The Combination of Lactoferrin and Creatine Ameliorates Muscle Decay in a Sarcopenia Murine Model
Abstract
1. Introduction
2. Material and Methods
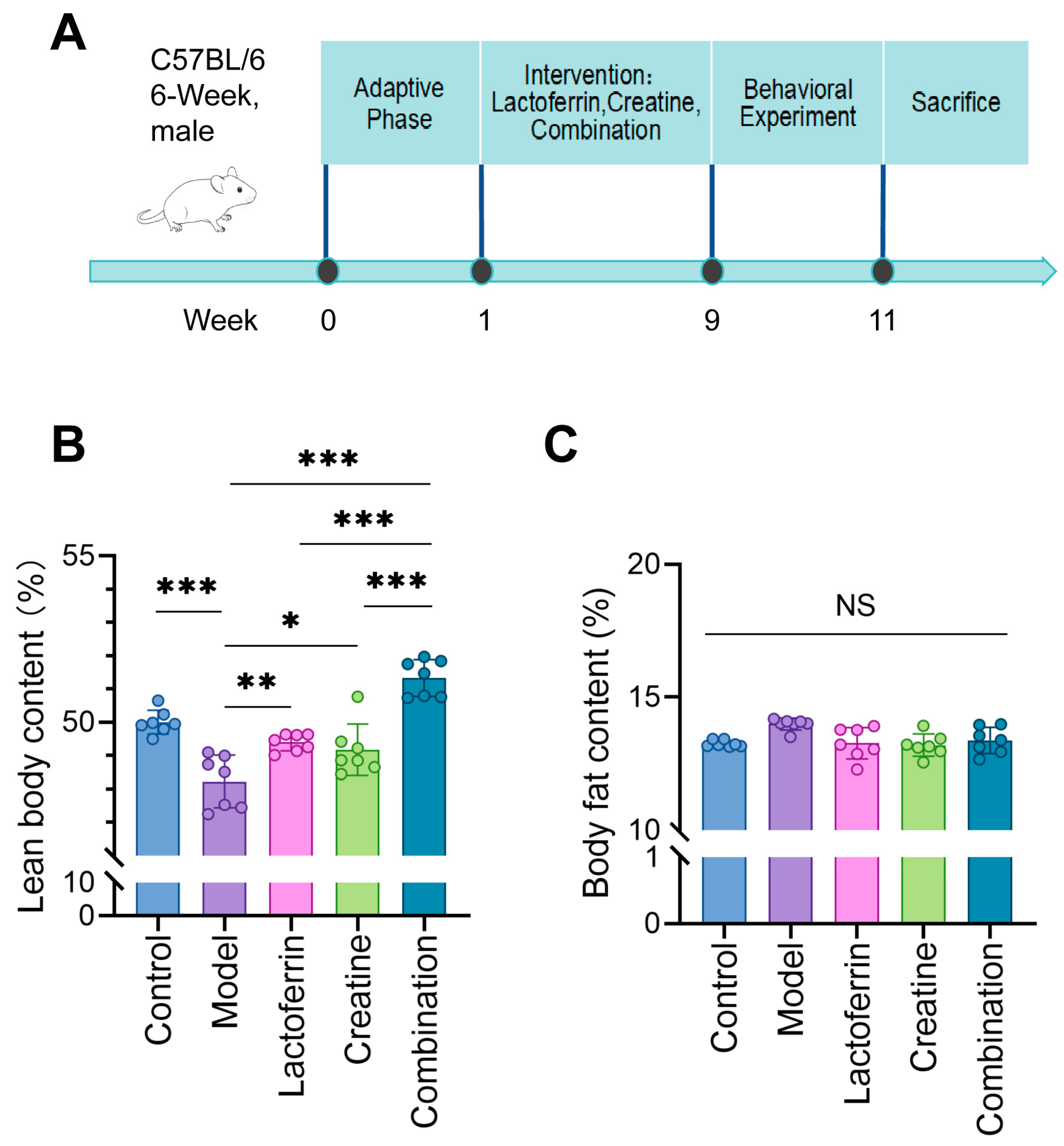
2.1. Animals
2.2. Analysis of Body Composition
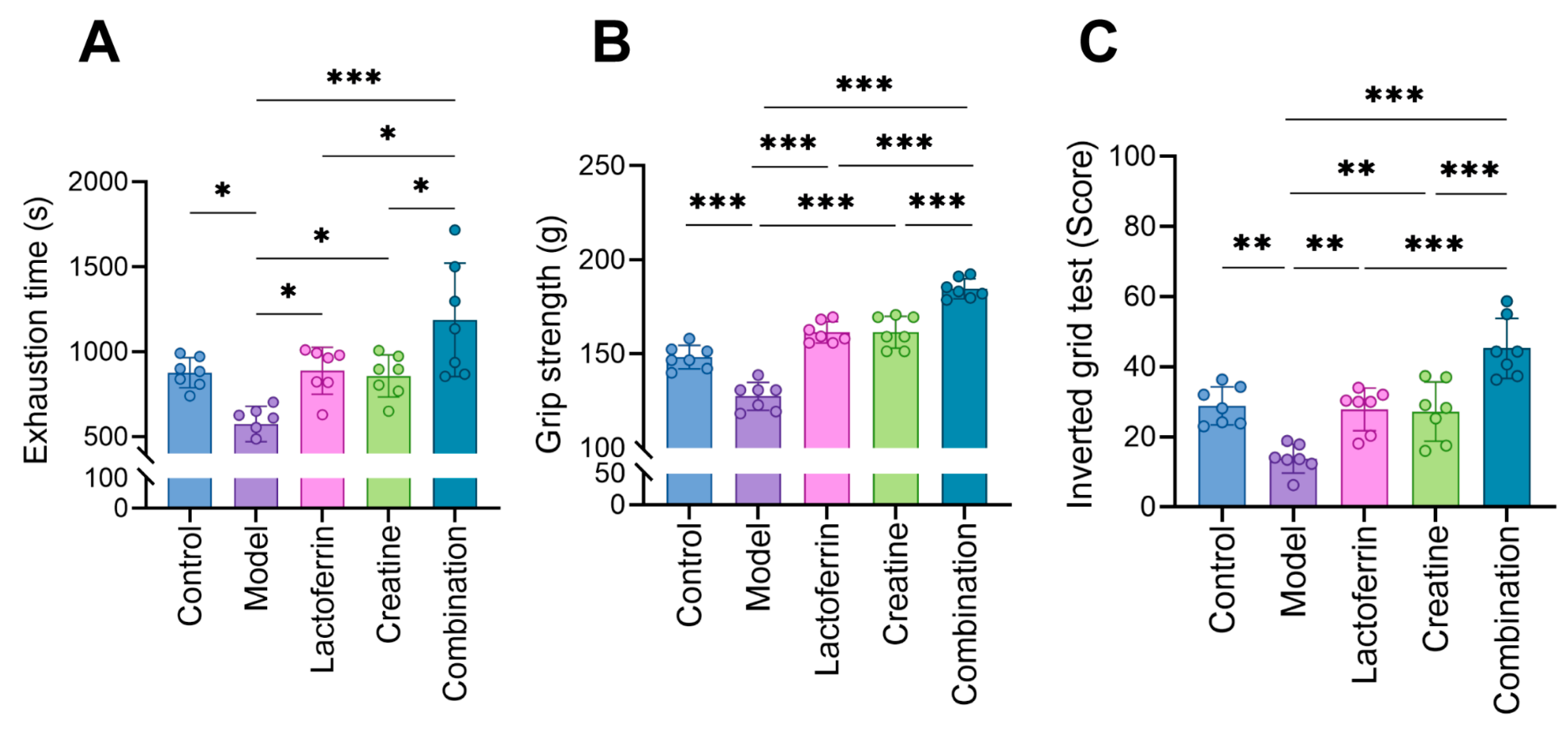
2.3. Grid Hanging Test
2.4. Grip Strength Test
2.5. Endurance Test
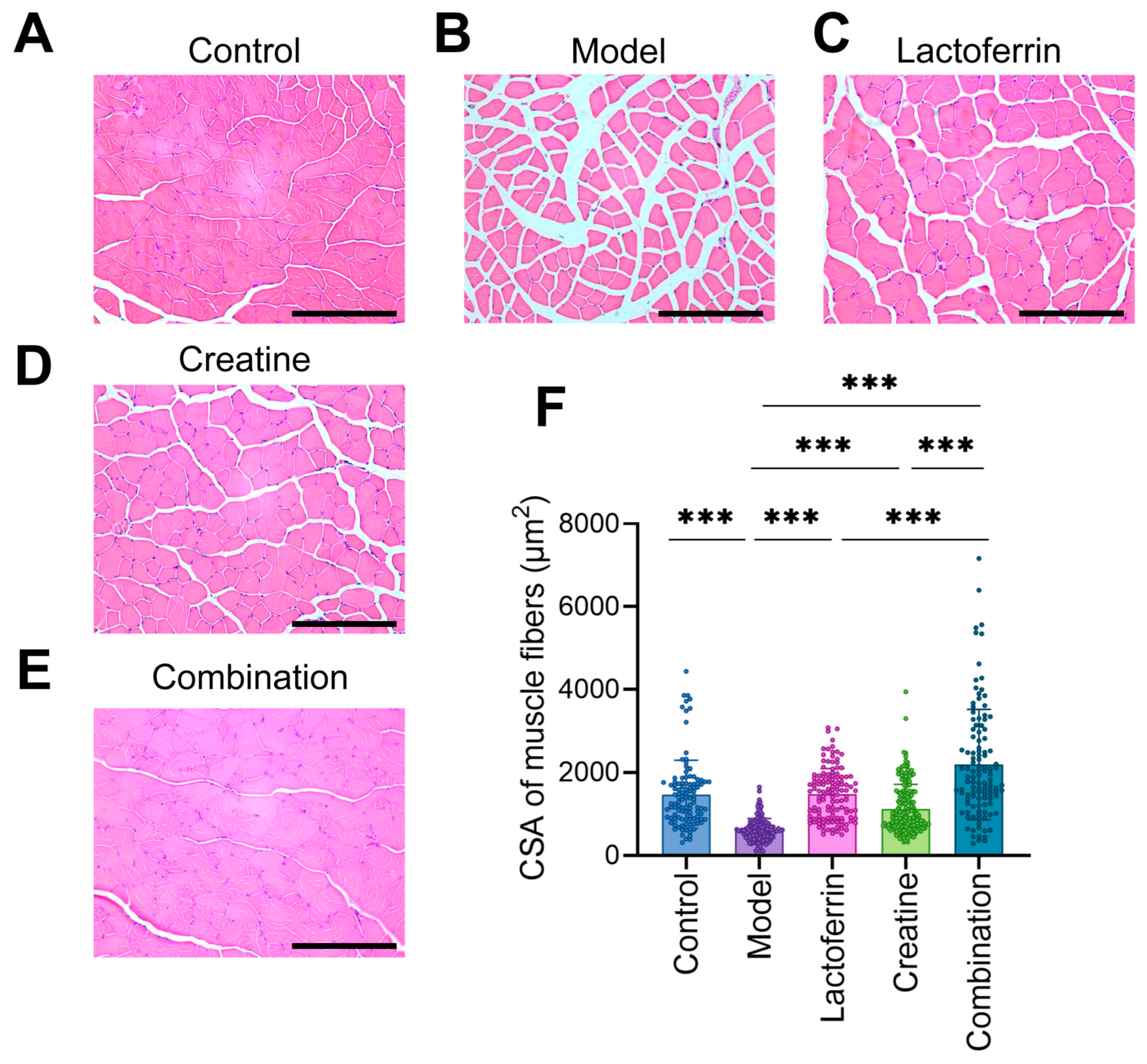
2.6. Hematoxylin–Eosin (HE) Staining for GAS
2.7. RNA-Seq
2.8. Functional Enrichment Analysis
2.9. Quantitative Real-Time PCR and Western Blotting
2.10. Statistical Analysis
3. Result
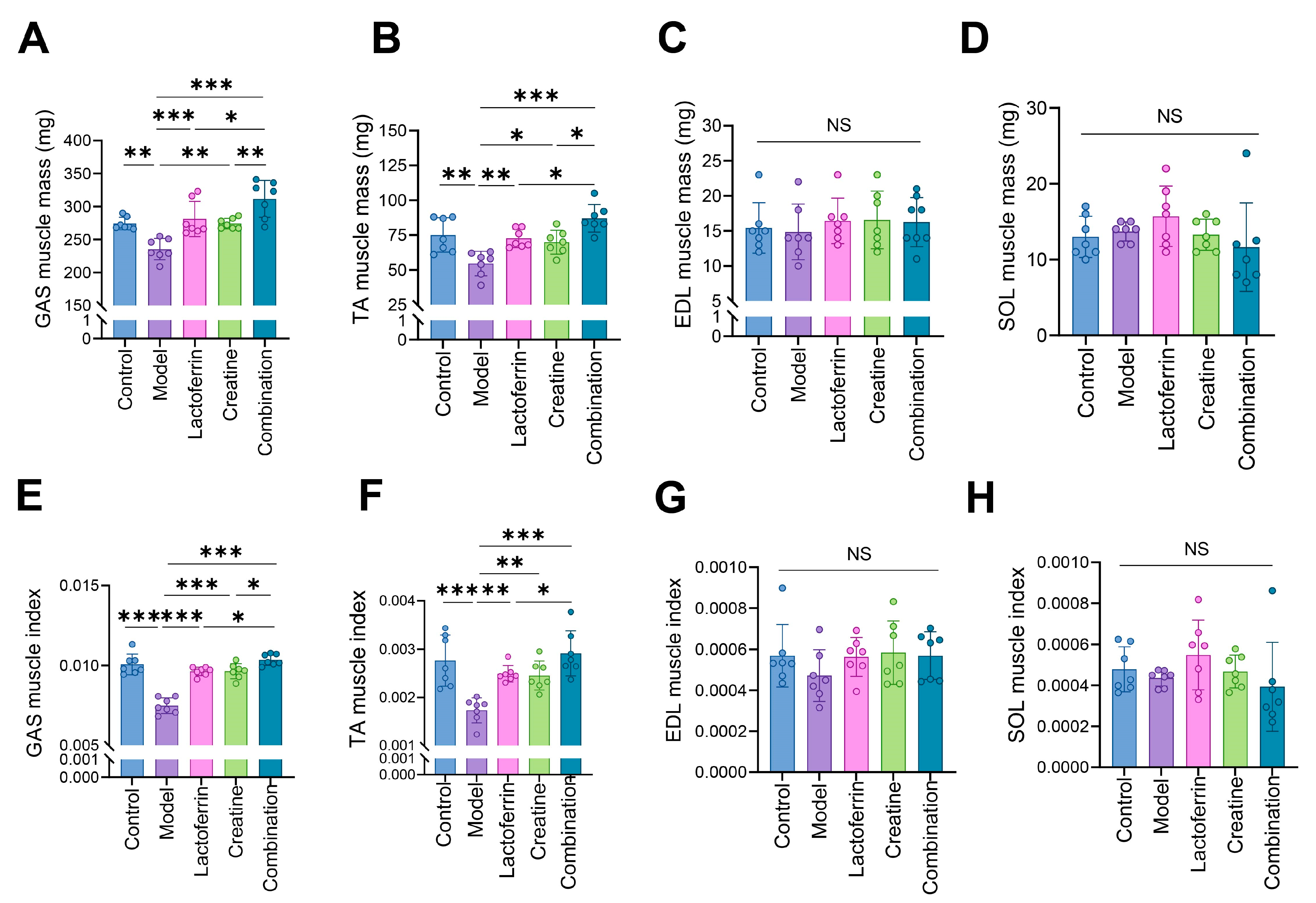
3.1. Lf and Cr Delayed Muscle Mass Loss
3.2. Lf and Cr Improved Muscle Function
3.3. Lf and Cr Improved the Condition of Muscle Tissue
3.4. RNA-Seq in Mice Muscle
3.4.1. Gene Ontology (GO) Enrichment Analysis
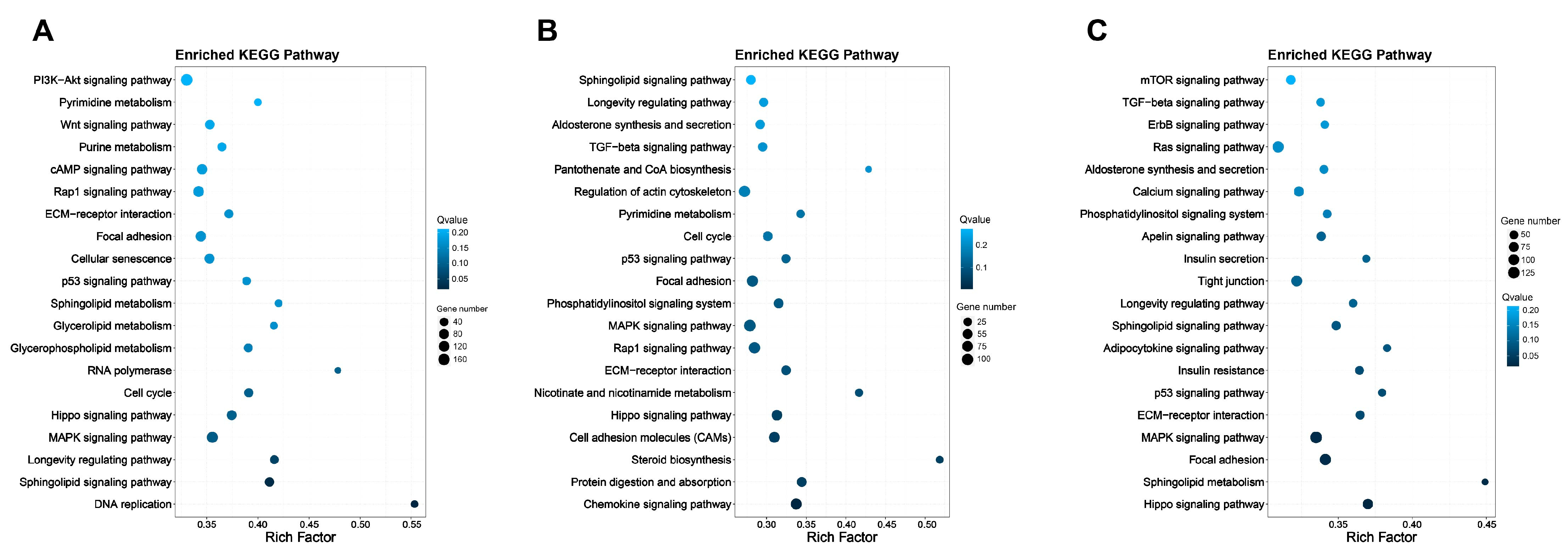
3.4.2. KEGG Pathway Enrichment Analysis
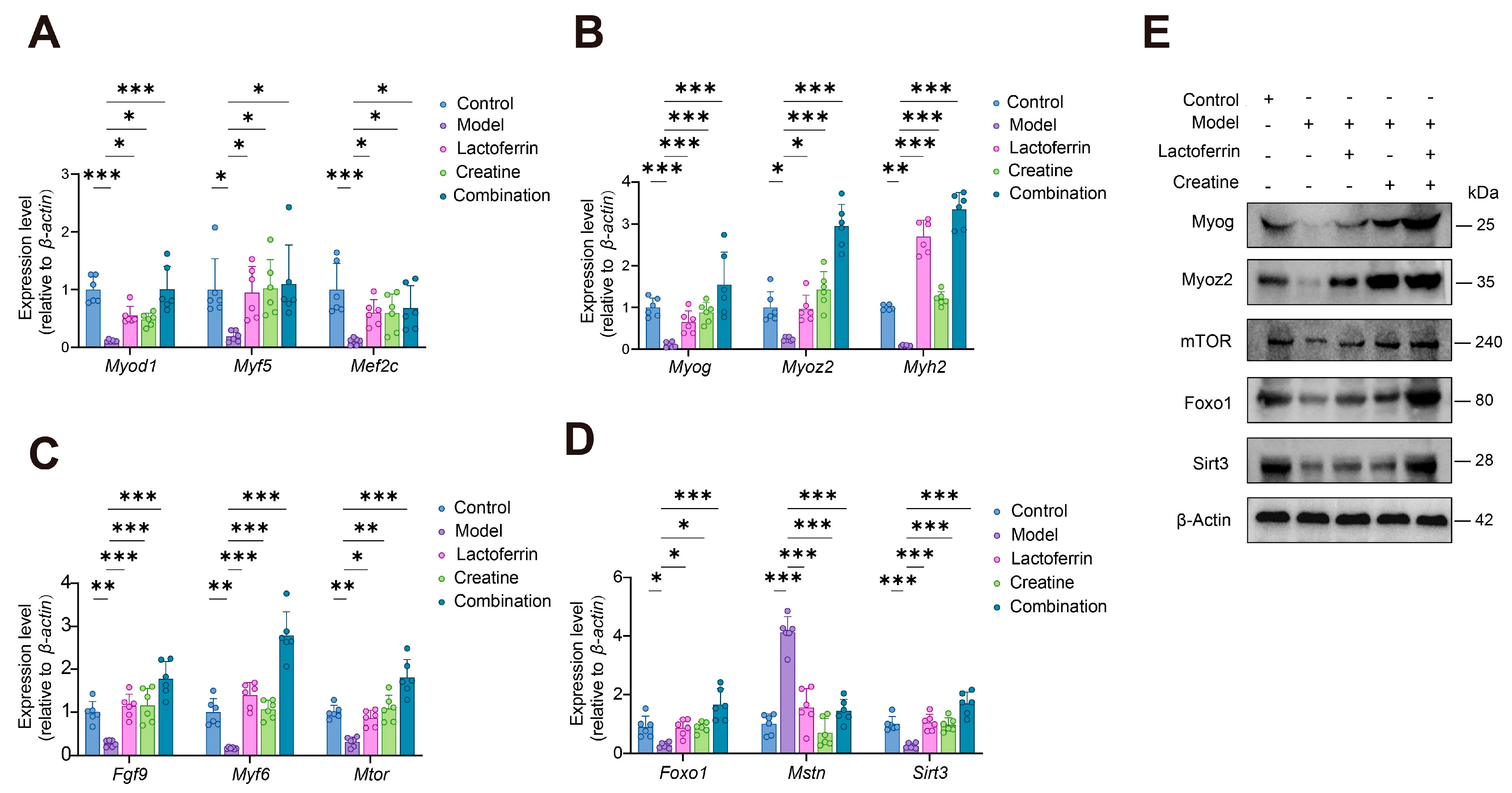
3.4.3. Validation of the mRNA and Protein Expression Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| D-gal | D-galactose. |
| Lf | Lactoferrin. |
| Cr | Creatine. |
| GAS | Gastrocnemius. |
| TA | Tibialis anterior. |
| EDL | Extensor digitorum longus. |
| SOL | Soleus. |
| HE | Hematoxylin–Eosin. |
| HISAT | Hierarchical Indexing for Spliced Alignment of Transcripts. |
| DEGs | Differentially expressed genes. |
| FDR | False-discovery rate. |
| GO | Gene Ontology. |
| KEGG | Kyoto encyclopedia of genes and genomes. |
| SCs | Satellite cells. |
| CAMs | Cell adhesion molecules. |
References
- Guo, X.; Luo, J.; Qi, J.; Zhao, X.; An, P.; Luo, Y.; Wang, G. The Role and Mechanism of Polysaccharides in Anti-Aging. Nutrients 2022, 14, 5330. [Google Scholar] [CrossRef]
- Dao, T.; Green, A.E.; Kim, Y.A.; Bae, S.J.; Ha, K.T.; Gariani, K.; Lee, M.R.; Menzies, K.J.; Ryu, D. Sarcopenia and Muscle Aging: A Brief Overview. Endocrinol. Metab. 2020, 35, 716–732. [Google Scholar] [CrossRef]
- Kitamura, A.; Seino, S.; Abe, T.; Nofuji, Y.; Yokoyama, Y.; Amano, H.; Nishi, M.; Taniguchi, Y.; Narita, M.; Fujiwara, Y.; et al. Sarcopenia: Prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J. Cachexia Sarcopenia Muscle 2021, 12, 30–38. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990s–991s. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, D.; Marco, E.; Cruz-Jentoft, A.J. Defining sarcopenia: Some caveats and challenges. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 127–132. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging 2018, 13, 913–927. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, G.; Xiao, Y.; Liu, H.; Chen, X.; Shen, M. Allergic Phenotypes and Sarcopenia: Evidence from Observational Studies and Mendelian Randomization Analysis. Phenomics 2024, 4, 46–50. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R. Molecular Mechanism and Pathogenesis of Sarcopenia: An Overview. Int. J. Mol. Sci. 2021, 22, 3032. [Google Scholar] [CrossRef]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical Pathways of Sarcopenia and Their Modulation by Physical Exercise: A Narrative Review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef]
- Ali, S.; Garcia, J.M. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options—A mini-review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Kirk, B.; Duque, G. Current Evidence and Possible Future Applications of Creatine Supplementation for Older Adults. Nutrients 2021, 13, 745. [Google Scholar] [CrossRef]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef]
- Rossi, A.P.; D’Introno, A.; Rubele, S.; Caliari, C.; Gattazzo, S.; Zoico, E.; Mazzali, G.; Fantin, F.; Zamboni, M. The Potential of β-Hydroxy-β-Methylbutyrate as a New Strategy for the Management of Sarcopenia and Sarcopenic Obesity. Drugs Aging 2017, 34, 833–840. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Zhong, H.; Jiang, Y.; Shi, X.; Yuan, B.; Yu, N.; Zhang, S.; Yuan, X.; Guo, S.; et al. Carrier strategies boost the application of CRISPR/Cas system in gene therapy. Exploration 2022, 2, 20210081. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Jakobi, J.M.; Rice, C.L.; Curtin, S.V.; Marsh, G.D. Neuromuscular properties and fatigue in older men following acute creatine supplementation. Eur. J. Appl. Physiol. 2001, 84, 321–328. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef]
- Forbes, S.C.; Cordingley, D.M.; Cornish, S.M.; Gualano, B.; Roschel, H.; Ostojic, S.M.; Rawson, E.S.; Roy, B.D.; Prokopidis, K.; Giannos, P.; et al. Effects of Creatine Supplementation on Brain Function and Health. Nutrients 2022, 14, 921. [Google Scholar] [CrossRef]
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Casciola, R.; Leoni, L.; Cuffari, B.; Pecchini, M.; Menozzi, R.; Colecchia, A.; Ravaioli, F. Creatine Supplementation to Improve Sarcopenia in Chronic Liver Disease: Facts and Perspectives. Nutrients 2023, 15, 863. [Google Scholar] [CrossRef]
- Candow, D.G.; Vogt, E.; Johannsmeyer, S.; Forbes, S.C.; Farthing, J.P. Strategic creatine supplementation and resistance training in healthy older adults. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Metab. 2015, 40, 689–694. [Google Scholar] [CrossRef]
- Ni Lochlainn, M.; Bowyer, R.C.E.; Welch, A.A.; Whelan, K.; Steves, C.J. Higher dietary protein intake is associated with sarcopenia in older British twins. Age Ageing 2023, 52, afad018. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; An, Q.; Wang, W.; Cheng, Z.; Dai, Y.; Meng, Q.; Zhang, Y. Lactoferrin Deficiency Impairs Proliferation of Satellite Cells via Downregulating the ERK1/2 Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 7478. [Google Scholar] [CrossRef]
- Wang, C.C.; Fang, C.C.; Lee, Y.H.; Yang, M.T.; Chan, K.H. Effects of 4-Week Creatine Supplementation Combined with Complex Training on Muscle Damage and Sport Performance. Nutrients 2018, 10, 1640. [Google Scholar] [CrossRef]
- Yáñez-Silva, A.; Buzzachera, C.F.; Piçarro, I.D.C.; Januario, R.S.B.; Ferreira, L.H.B.; McAnulty, S.R.; Utter, A.C.; Souza-Junior, T.P. Effect of low dose, short-term creatine supplementation on muscle power output in elite youth soccer players. J. Int. Soc. Sports Nutr. 2017, 14, 5. [Google Scholar] [CrossRef]
- Takakura, N.; Wakabayashi, H.; Ishibashi, H.; Teraguchi, S.; Tamura, Y.; Yamaguchi, H.; Abe, S. Oral lactoferrin treatment of experimental oral candidiasis in mice. Antimicrob. Agents Chemother. 2003, 47, 2619–2623. [Google Scholar] [CrossRef]
- Pino, A.; Giunta, G.; Randazzo, C.L.; Caruso, S.; Caggia, C.; Cianci, A. Bacterial biota of women with bacterial vaginosis treated with lactoferrin: An open prospective randomized trial. Microb. Ecol. Health Dis. 2017, 28, 1357417. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, X.; Zhang, H.; Tian, B.; Weng, Y.; Huang, J.; Lu, C.D.; Shi, H. Effects of Dietary Supplementation of Bovine Lactoferricin on Rumen Microbiota, Lactation, and Health in Dairy Goats. Front. Nutr. 2021, 8, 722303. [Google Scholar] [CrossRef]
- Qian, Y.; Yan, Z.; Ye, T.; Shahin, V.; Jiang, J.; Fan, C. Decoding the regulatory role of ATP synthase inhibitory factor 1 (ATPIF1) in Wallerian degeneration and peripheral nerve regeneration. Exploration 2024, 4, 20230098. [Google Scholar] [CrossRef]
- Tierney, M.T.; Sacco, A. Satellite Cell Heterogeneity in Skeletal Muscle Homeostasis. Trends Cell Biol. 2016, 26, 434–444. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Sosa, P.; Alcalde-Estévez, E.; Asenjo-Bueno, A.; Plaza, P.; Carrillo-López, N.; Olmos, G.; López-Ongil, S.; Ruiz-Torres, M.P. Aging-related hyperphosphatemia impairs myogenic differentiation and enhances fibrosis in skeletal muscle. J. Cachexia Sarcopenia Muscle 2021, 12, 1266–1279. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, J.; Raza, S.H.A.; Song, Y.; Jiang, C.; Song, X.; Wu, H.; Alotaibi, M.A.; Albiheyri, R.; Al-Zahrani, M.; et al. Interaction of MyoD and MyoG with Myoz2 gene in bovine myoblast differentiation. Res. Vet. Sci. 2022, 152, 569–578. [Google Scholar] [CrossRef]
- Said, S.S.; Yin, H.; Elfarnawany, M.; Nong, Z.; O’Neil, C.; Leong, H.; Lacefield, J.C.; Mequanint, K.; Pickering, J.G. Fortifying Angiogenesis in Ischemic Muscle with FGF9-Loaded Electrospun Poly(Ester Amide) Fibers. Adv. Healthc. Mater. 2019, 8, e1801294. [Google Scholar] [CrossRef]
- Lazure, F.; Blackburn, D.M.; Corchado, A.H.; Sahinyan, K.; Karam, N.; Sharanek, A.; Nguyen, D.; Lepper, C.; Najafabadi, H.S.; Perkins, T.J.; et al. Myf6/MRF4 is a myogenic niche regulator required for the maintenance of the muscle stem cell pool. EMBO Rep. 2020, 21, e49499. [Google Scholar] [CrossRef]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.A.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8, 998. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Chen, D.; Yu, B.; Huang, Z. FoxO1: A novel insight into its molecular mechanisms in the regulation of skeletal muscle differentiation and fiber type specification. Oncotarget 2017, 8, 10662–10674. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, J.; Liu, Y.; Wang, Y.; Duan, M.; Zeng, Z. Effects of myostatin gene knockout on white fat browning and related gene expression in type 2 diabetic mice. Adv. Clin. Exp. Med. 2024. ahead of print. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Gu, M.; Zhu, L.; Hai, C.; Di, A.; Wu, D.; Bai, C.; Su, G.; Liu, X.; et al. Loss of Myostatin Alters Mitochondrial Oxidative Phosphorylation, TCA Cycle Activity, and ATP Production in Skeletal Muscle. Int. J. Mol. Sci. 2022, 23, 15707. [Google Scholar] [CrossRef]
- Bugga, P.; Alam, M.J.; Kumar, R.; Pal, S.; Chattopadyay, N.; Banerjee, S.K. Sirt3 ameliorates mitochondrial dysfunction and oxidative stress through regulating mitochondrial biogenesis and dynamics in cardiomyoblast. Cell. Signal. 2022, 94, 110309. [Google Scholar] [CrossRef]
- Zhou, L.; Pinho, R.; Gu, Y.; Radak, Z. The Role of SIRT3 in Exercise and Aging. Cells 2022, 11, 2596. [Google Scholar] [CrossRef]
- Bi, D.; Shi, L.; Li, B.; Li, Y.; Liu, C.; Le, L.H.; Luo, J.; Wang, S.; Ta, D. The Protocol of Ultrasonic Backscatter Measurements of Musculoskeletal Properties. Phenomics 2024, 4, 72–80. [Google Scholar] [CrossRef]
- Hambright, W.S.; Niedernhofer, L.J.; Huard, J.; Robbins, P.D. Murine models of accelerated aging and musculoskeletal disease. Bone 2019, 125, 122–127. [Google Scholar] [CrossRef]
- Xie, W.Q.; He, M.; Yu, D.J.; Wu, Y.X.; Wang, X.H.; Lv, S.; Xiao, W.F.; Li, Y.S. Mouse models of sarcopenia: Classification and evaluation. J. Cachexia Sarcopenia Muscle 2021, 12, 538–554. [Google Scholar] [CrossRef]
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010, 21, 543–559. [Google Scholar] [CrossRef]
- Chen, Z.L.; Guo, C.; Zou, Y.Y.; Feng, C.; Yang, D.X.; Sun, C.C.; Wen, W.; Jian, Z.J.; Zhao, Z.; Xiao, Q.; et al. Aerobic exercise enhances mitochondrial homeostasis to counteract D-galactose-induced sarcopenia in zebrafish. Exp. Gerontol. 2023, 180, 112265. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Peng, Y.; Du, J.; Günther, S.; Guo, X.; Wang, S.; Schneider, A.; Zhu, L.; Braun, T. Mechano-signaling via Piezo1 prevents activation and p53-mediated senescence of muscle stem cells. Redox Biol. 2022, 52, 102309. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.A.; Antoun, E.; Garratt, E.S.; Westbury, L.; Baczynska, A.; Dennison, E.M.; Harvey, N.C.; Cooper, C.; Patel, H.P.; Godfrey, K.M.; et al. Adiposity is associated with widespread transcriptional changes and downregulation of longevity pathways in aged skeletal muscle. J. Cachexia Sarcopenia Muscle 2023, 14, 1762–1774. [Google Scholar] [CrossRef]
- Pacini, E.S.A.; Sanders-Silveira, S.; Godinho, R.O. The Extracellular cAMP-Adenosine Pathway in Airway Smooth Muscle. J. Pharmacol. Exp. Ther. 2018, 366, 75–83. [Google Scholar] [CrossRef]
- Ribeiro-Silva, J.C.; Miyakawa, A.A.; Krieger, J.E. Focal adhesion signaling: Vascular smooth muscle cell contractility beyond calcium mechanisms. Clin. Sci. 2021, 135, 1189–1207. [Google Scholar] [CrossRef]
- Kim, K.M.; Yoo, G.D.; Heo, W.; Oh, H.T.; Park, J.; Shin, S.; Do, Y.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. TAZ stimulates exercise-induced muscle satellite cell activation via Pard3-p38 MAPK-TAZ signalling axis. J. Cachexia Sarcopenia Muscle 2023, 14, 2733–2746. [Google Scholar] [CrossRef]
- Cui, C.; Bao, Z.; Chow, S.K.; Wong, R.M.Y.; Welch, A.; Qin, L.; Cheung, W.H. Coapplication of Magnesium Supplementation and Vibration Modulate Macrophage Polarization to Attenuate Sarcopenic Muscle Atrophy through PI3K/Akt/mTOR Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 12944. [Google Scholar] [CrossRef]
- Son, J.S.; Chae, S.A.; Wang, H.; Chen, Y.; Bravo Iniguez, A.; de Avila, J.M.; Jiang, Z.; Zhu, M.J.; Du, M. Maternal Inactivity Programs Skeletal Muscle Dysfunction in Offspring Mice by Attenuating Apelin Signaling and Mitochondrial Biogenesis. Cell Rep. 2020, 33, 108461. [Google Scholar] [CrossRef]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachexia Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]

| Speed (m/min) | Acceleration Time (s) | Velocity Duration (min) | |
|---|---|---|---|
| Initial velocity | 12 | 5 | 4 |
| First-order velocity | 16 | 5 | 4 |
| Second-order velocity | 20 | 5 | 2 |
| Speed (m/min) | Acceleration Time (s) | Velocity Duration (min) | |
|---|---|---|---|
| Initial velocity | 12 | 5 | 4 |
| First-order velocity | 20 | 5 | exhaustion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Guo, X.; Qu, T.; Huang, Y.; Tao, J.; He, J.; Wang, X.; Luo, J.; An, P.; Zhu, Y.; et al. The Combination of Lactoferrin and Creatine Ameliorates Muscle Decay in a Sarcopenia Murine Model. Nutrients 2024, 16, 1958. https://doi.org/10.3390/nu16121958
Wu W, Guo X, Qu T, Huang Y, Tao J, He J, Wang X, Luo J, An P, Zhu Y, et al. The Combination of Lactoferrin and Creatine Ameliorates Muscle Decay in a Sarcopenia Murine Model. Nutrients. 2024; 16(12):1958. https://doi.org/10.3390/nu16121958
Chicago/Turabian StyleWu, Wenbin, Xinlu Guo, Taiqi Qu, Yuejia Huang, Jin Tao, Jian He, Xiaoping Wang, Junjie Luo, Peng An, Yinhua Zhu, and et al. 2024. "The Combination of Lactoferrin and Creatine Ameliorates Muscle Decay in a Sarcopenia Murine Model" Nutrients 16, no. 12: 1958. https://doi.org/10.3390/nu16121958
APA StyleWu, W., Guo, X., Qu, T., Huang, Y., Tao, J., He, J., Wang, X., Luo, J., An, P., Zhu, Y., Sun, Y., & Luo, Y. (2024). The Combination of Lactoferrin and Creatine Ameliorates Muscle Decay in a Sarcopenia Murine Model. Nutrients, 16(12), 1958. https://doi.org/10.3390/nu16121958










