Effects of Evodiamine on Behavior and Hippocampal Neurons through Inhibition of Angiotensin-Converting Enzyme and Modulation of the Renin Angiotensin Pathway in a Mouse Model of Post-Traumatic Stress Disorder
Abstract
1. Introduction
2. Results
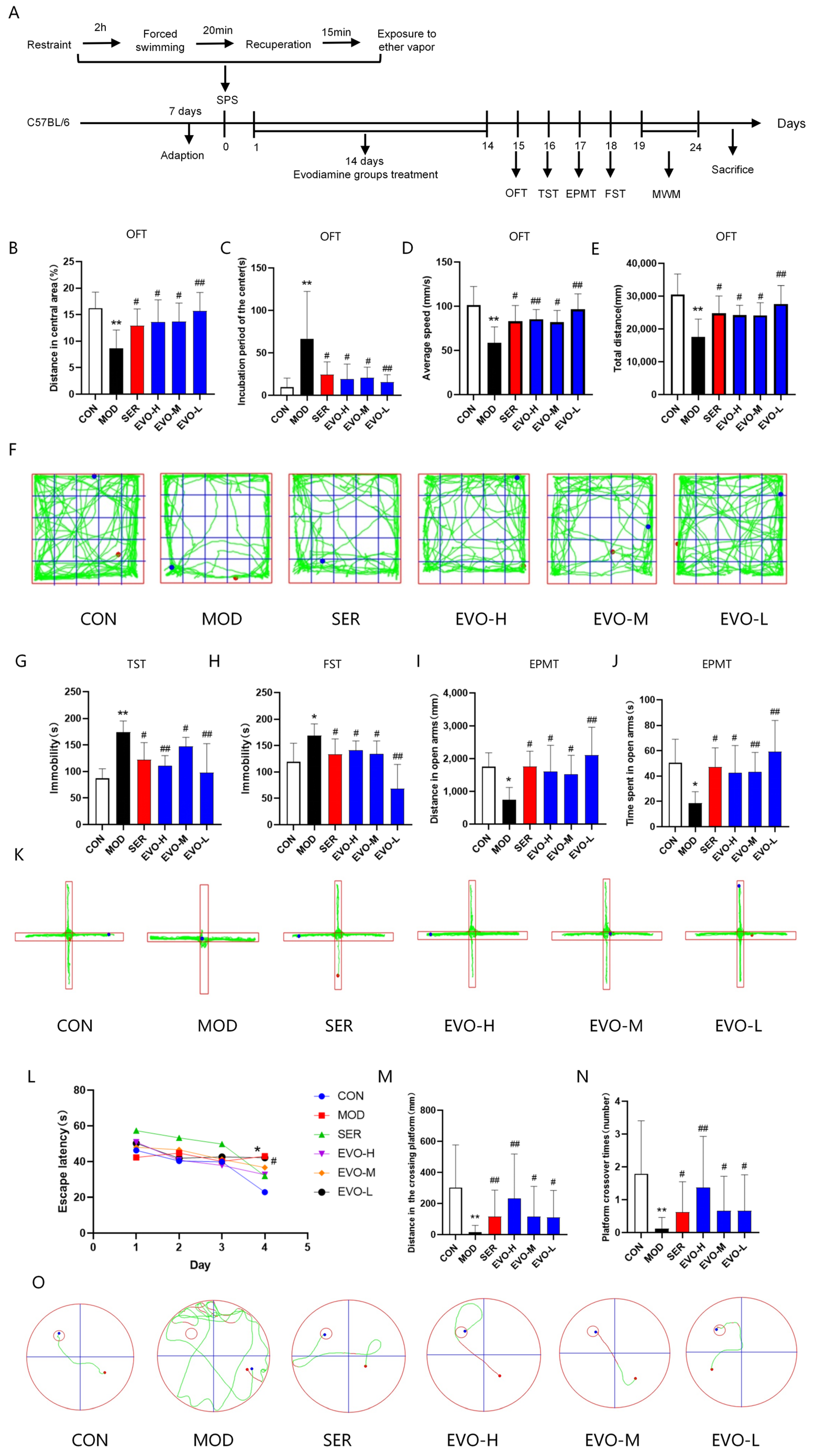
2.1. Effects of EVO on Behavioral Deficits in SPS-Induced PTSD Mice
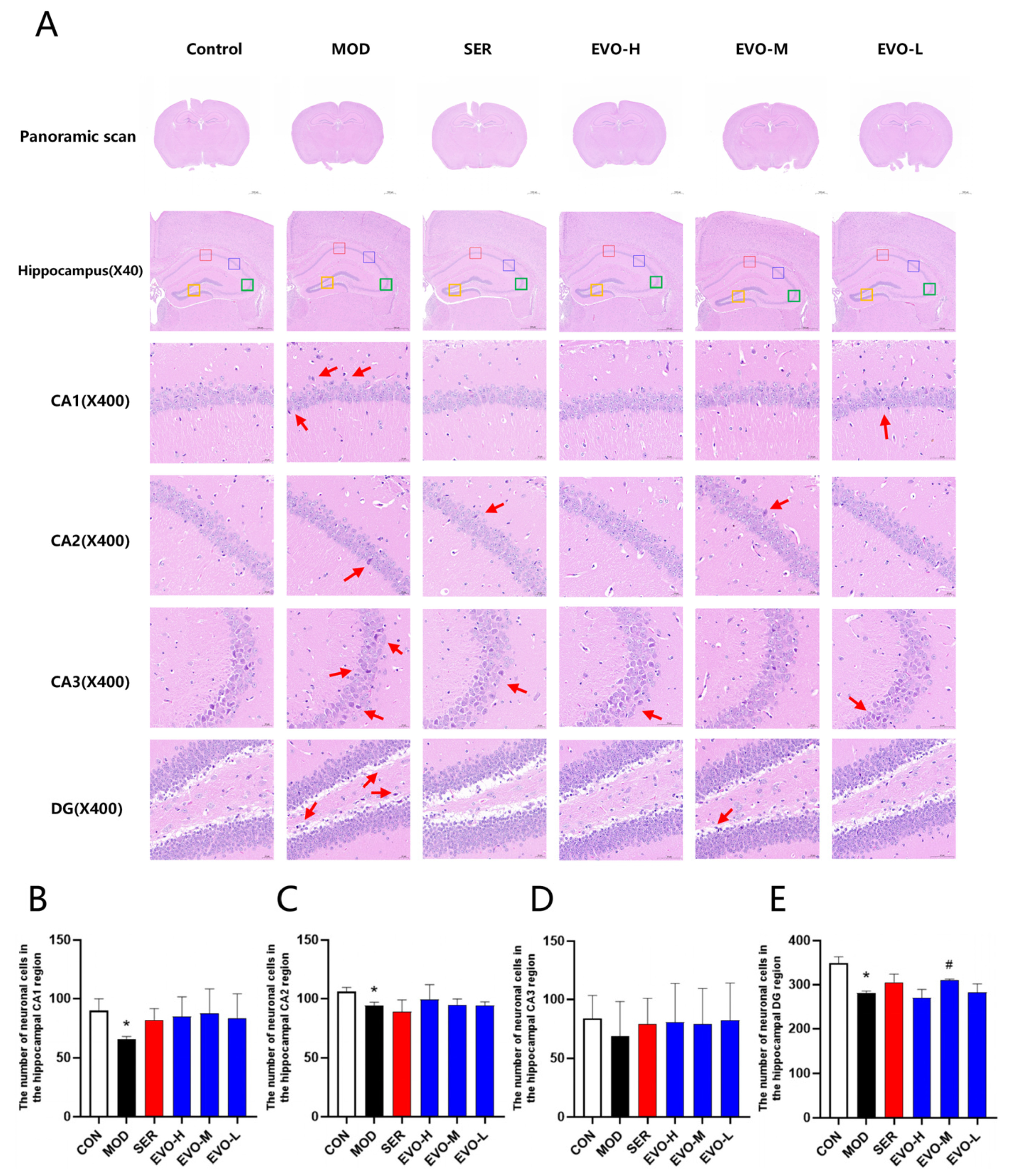
2.2. Effects of EVO on Hippocampal Morphology in SPS-Induced PTSD Mice
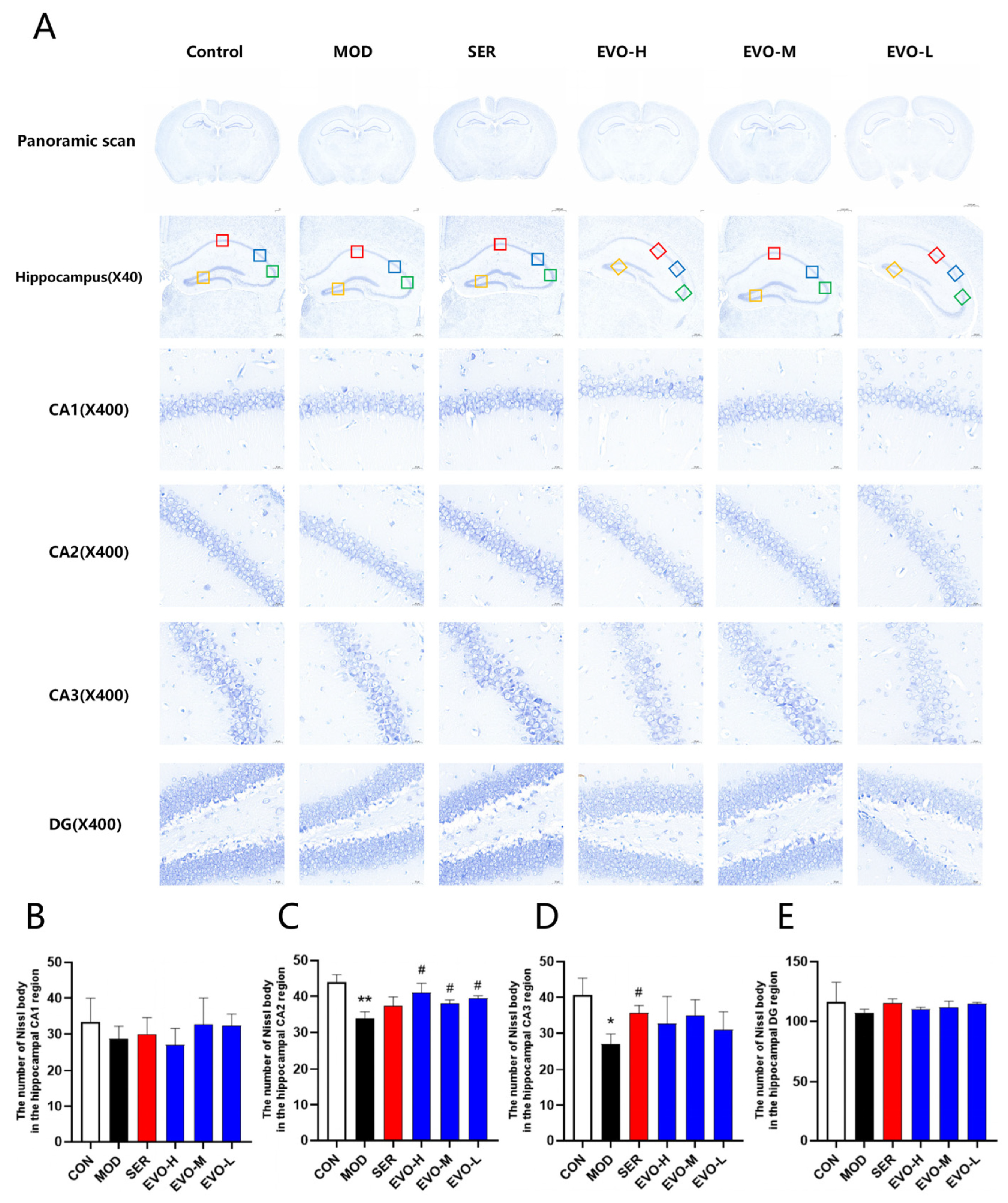
2.3. Effect of EVO on Nissl Bodies in the Hippocampus of PTSD Mice Induced by SPS
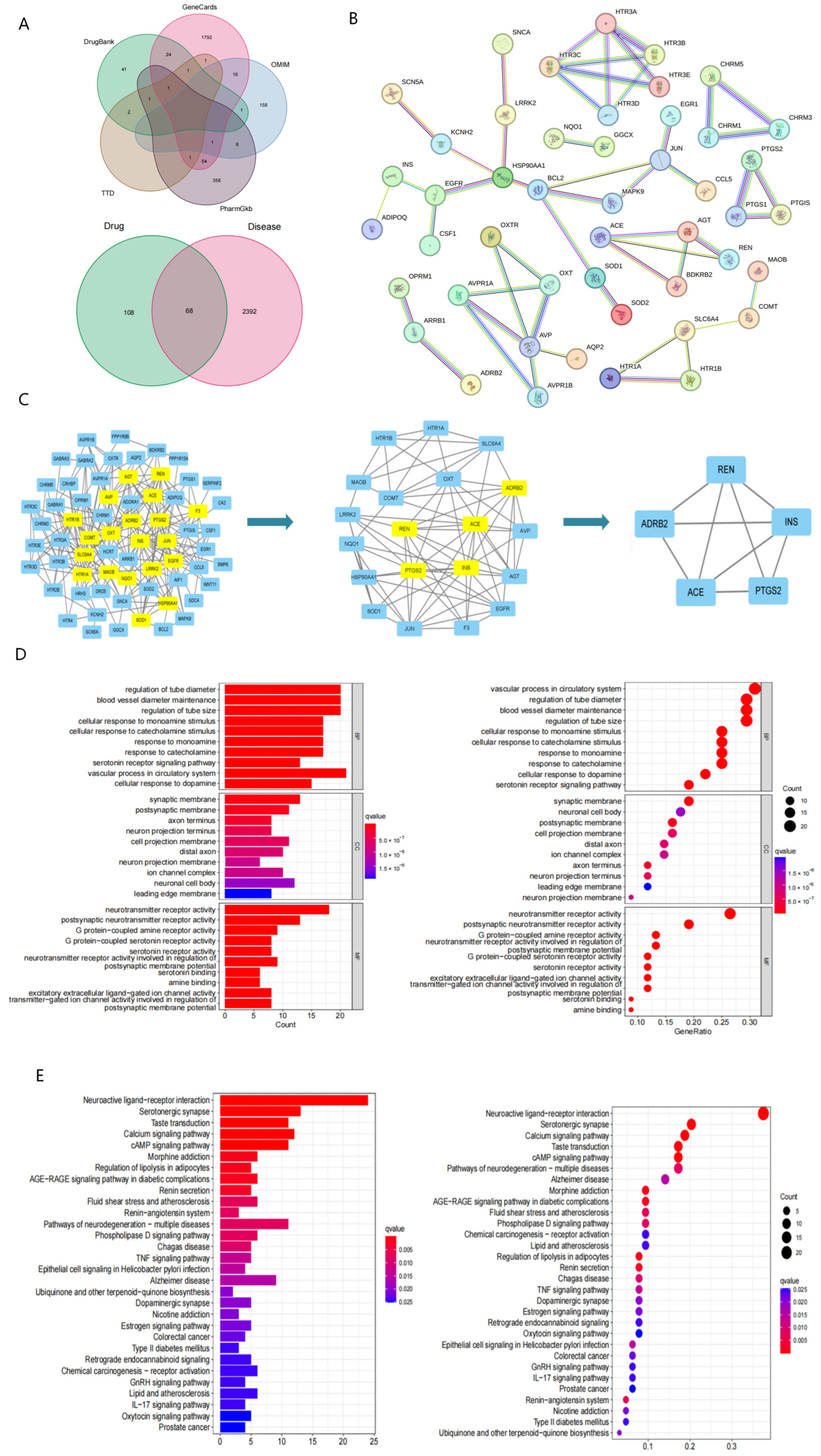
2.4. Network Pharmacology Reveals Potential Targets and Pathways Associated with Evodiamine Intervention in PTSD
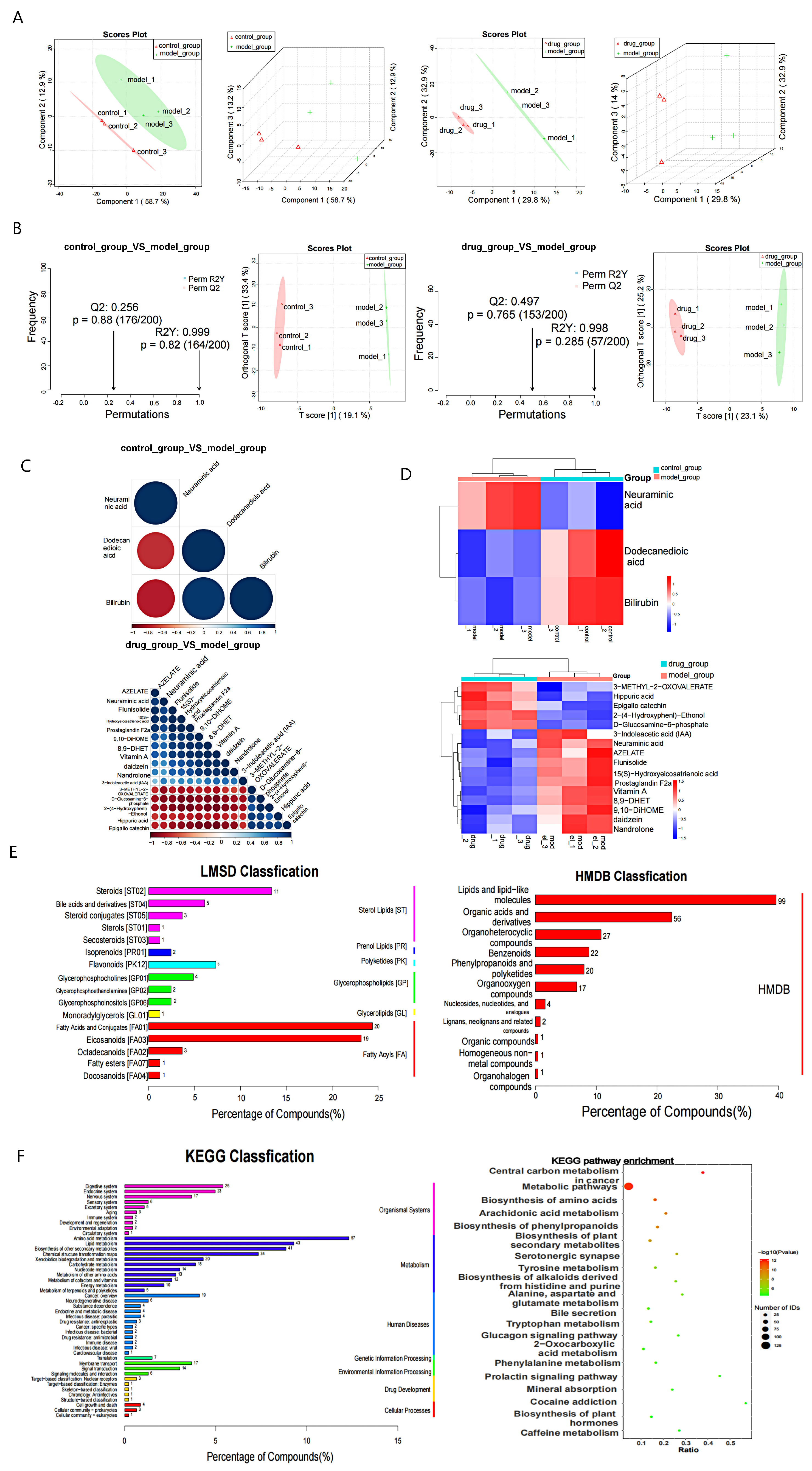
2.5. Effect of EVO on Serum Metabolites in SPS-Induced PTSD
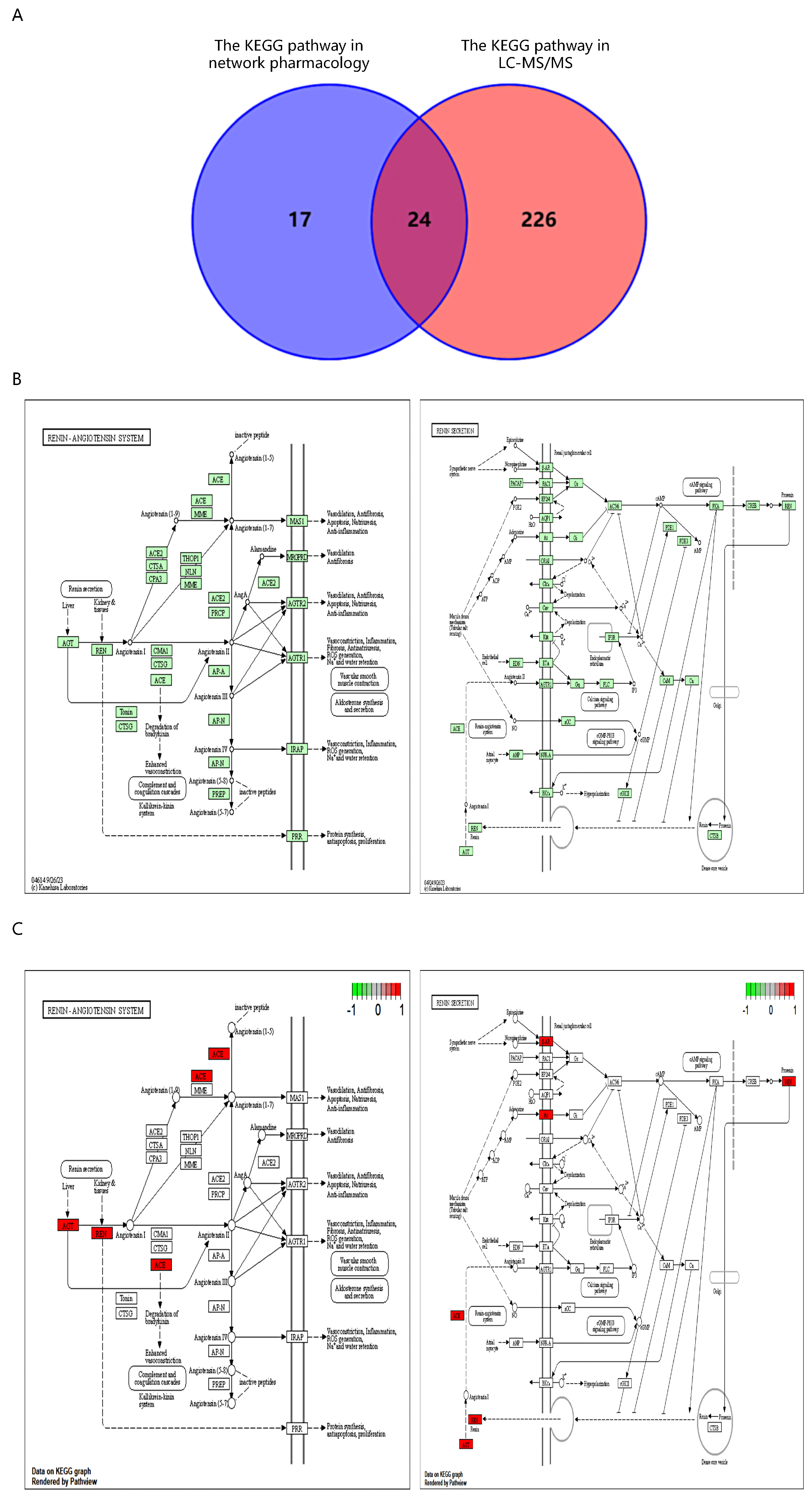
2.6. Intersection of Network Pharmacology and LC-MS/MS KEGG Pathways
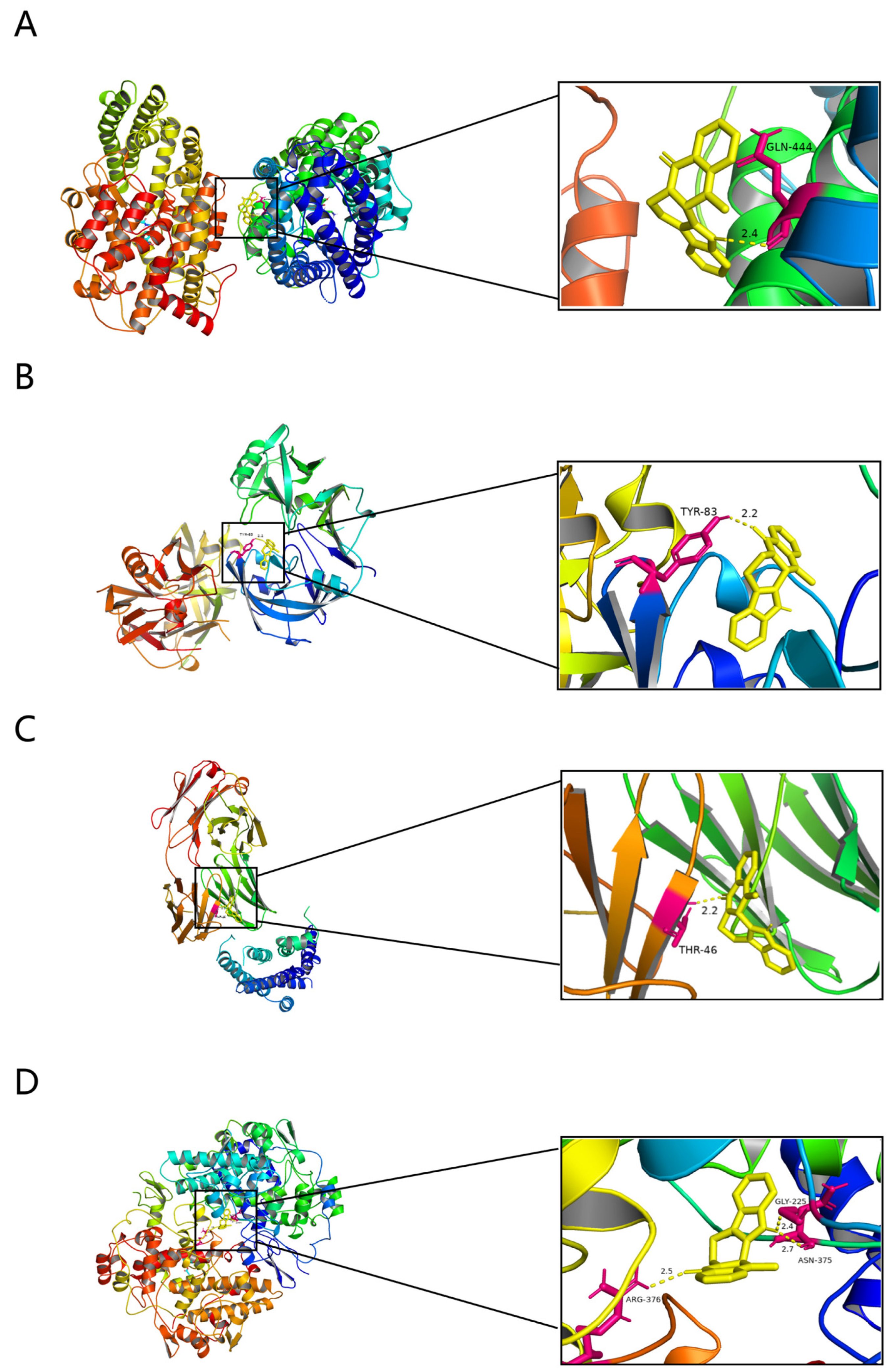
2.7. Molecular Docking Results for EVO and Potential Targets
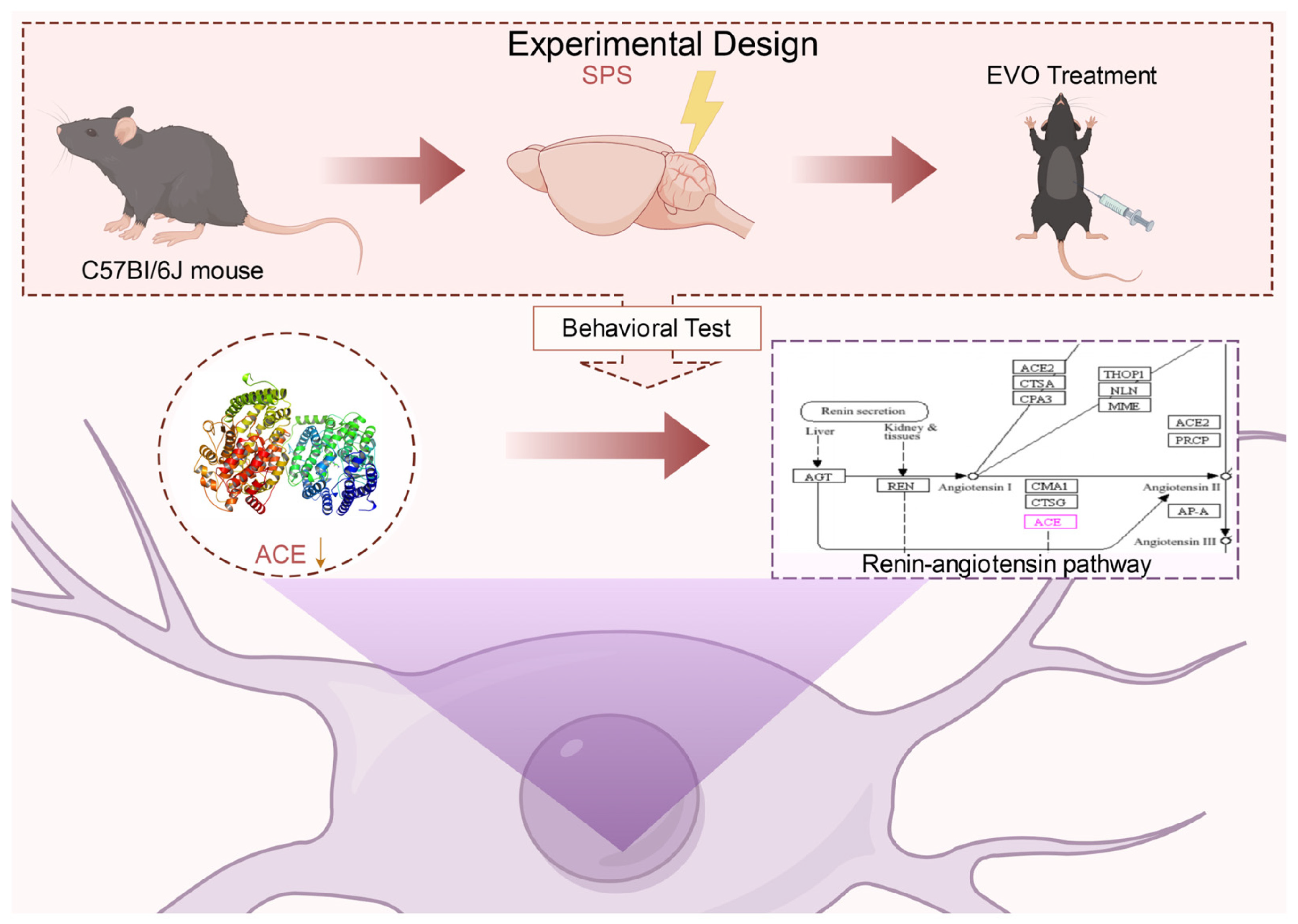
2.8. Effects of EVO on Core Target Levels and the Renin–Angiotensin Pathway in the Hippocampus of SPS-Induced PTSD Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Stimulation of the SPS Model
4.3. Experimental Design
4.4. Behavioral Tests
4.4.1. Open Field Test (OFT)
4.4.2. Tail Suspension Test (TST)
4.4.3. Elevated Plus Maze Test (EPMT)
4.4.4. Forced Swimming Test (FST)
4.4.5. Morris Water Maze (MWM) Test
4.5. Histopathologic Analysis
4.6. Nissl Staining and Neuron Counting
4.7. Network Pharmacological Analysis
4.7.1. Collection and Screening of EVO Targets
4.7.2. Collection of Disease Targets
4.7.3. Constructing the PPI Network Diagram and Obtaining Core Targets
4.7.4. Analysis of GO and KEGG Pathway Enrichment
4.8. LC-MS/MS-Based Serum Metabolomics Studies
4.9. Molecular Docking
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Immunofluorescence
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, B.; Choi, G.M.; Sur, B. Silibinin prevents depression-like behaviors in a single prolonged stress rat model: The possible role of serotonin. BMC Complement Med. Ther. 2020, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Feduccia, A.A.; Jerome, L.; Yazar-Klosinski, B.; Emerson, A.; Mithoefer, M.C.; Doblin, R. Breakthrough for Trauma Treatment: Safety and Efficacy of MDMA-Assisted Psychotherapy Compared to Paroxetine and Sertraline. Front. Psychiatry 2019, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Yunitri, N.; Chu, H.; Kang, X.L.; Jen, H.J.; Pien, L.C.; Tsai, H.T.; Kamil, A.R.; Chou, K.R. Global prevalence and associated risk factors of posttraumatic stress disorder during COVID-19 pandemic: A meta-analysis. Int. J. Nurs. Stud. 2022, 126, 104136. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.B.; Singewald, N. Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol. Ther. 2019, 204, 107402. [Google Scholar] [CrossRef] [PubMed]
- Stübner, S.; Grohmann, R.; Greil, W.; Zhang, X.; Müller-Oerlinghausen, B.; Bleich, S.; Rüther, E.; Möller, H.J.; Engel, R.; Falkai, P.; et al. Suicidal Ideation and Suicidal Behavior as Rare Adverse Events of Antidepressant Medication: Current Report from the AMSP Multicenter Drug Safety Surveillance Project. Int. J. Neuropsychopharmacol. 2018, 21, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, X.; Zhao, Y.; Zhang, L.; Bai, X.; Zhang, J.; Zhao, X.; Chen, L.; Wang, L.; Cui, L. Pretreatment by evodiamine is neuroprotective in cerebral ischemia: Up-regulated pAkt, pGSK3β, down-regulated NF-κB expression, and ameliorated BBB permeability. Neurochem. Res. 2014, 39, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M.; Gao, K.; Wang, D.M.; Quan, X.Z.; Liu, J.N.; Ma, C.M.; Qin, C.; Zhang, L.F. Evodiamine improves congnitive abilities in SAMP8 and APP(swe)/PS1(ΔE9) transgenic mouse models of Alzheimer’s disease. Acta Pharmacol. Sin. 2011, 32, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Chang, J.Y.; Wang, F.Y.; Tseng, J.; Chang, J.G. The effect of Evodia rutaecarpa extract on cytokine secretion by human mononuclear cells in vitro. Am. J. Chin. Med. 1995, 23, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yang, F.; Luo, F.; Liu, Y.; Zhang, F.; Zou, M.; Liu, Q. Evodiamine exerts anti-tumor effects against hepatocellular carcinoma through inhibiting beta-catenin-mediated angiogenesis. Tumour. Biol. 2016, 37, 12791–12803. [Google Scholar] [CrossRef]
- Wei, J.; Ching, L.C.; Zhao, J.F.; Shyue, S.K.; Lee, H.F.; Kou, Y.R.; Lee, T.S. Essential role of transient receptor potential vanilloid type 1 in evodiamine-mediated protection against atherosclerosis. Acta Physiol. 2013, 207, 299–307. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, X.; Xu, Y.; Lu, X.; He, S.; He, R.; Gong, M. Optimization of combinations of ginsenoside-Rg1, ginsenoside-Rb1, evodiamine and rutaecarpine for effective therapy of mouse migraine. J. Nat. Med. 2016, 70, 207–216. [Google Scholar] [CrossRef]
- Smith, H.C.; Yu, Z.; Iyer, L.; Marvar, P.J. Sex-dependent effects of angiotensin type 2 receptor expressing medial prefrontal cortex (mPFC) interneurons in fear extinction learning. Biol. Psychiatry Glob. Open Sci. 2023; in press. [Google Scholar] [CrossRef]
- Seligowski, A.V.; Duffy, L.A.; Merker, J.B.; Michopoulos, V.; Gillespie, C.F.; Marvar, P.J.; Stein, M.B.; Ressler, K.J. The renin-angiotensin system in PTSD: A replication and extension. Neuropsychopharmacology 2021, 46, 750–755. [Google Scholar] [CrossRef]
- Osborn, J.W.; Fink, G.D. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp. Physiol. 2010, 95, 61–68. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Liu, L.; Li, S. Protective effects of evodiamine in experimental paradigm of Alzheimer’s disease. Cogn. Neurodyn. 2018, 12, 303–313. [Google Scholar] [CrossRef]
- Yamamoto, S.; Morinobu, S.; Takei, S.; Fuchikami, M.; Matsuki, A.; Yamawaki, S.; Liberzon, I. Single prolonged stress: Toward an animal model of posttraumatic stress disorder. Depress Anxiety 2009, 26, 1110–1117. [Google Scholar] [CrossRef]
- Keller, S.M.; Schreiber, W.B.; Stanfield, B.R.; Knox, D. Inhibiting corticosterone synthesis during fear memory formation exacerbates cued fear extinction memory deficits within the single prolonged stress model. Behav. Brain Res. 2015, 287, 182–186. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; Rhee, D.; Martin, L.; Smith, J.A.; Sonti, A.N.; Padmanaban, V.; Cameron, H.A. New neurons restore structural and behavioral abnormalities in a rat model of PTSD. Hippocampus 2019, 29, 848–861. [Google Scholar] [CrossRef]
- Heo, S.-K.; Yun, H.-J.; Yi, H.-S.; Noh, E.-K.; Park, S.-D. Evodiamine and rutaecarpine inhibit migration by LIGHT via suppression of NADPH oxidase activation. J. Cell. Biochem. 2009, 107, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ehli, E.A.; Hudziak, J.J.; Davies, G.E. Berberine and evodiamine influence serotonin transporter (5-HTT) expression via the 5-HTT-linked polymorphic region. Pharmacogenomics J. 2012, 12, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Terock, J.; Hannemann, A.; Janowitz, D.; Freyberger, H.J.; Felix, S.B.; Dorr, M.; Nauck, M.; Volzke, H.; Grabe, H.J. Associations of trauma exposure and post-traumatic stress disorder with the activity of the renin-angiotensin-aldosterone-system in the general population. Psychol. Med. 2019, 49, 843–851. [Google Scholar] [CrossRef]
- Zanchetti, A.; Elmfeldt, D. Findings and implications of the Study on COgnition and Prognosis in the Elderly (SCOPE)—A review. Blood Press 2006, 15, 71–79. [Google Scholar] [CrossRef]
- Chiou, W.F.; Chou, C.J.; Shum, A.Y.; Chen, C.F. The vasorelaxant effect of evodiamine in rat isolated mesenteric arteries: Mode of action. Eur. J. Pharmacol. 1992, 215, 277–283. [Google Scholar] [CrossRef]
- Hung, P.H.; Lin, L.C.; Wang, G.J.; Chen, C.F.; Wang, P.S. Inhibitory effect of evodiamine on aldosterone release by Zona glomerulosa cells in male rats. Chin. J. Physiol. 2001, 44, 53–57. [Google Scholar]
- Carobrez, A.P.; Bertoglio, L.J. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 2005, 29, 1193–1205. [Google Scholar] [CrossRef]
- Malik, H.; Usman, M.; Arif, M.; Ahmed, Z.; Ali, G.; Rauf, K.; Sewell, R.D.E. Diosgenin normalization of disrupted behavioral and central neurochemical activity after single prolonged stress. Front. Pharmacol. 2023, 14, 1232088. [Google Scholar] [CrossRef]
- Zhang, J.P.; Zhang, K.Y.; Guo, L.; Chen, Q.L.; Gao, P.; Wang, T.; Li, J.; Guo, G.Z.; Ding, G.R. Effects of 1.8 GHz Radiofrequency Fields on the Emotional Behavior and Spatial Memory of Adolescent Mice. Int. J. Environ. Res. Public Health 2017, 14, 1344. [Google Scholar] [CrossRef]
- Belzung, C.; Turiault, M.; Griebel, G. Optogenetics to study the circuits of fear- and depression-like behaviors: A critical analysis. Pharmacol. Biochem. Behav. 2014, 122, 144–157. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef]
- Rossato, J.I.; Zinn, C.G.; Furini, C.; Bevilaqua, L.R.; Medina, J.H.; Cammarota, M.; Izquierdo, I. A link between the hippocampal and the striatal memory systems of the brain. Acad Bras. Cienc. 2006, 78, 515–523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kenney, J.W.; Gould, T.J. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol. Neurobiol. 2008, 38, 101–121. [Google Scholar] [CrossRef]
- Bossini, L.; Tavanti, M.; Lombardelli, A.; Calossi, S.; Polizzotto, N.R.; Galli, R.; Vatti, G.; Pieraccini, F.; Castrogiovanni, P. Changes in hippocampal volume in patients with post-traumatic stress disorder after sertraline treatment. J. Clin. Psychopharmacol. 2007, 27, 233–235. [Google Scholar] [CrossRef]
- Daniels, J.K.; McFarlane, A.C.; Bluhm, R.L.; Moores, K.A.; Clark, C.R.; Shaw, M.E.; Williamson, P.C.; Densmore, M.; Lanius, R.A. Switching between executive and default mode networks in posttraumatic stress disorder: Alterations in functional connectivity. J. Psychiatry Neurosci. 2010, 35, 258–266. [Google Scholar] [CrossRef]
- Carrion, V.G.; Weems, C.F.; Watson, C.; Eliez, S.; Menon, V.; Reiss, A.L. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: An MRI study. Psychiatry Res. 2009, 172, 226–234. [Google Scholar] [CrossRef]
- Schauer, R. Sialic acids: Fascinating sugars in higher animals and man. Zoology 2004, 107, 49–64. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- Yerlikaya, F.H.; Toker, A.; Cicekler, H.; Aribas, A. The association of total sialic acid and malondialdehyde levels with metabolic and anthropometric variables in obesity. Biotech. Histochem. 2015, 90, 31–37. [Google Scholar] [CrossRef]
- Otsuka, T.; Hori, H.; Yoshida, F.; Itoh, M.; Lin, M.; Niwa, M.; Ino, K.; Imai, R.; Ogawa, S.; Matsui, M.; et al. Association of CRP genetic variation with symptomatology, cognitive function, and circulating proinflammatory markers in civilian women with PTSD. J. Affect Disord. 2021, 279, 640–649. [Google Scholar] [CrossRef]
- Bansal, P.; Bansal, P.; Verma, R. Association of serum sialic acid concentration with diabetic complications and cardiovascular risk factors in an Indian population. Arch. Med. Sci. Atheroscler. Dis. 2021, 6, e14–e17. [Google Scholar] [CrossRef]
- Lee, T.; Jarome, T.; Li, S.J.; Kim, J.J.; Helmstetter, F.J. Chronic stress selectively reduces hippocampal volume in rats: A longitudinal magnetic resonance imaging study. Neuroreport 2009, 20, 1554–1558. [Google Scholar] [CrossRef]
- Geuze, E.; Westenberg, H.G.; Heinecke, A.; de Kloet, C.S.; Goebel, R.; Vermetten, E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage 2008, 41, 675–681. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Liu, Y.; Li, Y.Y.; Chen, C.; Liu, L.M.; Wu, Y.M.; Li, S.; Yang, C. Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress. Med. Sci. Monit. 2014, 20, 499–512. [Google Scholar] [CrossRef]
- Lee, A.L.; Ogle, W.O.; Sapolsky, R.M. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar. Disord. 2002, 4, 117–128. [Google Scholar] [CrossRef]
- Wang, F.; Song, J.; Yan, Y.; Zhou, Q.; Li, X.; Wang, P.; Yang, Z.; Zhang, Q.; Zhang, H. Integrated Network Pharmacology Analysis and Serum Metabolomics to Reveal the Anti-malaria Mechanism of Artesunate. ACS Omega 2022, 7, 31482–31494. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Rodriguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef]
- Zhang, T.L.; Fu, J.L.; Geng, Z.; Yang, J.J.; Sun, X.J. The neuroprotective effect of losartan through inhibiting AT1/ASK1/MKK4/JNK3 pathway following cerebral I/R in rat hippocampal CA1 region. CNS Neurosci. Ther. 2012, 18, 981–987. [Google Scholar] [CrossRef]
- Tota, S.; Kamat, P.K.; Saxena, G.; Hanif, K.; Najmi, A.K.; Nath, C. Central angiotensin converting enzyme facilitates memory impairment in intracerebroventricular streptozotocin treated rats. Behav. Brain Res. 2012, 226, 317–330. [Google Scholar] [CrossRef]
- Kumaran, D.; Udayabanu, M.; Kumar, M.; Aneja, R.; Katyal, A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience 2008, 155, 626–639. [Google Scholar] [CrossRef]
- Gadelha, A.; Vendramini, A.M.; Yonamine, C.M.; Nering, M.; Berberian, A.; Suiama, M.A.; Oliveira, V.; Lima-Landman, M.T.; Breen, G.; Bressan, R.A.; et al. Convergent evidences from human and animal studies implicate angiotensin I-converting enzyme activity in cognitive performance in schizophrenia. Transl. Psychiatry 2015, 5, e691. [Google Scholar] [CrossRef]
- Schwengel, K.; Namsolleck, P.; Lucht, K.; Clausen, B.H.; Lambertsen, K.L.; Valero-Esquitino, V.; Thone-Reineke, C.; Muller, S.; Widdop, R.E.; Denton, K.M.; et al. Angiotensin AT2-receptor stimulation improves survival and neurological outcome after experimental stroke in mice. J. Mol. Med. 2016, 94, 957–966. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Du, Y.; Sun, H.; Zhang, W.; Wang, A.; Li, Q.; Li, C.; Wang, Y.; Du, Z.; et al. Effect of ketamine on mood dysfunction and spatial cognition deficits in PTSD mouse models via HCN1-BDNF signaling. J. Affect Disord. 2021, 286, 248–258. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Qiu, Z.K.; He, J.L.; Liu, X.; Chen, J.S.; Wang, Y.L. Resveratrol ameliorated the behavioral deficits in a mouse model of post-traumatic stress disorder. Pharmacol. Biochem. Behav. 2017, 161, 68–76. [Google Scholar] [CrossRef]
- Kurosawa, N.; Shimizu, K.; Seki, K. The development of depression-like behavior is consolidated by IL-6-induced activation of locus coeruleus neurons and IL-1β-induced elevated leptin levels in mice. Psychopharmacology 2016, 233, 1725–1737. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Qiao, H. Downregulation of hippocampal SIRT6 activates AKT/CRMP2 signaling and ameliorates chronic stress-induced depression-like behavior in mice. Acta Pharmacol. Sin. 2020, 41, 1557–1567. [Google Scholar] [CrossRef]
- Lo Iacono, L.; Ielpo, D.; Accoto, A.; Di Segni, M.; Babicola, L.; D’Addario, S.L.; Ferlazzo, F.; Pascucci, T.; Ventura, R.; Andolina, D. MicroRNA-34a Regulates the Depression-like Behavior in Mice by Modulating the Expression of Target Genes in the Dorsal Raphe. Mol. Neurobiol. 2020, 57, 823–836. [Google Scholar] [CrossRef]
- Taniguchi, E.; Tashiro, A.; Hattori, A.; Furuse, M.; Yasuo, S. Photoperiodic changes in hippocampal neurogenesis and plasma metabolomic profiles in relation to depression-like behavior in mice. Behav. Brain Res. 2021, 403, 113136. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Lai, C.; Shen, B.; Li, B.; Chen, J.; Shen, X.; Huang, Z.; Yang, C.; Gao, Y. Effects of Evodiamine on Behavior and Hippocampal Neurons through Inhibition of Angiotensin-Converting Enzyme and Modulation of the Renin Angiotensin Pathway in a Mouse Model of Post-Traumatic Stress Disorder. Nutrients 2024, 16, 1957. https://doi.org/10.3390/nu16121957
Wang Z, Lai C, Shen B, Li B, Chen J, Shen X, Huang Z, Yang C, Gao Y. Effects of Evodiamine on Behavior and Hippocampal Neurons through Inhibition of Angiotensin-Converting Enzyme and Modulation of the Renin Angiotensin Pathway in a Mouse Model of Post-Traumatic Stress Disorder. Nutrients. 2024; 16(12):1957. https://doi.org/10.3390/nu16121957
Chicago/Turabian StyleWang, Zhixing, Chengcai Lai, Baoying Shen, Bowei Li, Junru Chen, Xin Shen, Zhengping Huang, Chunqi Yang, and Yue Gao. 2024. "Effects of Evodiamine on Behavior and Hippocampal Neurons through Inhibition of Angiotensin-Converting Enzyme and Modulation of the Renin Angiotensin Pathway in a Mouse Model of Post-Traumatic Stress Disorder" Nutrients 16, no. 12: 1957. https://doi.org/10.3390/nu16121957
APA StyleWang, Z., Lai, C., Shen, B., Li, B., Chen, J., Shen, X., Huang, Z., Yang, C., & Gao, Y. (2024). Effects of Evodiamine on Behavior and Hippocampal Neurons through Inhibition of Angiotensin-Converting Enzyme and Modulation of the Renin Angiotensin Pathway in a Mouse Model of Post-Traumatic Stress Disorder. Nutrients, 16(12), 1957. https://doi.org/10.3390/nu16121957





