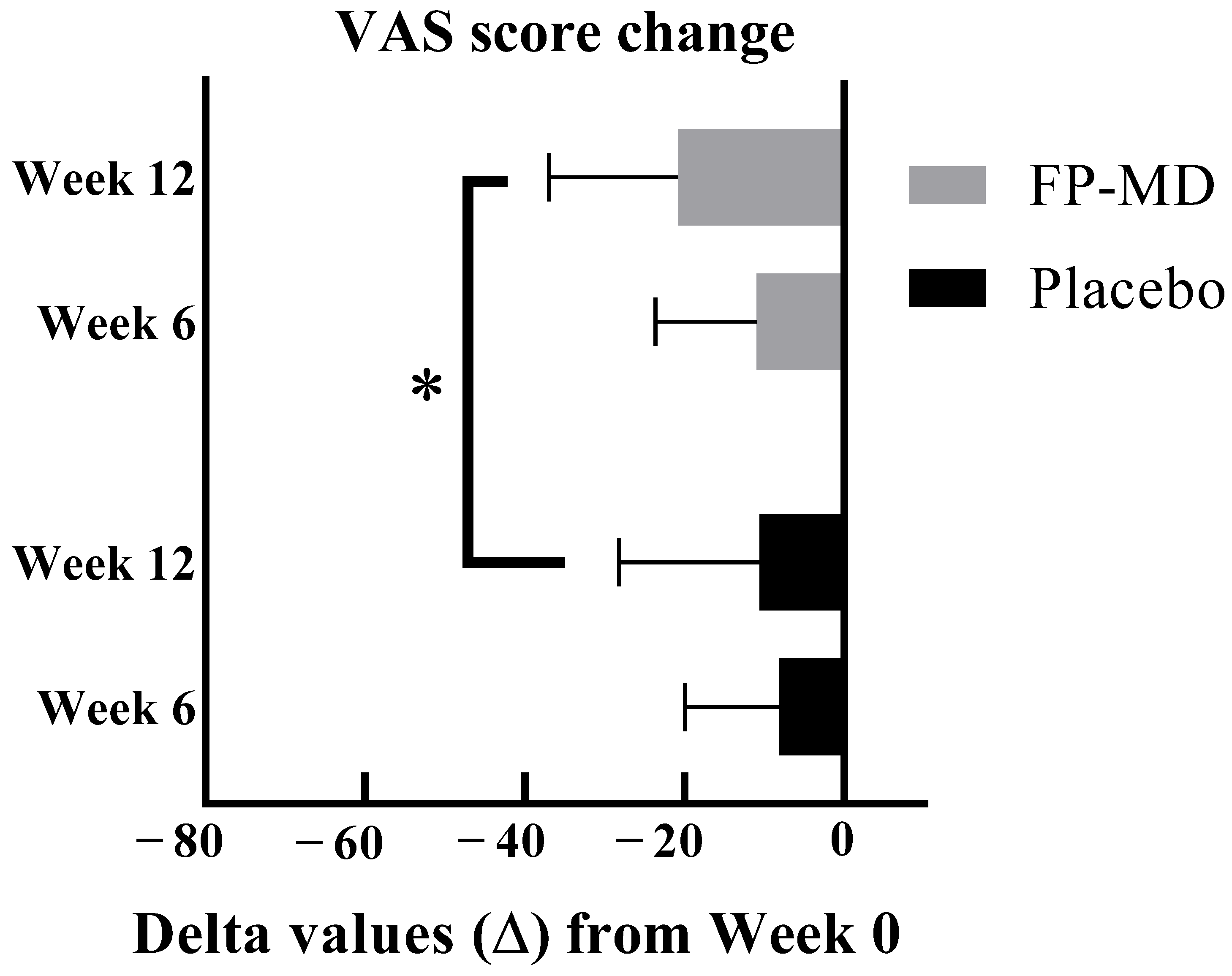
3.2. Joint Pain K-VAS
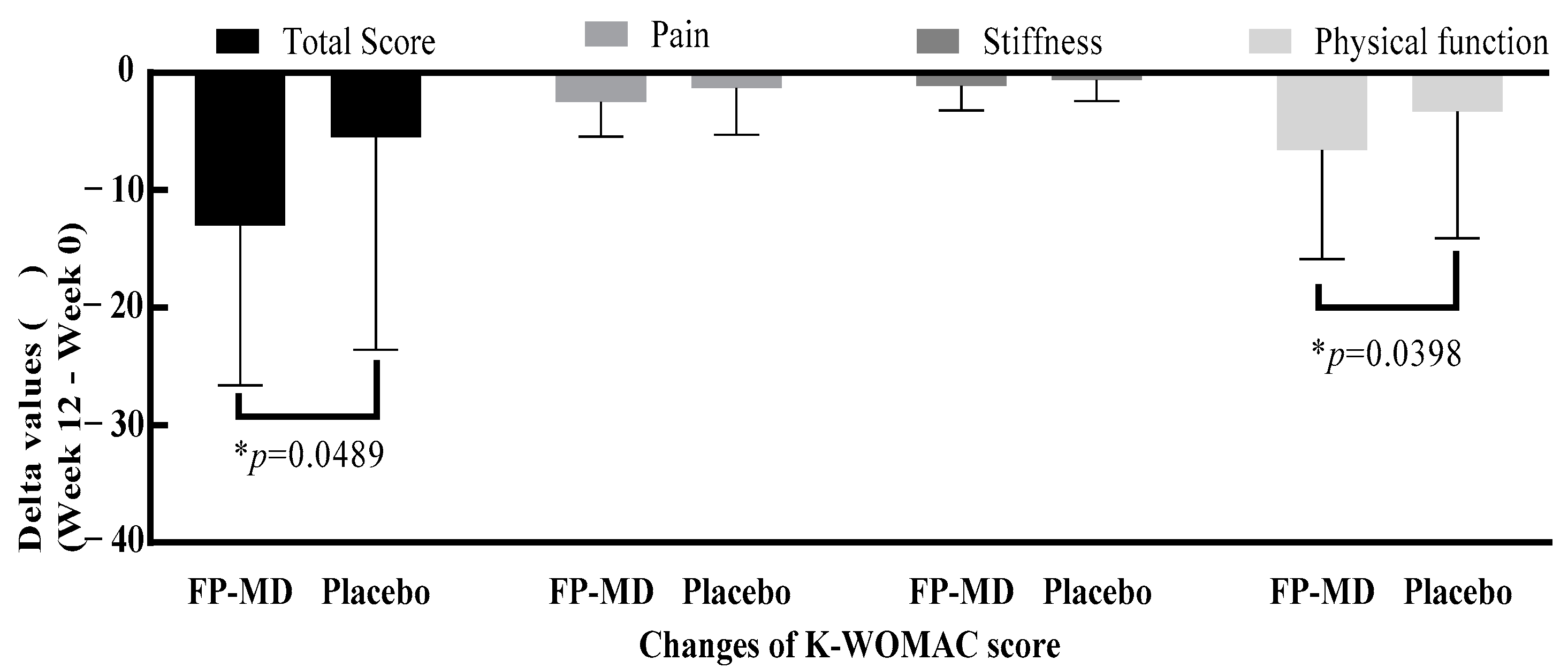
3.3. K-WOMAC
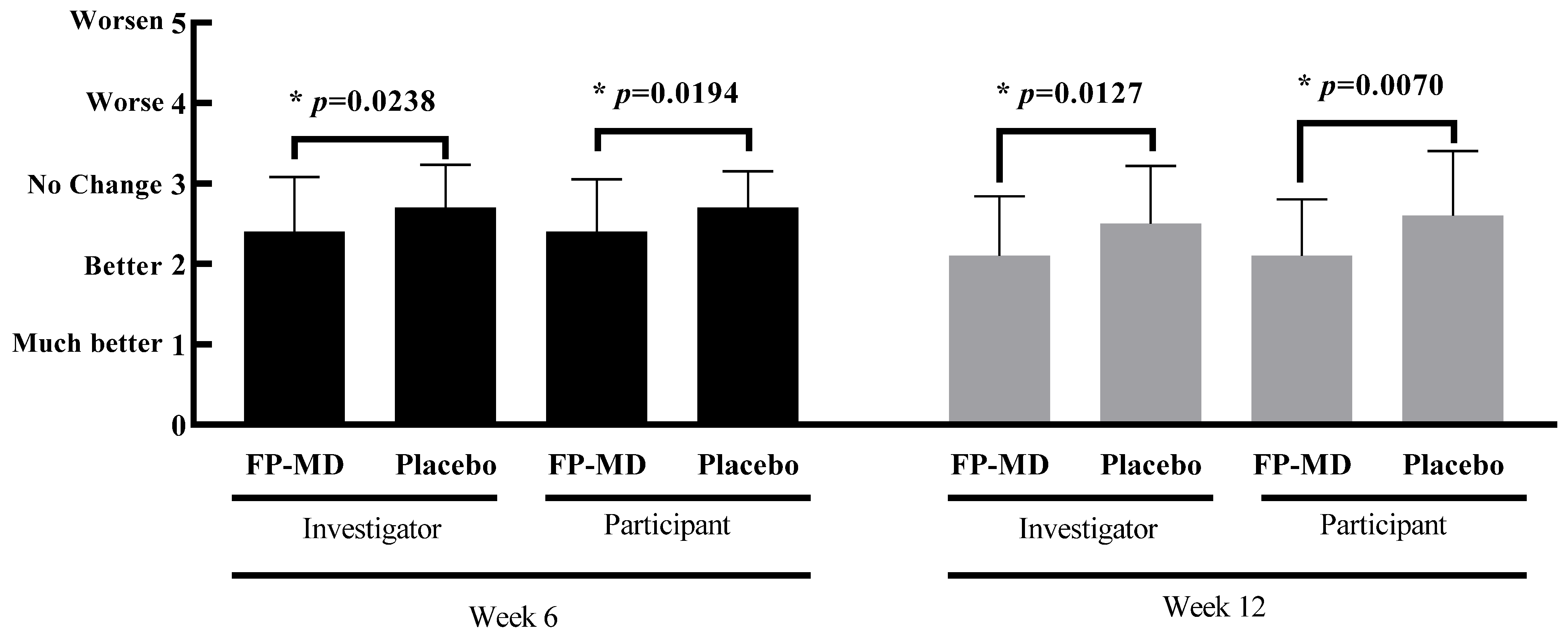
3.4. Investigator and Participant Improvement Assessment Scores
3.5. Serum CRP and Urinary CTX-II Levels
3.6. Safety Evaluation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safiri, S.; Kolahi, A.A.; Cross, M.; Hill, C.; Smith, E.; Carson-Chahhoud, K.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Kaufman, J.; et al. Prevalence, Deaths, and Disability-Adjusted Life Years Due to Musculoskeletal Disorders for 195 Countries and Territories 1990–2017. Arthritis Rheumatol. 2021, 73, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Berteau, J.P. Knee Pain from Osteoarthritis: Pathogenesis, Risk Factors, and Recent Evidence on Physical Therapy Interventions. J. Clin. Med. 2022, 11, 3252. [Google Scholar] [CrossRef]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- Debbi, E.M.; Agar, G.; Fichman, G.; Ziv, Y.B.; Kardosh, R.; Halperin, N.; Elbaz, A.; Beer, Y.; Debi, R. Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: A randomized controlled study. BMC Complement. Altern. Med. 2011, 11, 50. [Google Scholar] [CrossRef]
- Wei, P.; Liu, M.; Chen, Y.; Chen, D.C. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 2012, 5, 243–248. [Google Scholar] [CrossRef]
- Tomonaga, A.; Watanabe, K.; Fukagawa, M.; Suzuki, A.; Kurokawa, M.; Nagaoka, I. Evaluation of the effect of N-acetyl-glucosamine administration on biomarkers for cartilage metabolism in healthy individuals without symptoms of arthritis: A randomized double-blind placebo-controlled clinical study. Exp. Ther. Med. 2016, 12, 1481–1489. [Google Scholar] [CrossRef][Green Version]
- Huang, S.L.; Ling, P.X.; Zhang, T.M. Oral absorption of hyaluronic acid and phospholipids complexes in rats. World J. Gastroenterol. 2007, 13, 945–949. [Google Scholar] [CrossRef]
- Mercke Odeberg, J.; Lignell, A.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, S.H.; Lee, H.Y.; Lee, H.J.; Chang, S.B.; Chung, S.K.; Kim, H.J. Validation of the Korean version of the oswestry disability index. Spine 2005, 30, E123–E127. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Oh, H.; Lee, S.; Lee, K.; Kim, K. Effects of resistance exercise using the elastic band on the pain and function of patients with degenerative knee arthritis. J. Phys. Ther. Sci. 2020, 32, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Jinks, C.; Jordan, K.; Croft, P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Pain 2002, 100, 55–64. [Google Scholar] [CrossRef]
- Bae, S.C.; Lee, H.S.; Yun, H.R.; Kim, T.H.; Yoo, D.H.; Kim, S.Y. Cross-cultural adaptation and validation of Korean Western Ontario and McMaster Universities (WOMAC) and Lequesne osteoarthritis indices for clinical research. Osteoarthr. Cartil. 2001, 9, 746–750. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, K.H.; Kang, J.W.; Lee, M.; Kang, K.W.; Kim, J.E.; Kim, J.H.; Lee, S.; Shin, M.S.; Jung, S.Y.; et al. Moxibustion treatment for knee osteoarthritis: A multi-centre, non-blinded, randomised controlled trial on the effectiveness and safety of the moxibustion treatment versus usual care in knee osteoarthritis patients. PLoS ONE 2014, 9, e101973. [Google Scholar] [CrossRef]
- Garnero, P.; Peterfy, C.; Zaim, S.; Schoenharting, M. Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis: A three-month longitudinal study. Arthritis Rheum. 2005, 52, 2822–2829. [Google Scholar] [CrossRef]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 3–6. [Google Scholar]
- Kare, S.K.; Vinay, V.; Maresz, K.; Prisk, V.; Vik, H. Tamarindus indica Seed Extract-Based Botanical Compositions Alleviate Knee Pain and Improve Joint Function in Mild-to-Moderate Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Clinical Study. Evid. Based Complement. Alternat Med. 2022, 2022, 2226139. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Cai, X.; Yuan, S.; Zeng, Y.; Wang, C.; Yu, N.; Ding, C. New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 2021, 12, 645842. [Google Scholar] [CrossRef] [PubMed]
- Walzer, S.M.; Weinmann, D.; Toegel, S. Medical Plant Extracts for Treating Knee Osteoarthritis: A Snapshot of Recent Clinical Trials and Their Biological Background. Curr. Rheumatol. Rep. 2015, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Lubis, A.M.T.; Siagian, C.; Wonggokusuma, E.; Marsetyo, A.F.; Setyohadi, B. Comparison of Glucosamine-Chondroitin Sulfate with and without Methylsulfonylmethane in Grade I-II Knee Osteoarthritis: A Double Blind Randomized Controlled Trial. Acta Med. Indones. 2017, 49, 105–111. [Google Scholar] [PubMed]
- Sawitzke, A.D.; Shi, H.; Finco, M.F.; Dunlop, D.D.; Harris, C.L.; Singer, N.G.; Bradley, J.D.; Silver, D.; Jackson, C.G.; Lane, N.E.; et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann. Rheum. Dis. 2010, 69, 1459–1464. [Google Scholar] [CrossRef]
- Park, D.R.; Ko, R.; Kwon, S.H.; Min, B.; Yun, S.H.; Kim, M.H.; Minatelli, J.; Hill, S.; Lee, S.Y. FlexPro MD, a Mixture of Krill Oil, Astaxanthin, and Hyaluronic Acid, Suppresses Lipopolysaccharide-Induced Inflammatory Cytokine Production Through Inhibition of NF-κB. J. Med. Food 2016, 19, 1196–1203. [Google Scholar] [CrossRef]
- Park, M.H.; Jung, J.C.; Hill, S.; Cartwright, E.; Dohnalek, M.H.; Yu, M.; Jun, H.J.; Han, S.B.; Hong, J.T.; Son, D.J. FlexPro MD®, a Combination of Krill Oil, Astaxanthin and Hyaluronic Acid, Reduces Pain Behavior and Inhibits Inflammatory Response in Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Nutrients 2020, 12, 956. [Google Scholar] [CrossRef]
- Dohnalek, M.H.; Cartwright, E.J.; Hill, W.S. Efficacy and Safety of a Joint Health Nutritional Supplement for Subjects with Non-arthritic Knee Joint Pain: A Double-blind, Placebo- and Active-Controlled, Randomized Clinical Trial. J. Orthop. Res. Ther. 2023, 8, 1272. [Google Scholar] [CrossRef]
- Wann, A.K.; Mistry, J.; Blain, E.J.; Michael-Titus, A.T.; Knight, M.M. Eicosapentaenoic acid and docosahexaenoic acid reduce interleukin-1β-mediated cartilage degradation. Arthritis Res. Ther. 2010, 12, R207. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, C.K.; Hwang, S.W. Biological Roles of Resolvins and Related Substances in the Resolution of Pain. Biomed. Res. Int. 2015, 2015, 830930. [Google Scholar] [CrossRef]
- Stonehouse, W.; Benassi-Evans, B.; Bednarz, J.; Vincent, A.D.; Hall, S.; Hill, C.L. Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: A 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2022, 116, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- Berge, K.; Robertson, B.; Burri, L. Safety assessment of Superba™ krill powder: Subchronic toxicity study in rats. Toxicol. Rep. 2015, 2, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.; Burri, L.; Berge, K. Genotoxicity test and subchronic toxicity study with Superba™ krill oil in rats. Toxicol. Rep. 2014, 1, 764–776. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Meyer, K.; Palmer, J.W. The Polysaccharide of the Vitreous Humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.H.; Liu, S.Q.; Peng, H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J. Orthop. Res. 2008, 26, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef]
- Tashiro, T.; Seino, S.; Sato, T.; Matsuoka, R.; Masuda, Y.; Fukui, N. Oral administration of polymer hyaluronic acid alleviates symptoms of knee osteoarthritis: A double-blind, placebo-controlled study over a 12-month period. Sci. World J. 2012, 2012, 167928. [Google Scholar] [CrossRef]
- Nelson, F.R.; Zvirbulis, R.A.; Zonca, B.; Li, K.W.; Turner, S.M.; Pasierb, M.; Wilton, P.; Martinez-Puig, D.; Wu, W. The effects of an oral preparation containing hyaluronic acid (Oralvisc®) on obese knee osteoarthritis patients determined by pain, function, bradykinin, leptin, inflammatory cytokines, and heavy water analyses. Rheumatol. Int. 2015, 35, 43–52. [Google Scholar] [CrossRef]
- Ricci, M.; Micheloni, G.M.; Berti, M.; Perusi, F.; Sambugaro, E.; Vecchini, E.; Magnan, B. Clinical comparison of oral administration and viscosupplementation of hyaluronic acid (HA) in early knee osteoarthritis. Musculoskelet. Surg. 2017, 101, 45–49. [Google Scholar] [CrossRef]
- Armstrong, D.C.; Cooney, M.J.; Johns, M.R. Growth and amino acid requirements of hyaluronic-acid-producing Streptococcus zooepidemicus. Appl. Microbiol. Biotechnol. 1997, 47, 309–312. [Google Scholar] [CrossRef]
- Armstrong, D.C.; Johns, M.R. Culture Conditions Affect the Molecular Weight Properties of Hyaluronic Acid Produced by Streptococcus zooepidemicus. Appl. Environ. Microbiol. 1997, 63, 2759–2764. [Google Scholar] [CrossRef]
- Flores-Gatica, M.; Castañeda-Aponte, H.; Gil-Garzon, M.R.; Mora-Galvez, L.M.; Banda-Magaña, M.P.; Jáuregui-Jáuregui, J.A.; Torres-Acosta, M.A.; Mayolo-Deloisa, K.; Licona-Cassani, C. Primary recovery of hyaluronic acid produced in Streptococcus equi subsp. zooepidemicus using PEG-citrate aqueous two-phase systems. AMB Express 2021, 11, 123. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Wang, T.; Li, M.; Li, X. Sucrose-modified iron nanoparticles for highly efficient microbial production of hyaluronic acid by Streptococcus zooepidemicus. Colloids Surf. B Biointerfaces 2021, 205, 111854. [Google Scholar] [CrossRef] [PubMed]
- Oe, M.; Tashiro, T.; Yoshida, H.; Nishiyama, H.; Masuda, Y.; Maruyama, K.; Koikeda, T.; Maruya, R.; Fukui, N. Oral hyaluronan relieves knee pain: A review. Nutr. J. 2016, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Cubukcu, D.; Sarsan, A.; Alkan, H. Relationships between Pain, Function and Radiographic Findings in Osteoarthritis of the Knee: A Cross-Sectional Study. Arthritis 2012, 2012, 984060. [Google Scholar] [CrossRef] [PubMed]
- Ezzelle, J.; Rodriguez-Chavez, I.R.; Darden, J.M.; Stirewalt, M.; Kunwar, N.; Hitchcock, R.; Walter, T.; D’Souza, M.P. Guidelines on good clinical laboratory practice: Bridging operations between research and clinical research laboratories. J. Pharm. Biomed. Anal. 2008, 46, 18–29. [Google Scholar] [CrossRef]
| Composition | FP-MD 600 mg mg (%) | Placebo 600 mg mg (%) |
|---|---|---|
| FP-MD | 462 (77) | 0 |
| Antarctic krill oil | 321 (53.5) | 0 |
| Haematococcus pluvialis extract (to deliver 2 mg astaxanthin) | 25–35 (4–5) | 0 |
| Sodium hyaluronate | 33 (5.5) | 0 |
| Excipients | 73–83 (12–14) * | |
| Palm oil | 0 | 400 (67) |
| Olive oil | 0 | 62 (10) |
| Soybean oil | 114 (19) | 114 (19) |
| Beeswax | 24 (4) | 24 (4) |
| Score | Degree | Symptoms |
|---|---|---|
| 1 | Much better | Significant improvement in symptoms |
| 2 | Better | Overall improvement of symptoms |
| 3 | No change | No difference from baseline |
| 4 | Worse | Overall worsening of symptoms |
| 5 | Worsen | Significant worsening of symptoms |
| FP-MD n = 37 | Placebo n = 38 | Total n = 75 | p-Value | ||
|---|---|---|---|---|---|
| Sex n (%) | Male | 16 (43.24) | 15 (39.47) | 31 (41.33) | 0.7403 † |
| Female | 21 (56.76) | 23 (60.53) | 44 (58.67) | ||
| Age (y) | Mean ± SD | 57.0 ± 10.28 | 59.0 ± 11.82 | 58.0 ± 11.06 | 0.2370 & |
| Min, Max | 31.0, 70.0 | 35.0, 75.0 | 31.0, 75.0 | ||
| Exercise frequency (%) | None | 7 (18.9) | 6 (15.8) | 13 (17.3) | 0.9100 † |
| <3 sessions/week or <30 min/session | 15 (40.5) | 15 (39.5) | 30 (40.00) | ||
| ≥3 sessions/week or >30 min/session | 15 (40.5) | 17 (44.7) | 32 (42.7) | ||
| Smoking status n (%) | Non-smoker | 31 (83.8) | 32 (84.2) | 63 (84.0) | 0.1569 ‡ |
| Ex-smoker Stopped smoking > 6 months before screening visit | 0 | 3 (7.9) | 3 (4.0) | ||
| Smoker | 6 (16.2) | 3 (7.9) | 9 (12.0) | ||
| Cigarettes/day (among smokers) | Mean ± SD | 11.7 ± 4.08 | 11.7 ± 7.6 | 11.7 ± 5.0 | 0.8774 & |
| Min, max | 10.0, 20.0 | 5.0, 20.0 | 5.0, 20.0 | ||
| Smoking (years) (among smokers) | Mean ± SD | 26.0 ± 9.38 | 21.7 ± 7.64 | 24.6 ± 8.62 | 0.5141 * |
| Min, max | 10.0, 36.0 | 15.0, 30.0 | 10.0, 36.0 | ||
| Alcohol consumption n (%) | None | 22 (59.5) | 20 (52.6) | 42 (56.0) | 0.5829 ‡ |
| Quit | 1 (2.7) | 1 (2.6) | 2 (2.7) | ||
| <1 bottle/week | 5 (13.5) | 10 (26.3) | 15 (20.0) | ||
| 1~2 bottles/week | 7 (18.9) | 4 (10.5) | 11 (14.7) | ||
| >3 bottles/weeks | 2 (5.4) | 3 (7.9) | 5 (6.7) | ||
| Body height (cm) | Mean ± SD | 161.9 ± 10.24 | 160.8 ± 9.74 | 161.4 ± 9.94 | 0.6259 & |
| Min, max | 145.2, 184.1 | 145.1, 180.0 | 145.1, 184.1 |
| K-VAS (mm) | FP-MD (n = 37) | Placebo (n = 38) | p-Value | p-Value ‡ |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Baseline | 46.1 ± 9.77 | 42.7 ± 8.38 | 0.1596 † | |
| Week 6 | 35.1 ± 17.13 | 34.6 ± 16.49 | 0.1059 † | 0.0854 |
| Change from baseline | −11.0 ± 12.62 | −8.1 ± 11.87 | ||
| p-value | <0.0001 ** | <0.0001 # | ||
| Week 12 | 25.3 ± 16.39 | 32.1 ± 19.08 | 0.0105 * | 0.0255 |
| Change from baseline | −20.8 ± 16.16 | −10.6 ± 17.58 | ||
| p value ** | <0.0001 | 0.0007 |
| FP-MD (n = 38) | Placebo (n = 37) | p-Value | p-Value ‡ | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| ||||
| Baseline | 30.7 ± 14.81 | 28.3 ± 13.55 | 0.4737 * | |
| Week 6 | 21.2 ± 13.10 | 23.5 ± 13.75 | 0.1304 * | 0.1658 |
| Change from baseline | −9.5 ± 12.57 | −4.8 ± 14.10 | ||
| p-value ** | <0.0001 | 0.0432 | ||
| Week 12 | 17.7 ± 15.06 | 22.8 ± 15.07 | 0.0489 * | 0.1063 |
| Change from baseline | −13.0 ± 13.62 | −5.5 ± 18.08 | ||
| p-value ** | <0.0001 | 0.0674 | ||
| ||||
| Baseline | 6.0 ± 3.22 | 5.7 ± 2.64 | 0.6582 † | |
| Week 6 | 4.0 ± 2.74 | 4.7 ± 2.98 | 0.1675 * | 0.1149 |
| Change from baseline | −2.0 ± 3.14 | −1.0 ± 6.07 | ||
| p-value ** | 0.0004 | 0.0518 | ||
| Week 12 | 3.5 ± 2.99 | 4.5 ± 3.45 | 0.2635 † | 0.1779 |
| Change from baseline | −2.5 ± 2.92 | −1.3 ± 3.94 | ||
| p-value | <0.0001 ** | 0.0173 # | ||
| ||||
| Baseline | 2.9 ± 1.61 | 2.6 ± 1.44 | 0.5240 † | |
| Week 6 | 2.0 ± 1.31 | 2.1 ± 1.35 | 0.4294 † | 0.5298 |
| Change from baseline | −0.9 ± 1.78 | −0.5 ± 1.45 | ||
| p-value | 0.0040 # | 0.0310 ** | ||
| Week 12 | 1.8 ± 1.57 | 2.0 ± 1.62 | 0.2819 † | 0.6330 |
| Change from baseline | −1.1 ± 2.08 | −0.6 ± 1.79 | ||
| p-value | 0.0039 ** | 0.0282 # | ||
| ||||
| Baseline | 21.8 ± 11.03 | 20.0 ± 10.25 | 0.4639 * | |
| Week 6 | 15.2 ± 9.84 | 16.7 ± 10.14 | 0.1528 * | 0.2148 |
| Change from baseline | −6.6 ± 9.25 | −3.3 ± 10.80 | ||
| p-value ** | 0.0001 | 0.0705 | ||
| Week 12 | 12.4 ± 10.83 | 16.3 ± 10.86 | 0.0398 * | 0.0890 |
| Change from baseline | −9.4 ± 9.99 | −3.7 ± 13.38 | ||
| p-value ** | <0.0001 | 0.1005 |
| FP-MD (n = 37) | Placebo (n = 38) | p-Value † | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Investigator assessment score | |||
| Week 6 | 2.4 ± 0.68 | 2.7 ± 0.53 | 0.0238 |
| Week 12 | 2.1 ± 0.74 | 2.5 ± 0.72 | 0.0127 |
| Participant assessment score | |||
| Week 6 | 2.4 ± 0.65 | 2.7 ± 0.45 | 0.0194 |
| Week 12 | 2.1 ± 0.70 | 2.6 ± 0.80 | 0.0070 |
| FP-MD (n = 50) | Placebo (n = 50) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| n | Incidence (%) | Cases | n | Incidence (%) | Cases | ||
| Adverse events (AEs) | 2 | 4.0 | 2 | 8 | 16.0 | 10 | 0.0455 † |
| Serious AEs (SAE) | 1 | 2.0 | 1 | 2 | 4.0 | 2 | 1.0000 ‡ |
| Dropouts due to AEs | 0 | 0.0 | 0 | 1 | 2.0 | 1 | 1.0000 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, W.S.; Dohnalek, M.H.; Ha, Y.; Kim, S.-J.; Jung, J.-C.; Kang, S.-B. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients 2023, 15, 3769. https://doi.org/10.3390/nu15173769
Hill WS, Dohnalek MH, Ha Y, Kim S-J, Jung J-C, Kang S-B. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients. 2023; 15(17):3769. https://doi.org/10.3390/nu15173769
Chicago/Turabian StyleHill, W. Stephen, Margaret H. Dohnalek, Yejin Ha, Seok-Jung Kim, Jae-Chul Jung, and Seung-Baik Kang. 2023. "A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis" Nutrients 15, no. 17: 3769. https://doi.org/10.3390/nu15173769
APA StyleHill, W. S., Dohnalek, M. H., Ha, Y., Kim, S.-J., Jung, J.-C., & Kang, S.-B. (2023). A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients, 15(17), 3769. https://doi.org/10.3390/nu15173769






