Sucrose Solution Ingestion Exacerbates Dinitrofluorobenzene-Induced Allergic Contact Dermatitis in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Rats, Experimental Diets, and Sucrose Solution
2.2. Experimental Model of Rat ACD
2.3. Serum Uric Acid Quantification
2.4. Isolation of Total RNA, Reverse Transcription, and qPCR
2.5. Measurement of SCFAs
2.6. Measurement of Sodium in Fecal Samples
2.7. Microbiome Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of Ingestion of 10% Sucrose Solution on Body Weight and Metabolic Parameters
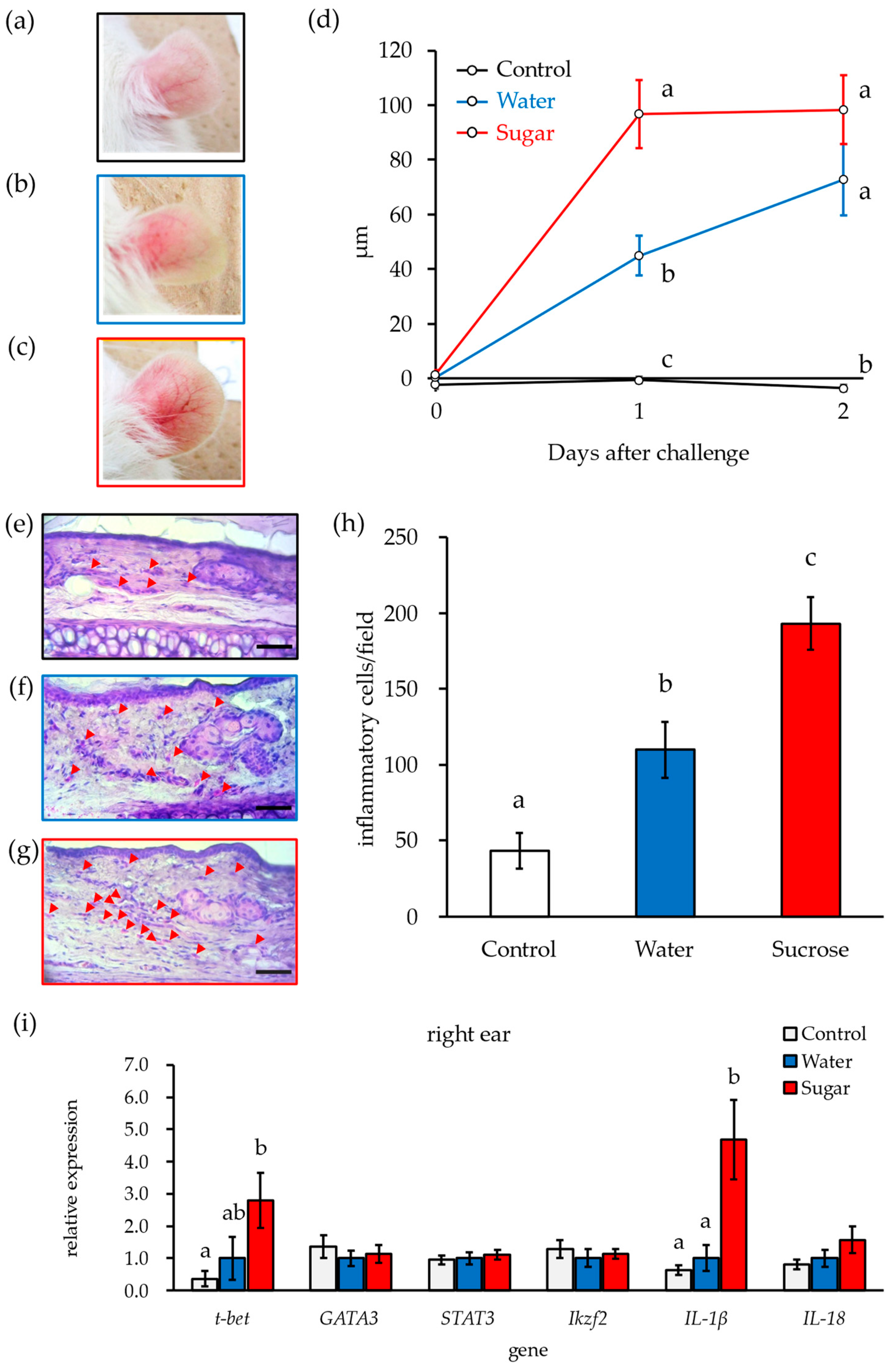
3.2. Ingestion of 10% Sucrose Solution Accelerates Early Deterioration of DNFB-ACD Symptoms
3.3. Ingestion of 10% Sucrose Solution Alters the Composition of the Intestinal Microbiota
3.4. Blood Acetate and Butyrate Concentrations Decrease after Liquid Sucrose Intake
3.5. Sucrose Ingestion Promotes the Expression of Monosaccharide Transporters in the Small Intestine but Has No Effect on SCFA Transporters
3.6. Triacetin Intake Reverses Early Exacerbation of DNFB-ACD Symptoms Associated with Sucrose Ingestion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.; Lim, S.; Andrews, K.G.; Engell, R.E.; Ezzati, M.; Mozaffarian, D.; Global Burden of Diseases, N.; et al. Global, Regional, and National Consumption of Sugar-Sweetened Beverages, Fruit Juices, and Milk: A Systematic Assessment of Beverage Intake in 187 Countries. PLoS ONE 2015, 10, e0124845. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Sharma, A.J.; Grellinger, L.; Vos, M.B. Consumption of added sugars is decreasing in the United States. Am. J. Clin. Nutr. 2011, 94, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Rosinger, A.; Herrick, K.; Gahche, J.; Park, S. Sugar-sweetened Beverage Consumption Among U.S. Youth, 2011–2014. In NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2017; pp. 1–8. [Google Scholar]
- Blecher, E.; Liber, A.C.; Drope, J.M.; Nguyen, B.; Stoklosa, M. Global Trends in the Affordability of Sugar-Sweetened Beverages, 1990–2016. Prev. Chronic Dis. 2017, 14, E37. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Xu, F.; Ye, Q.; Zhou, H.; Li, C.; He, J.; Wang, Z.; Hong, X.; Hou, X. Sugar-sweetened beverages and school students’ hypertension in urban areas of Nanjing, China. J. Hum. Hypertens. 2018, 32, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.V.; Azam-Ali, S.; McCullough, F.; Roy Mitra, S. The nutrition transition in Malaysia; key drivers and recommendations for improved health outcomes. BMC Nutr. 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Chen, B.; Li, J.; Yuan, X.; Li, J.; Wang, W.; Dai, T.; Chen, H.; Wang, Y.; et al. Dietary sugar consumption and health: Umbrella review. BMJ 2023, 381, e071609. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Kemps, E.; White, M.J.; Bartlett, S.E. The Impact of Free Sugar on Human Health—A Narrative Review. Nutrients 2023, 15, 889. [Google Scholar] [CrossRef]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef] [PubMed]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 12294. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wu, X.; Santos Rocha, C.; Rolston, M.; Garcia-Melchor, E.; Huynh, M.; Nguyen, M.; Law, T.; Haas, K.N.; Yamada, D.; et al. Short-Term Western Diet Intake Promotes IL-23—Mediated Skin and Joint Inflammation Accompanied by Changes to the Gut Microbiota in Mice. J. Investig. Dermatol. 2021, 141, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Fajstova, A.; Galanova, N.; Coufal, S.; Malkova, J.; Kostovcik, M.; Cermakova, M.; Pelantova, H.; Kuzma, M.; Sediva, B.; Hudcovic, T.; et al. Diet Rich in Simple Sugars Promotes Pro-Inflammatory Response via Gut Microbiota Alteration and TLR4 Signaling. Cells 2020, 9, 2701. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.F.; Casaro, M.B.; Ribeiro, W.R.; Mendes, E.; Murata, G.; Xander, P.; Lino-Dos-Santos-Franco, A.; Oliveira, F.A.; Ferreira, C.M. A short-term high-sugar diet is an aggravating factor in experimental allergic contact dermatitis. Heliyon 2023, 9, e21225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Zhu, M.J. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Beiko, R.G. 16S rRNA Gene Analysis with QIIME2. Methods Mol. Biol. 2018, 1849, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bellinghausen, I.; Brand, U.; Enk, A.H.; Knop, J.; Saloga, J. Signals involved in the early TH1/TH2 polarization of an immune response depending on the type of antigen. J. Allergy Clin. Immunol. 1999, 103, 298–306. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hamaguchi, M.; Kaji, A.; Sakai, R.; Osaka, T.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Uchiyama, K.; Takagi, T.; et al. Intake of sucrose affects gut dysbiosis in patients with type 2 diabetes. J. Diabetes Investig. 2020, 11, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Han, Z.; Kong, Q.; Wang, Y.; Mou, H.; Duan, X. Clostridium butyricum Prevents Dysbiosis and the Rise in Blood Pressure in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2023, 24, 4955. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Ding, Y.; Soh, M.; Low, A.; Seedorf, H. Cultivation and description of Duncaniella dubosii sp. nov., Duncaniella freteri sp. nov. and emended description of the species Duncaniella muris. Int. J. Syst. Evol. Microbiol. 2020, 70, 3105–3110. [Google Scholar] [CrossRef] [PubMed]
- van der Wielen, P.; Rovers, G.; Scheepens, J.M.A.; Biesterveld, S. Clostridium lactatifermen tans sp. nov., a lactate-fermenting anaerobe isolated from the caeca of a chicken. Int. J. Syst. Evol. Microbiol. 2002, 52, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Goto, K.; Ohtaki, Y.; Kaku, N.; Ueki, K. Description of Anaerotignum aminivorans gen. nov., sp. nov., a strictly anaerobic, amino-acid-decomposing bacterium isolated from a methanogenic reactor, and reclassification of Clostridium propionicum, Clostridium neopropionicum and Clostridium lactatifermentans as species of the genus Anaerotignum. Int. J. Syst. Evol. Microbiol. 2017, 67, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Kim, W. Cream Cheese-Derived Lactococcus chungangensis CAU 28 Modulates the Gut Microbiota and Alleviates Atopic Dermatitis in BALB/c Mice. Sci. Rep. 2019, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Investig. Dermatol. 2017, 137, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Gart, E.; Souto Lima, E.; Schuren, F.; de Ruiter, C.G.F.; Attema, J.; Verschuren, L.; Keijer, J.; Salic, K.; Morrison, M.C.; Kleemann, R. Diet-Independent Correlations between Bacteria and Dysfunction of Gut, Adipose Tissue, and Liver: A Comprehensive Microbiota Analysis in Feces and Mucosa of the Ileum and Colon in Obese Mice with NAFLD. Int. J. Mol. Sci. 2018, 20, 1. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, X.; Yao, J.; Cao, W.; Zou, Z.; Wang, L.; Qin, H.; Zhong, D.; Li, Y.; Xue, P.; et al. The role of short-chain fatty acids in inflammatory skin diseases. Front. Microbiol. 2022, 13, 1083432. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar] [PubMed]
- Kaji, I.; Iwanaga, T.; Watanabe, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D.; Akiba, Y. SCFA transport in rat duodenum. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 308, G188–G197. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Hui, S.; Lu, W.; Cowan, A.J.; Morscher, R.J.; Lee, G.; Liu, W.; Tesz, G.J.; Birnbaum, M.J.; Rabinowitz, J.D. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab. 2018, 27, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Kishi, K.; Tanaka, T.; Igawa, M.; Takase, S.; Goda, T. Sucrase-isomaltase and hexose transporter gene expressions are coordinately enhanced by dietary fructose in rat jejunum. J. Nutr. 1999, 129, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Ritze, Y.; Bardos, G.; D’Haese, J.G.; Ernst, B.; Thurnheer, M.; Schultes, B.; Bischoff, S.C. Effect of high sugar intake on glucose transporter and weight regulating hormones in mice and humans. PLoS ONE 2014, 9, e101702. [Google Scholar] [CrossRef]




| Target Gene | Forward Primer Sequence (5′->3′) | Reverse Primer Sequence (5′->3′) |
|---|---|---|
| actb | GGAGATTACTGCCCTGGCTCCTA | GACTCATCGTACTCCTGCTTGCTG |
| gata3 | GGCTACGGTGCAGAGGTATC | GATGGACGTCTTGGAGAAGG |
| hprt | CTCATGGACTGATTATGGACAGGAC | GCAGGTCAGCAAAGAACTTATAGCC |
| ikzf2 | GGCTTCCGAATGGTAAACTG | CATTTGAACGGCTTCTCTCC |
| slc16a1 | TCTTTGGATTTGCCTTTGGT | TGAGGCGGCCTAAAAGTG |
| slc16a3 | CTACAGCGACACAGCTTGGA | GACCCCTGTGGTGAGGTAGA |
| slc2a5 | AGAAGACAGGGAAGCTGACC | CTGCTGCATGAACTCTGAGG |
| slc5a1 | CATGCCTAACGGACTTCGAG | CGAGGATGAACAACCTTCCT |
| slc5a8 | TCAAGGTGGCATCAATACGA | TCCAGAACGTGTGTCTTTGC |
| stat3 | AAGAGTCTCGCCTCCTCCAG | ATCTGCTGCTTCTCCGTCAC |
| stat4 | GATCTGCCTCTATGGCCTCA | AGGAGTTGGCCCAAGGTAAC |
| tbx21 | GGAACCGCTTATACGTCCAC | CTTATGGAGGGACTGCAGGA |
| il-1β | AGGAGAGACAAGCAACGACAA | GTTTGGGATCCACACTCTCCA |
| il-18 | GACAAAAGAAACCCGCCTG | ACATCCTTCCATCCTTCACAG |
| Control | Water | Sucrose | |
|---|---|---|---|
| initial body weight (g) | 194 ± 4.78 | 198 ± 4.38 | 198 ± 5.89 |
| total body weight gain (g) | 107 ± 4.83 | 112 ± 2.62 | 113 ± 8.91 |
| total food ingestion (g) | 391 ± 12.6 a | 406 ± 11.6 a | 314 ± 20.3 b |
| total water ingestion (g) | 586 ± 48.5 a | 658 ± 87.6 a | 1240 ± 118 b |
| total energy intake (kcal) | 1140± 47.2 a | 1390 ± 43.5 a | 1570 ± 93.0 b |
| liver weight (g) | 15.1± 0.858 | 15.2 ± 0.879 | 15.3 ± 1.31 |
| liver triglyceride (mg/g) | 24.0 ± 2.24 | 20.7 ± 1.18 | 20.5 ± 1.47 |
| serum urate (mg/dL) | 0.631 ± 0.119 a | 1.94 ± 0.262 b | 2.89 ± 0.407 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, A.; Kimura, R.; Mori, A.; Yoshimura, Y. Sucrose Solution Ingestion Exacerbates Dinitrofluorobenzene-Induced Allergic Contact Dermatitis in Rats. Nutrients 2024, 16, 1962. https://doi.org/10.3390/nu16121962
Fujii A, Kimura R, Mori A, Yoshimura Y. Sucrose Solution Ingestion Exacerbates Dinitrofluorobenzene-Induced Allergic Contact Dermatitis in Rats. Nutrients. 2024; 16(12):1962. https://doi.org/10.3390/nu16121962
Chicago/Turabian StyleFujii, Aya, Ryuto Kimura, Azumi Mori, and Yukihiro Yoshimura. 2024. "Sucrose Solution Ingestion Exacerbates Dinitrofluorobenzene-Induced Allergic Contact Dermatitis in Rats" Nutrients 16, no. 12: 1962. https://doi.org/10.3390/nu16121962
APA StyleFujii, A., Kimura, R., Mori, A., & Yoshimura, Y. (2024). Sucrose Solution Ingestion Exacerbates Dinitrofluorobenzene-Induced Allergic Contact Dermatitis in Rats. Nutrients, 16(12), 1962. https://doi.org/10.3390/nu16121962






