Flavan-3-ols and Vascular Health: Clinical Evidence and Mechanisms of Action
Abstract
1. Introduction
2. Gut Microbiota and Cardiovascular Health
3. Clinical Evidence of Flavan-3-ols on Vascular Outcomes
3.1. An Overview of Randomized Clinical Trials Evaluating the Effect of Flavan-3-ol Intake on Vascular Health
3.2. A Summary of Randomized Clinical Trials Investigating the Impact of Catechin Supplementation on Vascular Health
| Author, Year, Country | Study Design | Participants (Mean Age) | Duration | Treatment | Polyphenol Constituent (Daily Intake) | Comparison | Main Findings |
|---|---|---|---|---|---|---|---|
| Fukino, 2005, Japan [81] | Controlled | 66 patients with borderline diabetes or diabetes (53 y) | 2 mo | One packet of GTE/powder | 544 mg polyphenols, 456 mg catechins | No intervention | No significant changes were observed between groups. |
| Diepvens, 2005, The Netherlands [77] | Double-blind, placebo-controlled | 46 overweight women (placebo: 41 y; green tea: 41 y) | 87 d | GTE capsules with low-calorie diet | 1125 mg tea catechins | Maltodextrin capsules | No changes were observed between groups. |
| Hill, 2007, Australia [82] | Placebo-controlled | 38 overweight or obese postmenopausal women (58 y) | 12 wk | EGCG capsules + walking for 45 min at 75% of age-predicted maximum heart rate | 300 mg EGCG | 2 lactose capsules/d + walking for 45 min at 75% of age-predicted maximum heart rate 3/wk | A significant reduction in HR was observed in the EGCG group (p < 0.01). |
| Nagao, 2007, Japan [83] | Double-blind, placebo-controlled | 240 women and men with visceral fat-type obesity (41 y) | 12 wk | GTE beverage high in catechins | 583 mg total catechins, 42.84 mg catechin, 40.12 mg catechin gallate, 127.5 mg gallocatechin, 139.74 mg gallocatechin gallate, 32.3 mg epicatechin, 30.94 mg epicatechin gallate, 60.36 mg epigallocatechin, and 100.3 mg epigallocatechin gallate | Control beverage (96 mg catechins) | Compared with the control group, in the catechin group, a greater decrease in SBP for subjects with initial SBP values of 130 mm Hg or higher was observed (p < 0.05). |
| Fukino, 2008, Japan [84] | Crossover | 60 volunteers with fasting blood glucose levels of ≥6.1 mmol/L or nonfasting blood glucose levels of ≥7.8 mmol/L (52 y) | 2 × 2 mo (no washout) | GTE powder | 544 mg polyphenols, 456 mg catechins | No intervention | No significant changes were observed after intervention. |
| Frank, 2009, UK [78] | Double-blind, placebo-controlled | 33 healthy men (treatment group: 41 y; control group 40 y) | 3 wk | GTE capsules | 714 mg of green tea polyphenols, ~670 mg flavanols | Placebo capsules (maltodextrin and caffeine) | No changes were observed after intervention. |
| Hsu, 2008, Taiwan [85] | Double-blind, placebo-controlled | 78 obese women (green tea: 43 y; placebo: 43 y) | 12 wk | Dried, powdered GTE capsules | 491 mg catechins, 302 mg EGCG | Placebo capsules (cellulose) | No significant differences were observed when comparing the two groups. |
| Brown, 2009, UK [86] | Double-blind, placebo-controlled | 88 overweight or obese male subjects (53 y) | 8 wk | EGCG capsules | 800 mg EGCG | Placebo capsule (lactose) | Compared to the placebo, EGCG treatment reduced DBP (p = 0.014). |
| Nagao, 2009, Japan [87] | Double-blind, placebo-controlled | 43 patients with T2DM (catechin group: 64 y; control group 62 y) | 12 wk | Catechin-rich beverage | 582.8 mg catechins | Control beverage with 96.3 mg catechins | No significant differences were observed between groups. |
| Nantz, 2009, USA [73] | Double-blind, placebo-controlled | 111 healthy adult volunteers (29 y) | 3 mo | Camellia sinensis compounds capsules | polyphenols > 80%, catechins > 80%, EGCG > 45% | Placebo capsules (microcrystalline cellulose) | Treatment led to a significant reduction in SBP (p < 0.05). |
| Basu, 2011, USA [88] | Single-blind, placebo-controlled | 35 obese subjects with MetS (42 y) | 8 wk | (i) Green tea; (ii) GTE | (i) 440 mg EGCG, 220 mg EGC, 180 mg ECG, and 88 mg EC; (ii) 460 mg EGCG, 240 mg EGC, and 120 mg ECG e 50 mg EC | Water | No effects were observed after treatment. |
| Brown, 2011, UK [89] | Double-blind, placebo-controlled, crossover | 64 overweight and obese sedentary males (placebo: 49 y; intervention: 49 y) | 2 × 6 wk (2 wk washout) | DGT capsules | EGCG 40.71%, EGC 16.27%, EC 8.74%, ECG 6.02%, GC 2.02%, GCG 1.27%, catechin 1.16%, gallic acid 0.75%, and catechin gallate 0.03% in each capsule | Placebo capsules (lactose) | No effect of treatment on any of the ambulatory BP-monitoring parameters. |
| Hsu, 2011, Taiwan [90] | Double-blind, placebo-controlled | 68 subjects with BMI > 25 kg/m2 and T2DM for more than one year (GTE: 50 y; placebo: 52 y) | 16 wk | DGT capsules | 856.8 mg EGCG, 236.1 mg ECG, 115.5 mg EGC, 71.9 mg EC, 63.7 mg GCG, and <1.05 mg GC | Placebo capsules (cellulose) | No statistically significant differences were observed between groups. |
| Sone, 2011, Japan [76] | Placebo-controlled | 51 individuals (high-catechin group: 43 y; low-catechin group: 48 y) | 9 wk treatment | Catechin-enriched green tea beverage | 400 mg catechins | Control beverage with 100 mg catechins | No significant differences were observed between groups. |
| Bogdanski, 2012, Poland [91] | Double-blind, placebo-controlled | 56 obese, hypertensive subjects (GTE group: 49 y; placebo group 51 y) | 3 mo | GTE capsules | 208 mg EGCG | Placebo capsules (microcrystalline cellulose) | In the treated group, both SBP and DBP significantly decreased (p < 0.01). |
| Suliburska, 2012, Poland [92] | Double-blind, placebo-controlled | 46 obese patients (GTE group: 48 y; placebo group: 52 y) | 3 mo | GTE capsules | 208 mg EGCG | Placebo capsules (microcrystalline cellulose) | No significant changes in SBP and DBP. |
| Miyazaki, 2013, Japan [79] | Double-blind, placebo-controlled | 50 older adults participating in a pedometer-based walking program (69 y) | 14 wk | Green tea catechins beverage | 630.9 mg total catechin, 125.7 mg GC, 114 mg EGC, 30 mg catechin, 34.7 mg EC, 143.2 mg EGCG, 112.6 mg GCG, 45.8 mg ECG, and 24.8 mg CG | Control beverage (88.7 mg total catechin) | No significant changes were shown when comparing groups. |
| Liu, 2014, Taiwan [93] | Double-blind, placebo-controlled | 77 subjects with type 2 diabetes and lipid abnormalities (GTE group: 55 y; cellulose group: 53 y) | 16 wk | GTE capsules | 856.8 mg EGCG, 236.1 mg ECG, 115.5 mg EGC, 71.9 mg EC, 63.7 mg GCG, and <1.05 mg GC | Placebo capsules (cellulose) | Treatment with GTE did not show significant changes in BP. |
| Takahashi, 2014, Japan [75] | Double-blind, placebo-controlled | 22 healthy postmenopausal women (placebo: 66 y; green tea: 66 y) | 4 wk | Green tea beverage | 615 mg catechins | Placebo beverage (92 mg catechins) | No differences in BP between the groups were observed. |
| Dower, 2015, The Netherlands [94] | Double-blind, placebo-controlled, crossover | 33 healthy (pre)hypertensive men and women (SBP between 125 and 160 mmHg) (66 y) | 3 × 4 wk (2 × 4 wk washout) | (i) Epicatechin capsules; (ii) quercetin capsules | (i) 100 mg epicatechin; (ii) 160 mg quercetin-3-glucoside | Placebo capsules | Epicatechin and quercetin supplementation significantly decreased soluble endothelial selectin (p = 0.03 and p = 0.03, respectively). |
| Chen, 2016, Taiwan [95] | Double-blind, placebo-controlled | 77 women with central obesity (GTE: 44 y; placebo: 44 y) | 12 wk | DGT capsules | EGCG 856.8 mg, ECG 236.1 mg, EGC 115.5 mg, EC 71.9 mg, GCG 63.7 mg, and GC < 1.05 mg | Placebo capsules (microcrystalline cellulose) | There were no significant percentage reductions in BP between groups. |
| Gutiérrez-Salmeàn, 2016, India [96] | Double-blind, placebo-controlled | 30 subjects with hypertriglyceridemia (37 y) | 4 wk | Epicatechin capsules | 100 mg epicatechin | Placebo capsules | No significant changes in SBP and DBP were observed when comparing groups. |
| Lu and Hsu, 2016, Taiwan [97] | Double-blind, placebo-controlled | 64 women with moderate or severe acne vulgaris (GTE 28 y, placebo 30 y) | 4 wk | DGT capsules | EGCG 856.8 mg ECG 236.1 mg, EGC 115.5 mg, EC 71.9 mg GCG 63.7 mg, and GC < 1.05 mg | Placebo capsules (microcrystalline cellulose) | No changes were observed after intervention. |
| Kafeshani, 2017, Iran [74] | Double-blind, placebo-controlled | 49 healthy adult men (green tea: 20 y; sour tea: 20 y; placebo: 21 y) | 6 wk | (i) Green tea tablets; (ii) sour tea tables | (i) ~240 mg catechins; (ii) at least 250 mg anthocyanins | Placebo tablets (maltodextrin) | A reduction in DBP in the sour tea group compared to the baseline (p = 0.007) and a significant reduction in SBP (p = 0.004) compared to the placebo. |
| Saarenhovi, 2017, Finland [98] | Double-blind, placebo-controlled, crossover | 57 otherwise healthy subjects with borderline hypertension (130–139/85–89 mmHg) or unmedicated mild hypertension (140–165/90–95 mmHg) (55 y) | 2 × 4 wk (4 to 5 wk washout) | Epicatechin capsules | 100 mg epicatechin | Placebo capsules (microcrystalline cellulose) | Treatment induced significant increase in FMD% at the first visit (p = 0.04), last visit (p = 0.02), and for both visits combined (p < 0.01) but not significantly compared to placebo. DBP at end of treatment was −3.3 mmHg (p = 0.008). |
| Kirch, 2018, Germany [99] | Double-blind, placebo-controlled, crossover | 47 overweight or obese nonsmokers with clear signs of MetS (M: 36 y; F: 35 y) | 2 × 2 wk (2 wk washout) | Epicatechin capsules | 25 mg epicatechin | Placebo capsules (mannitol) | No differences were observed between treatments. |
| Maeda-Yamamoto, 2018, Japan [80] | Double-blind, placebo-controlled | 114 healthy subjects, with an SBP value of ≤125 and <155 and a DBP value < 95, or a DBP of ≤75 mmHg and <95 mmHg and an SBP < 155 mmHg (40 y) | 12 wk | (i) Green tea cultivar Sunrouge extract; (ii) green tea cultivar Yabukita extract | (i) 11.2 mg anthocyanin, 323.6 mg EGCG, 85.7 mg EGC, 12.3 mg EGCG3”Me, 40.4 mg flavonols; (ii) 322.2 mg EGCG, 413.1 mg EGC, and 54.8 mg flavonols | Barley extract without catechin | After “Sunrouge” extract treatment, participants showed increased blood pressure (p < 0.05). |
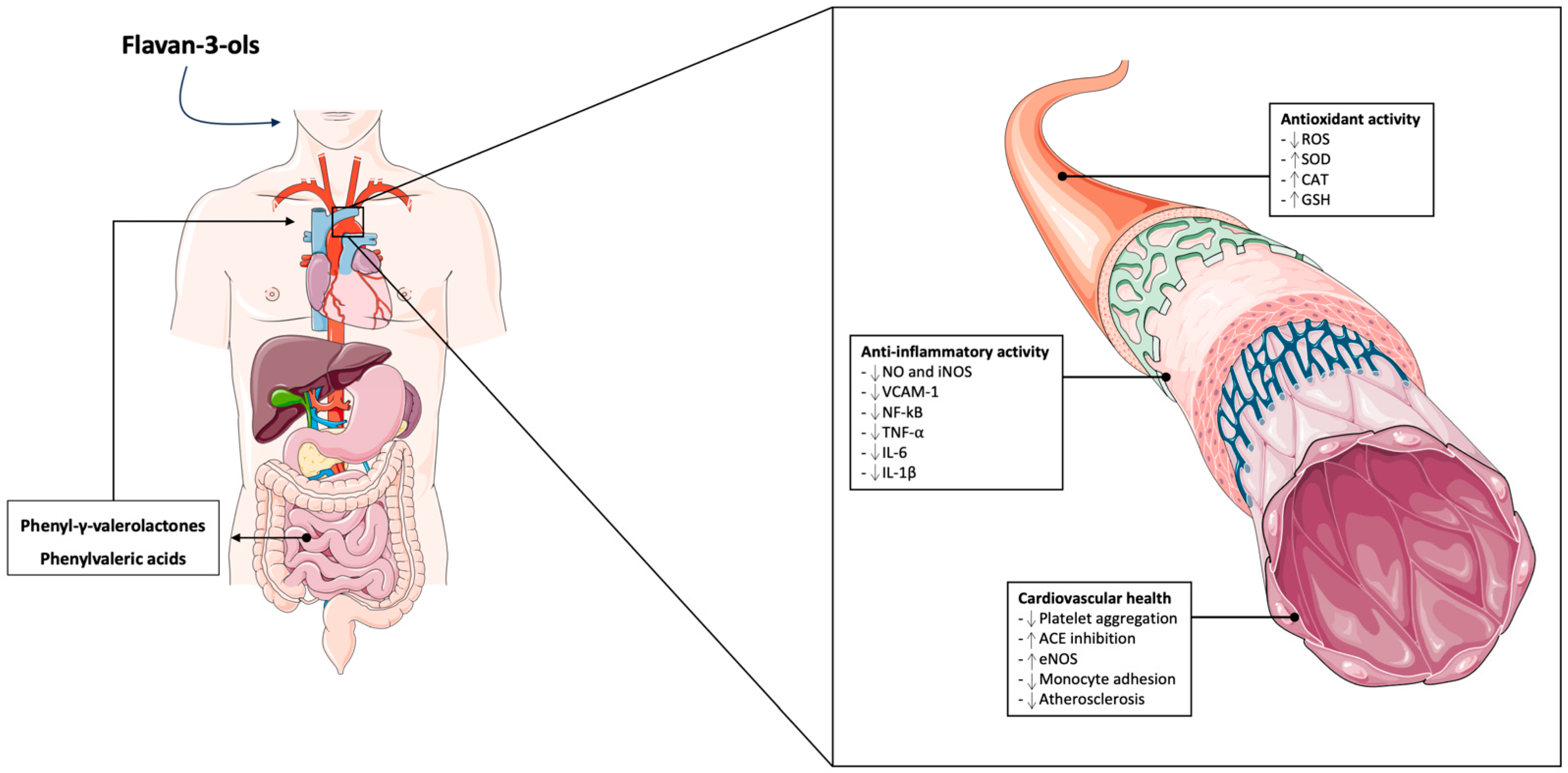
4. Mechanisms of Action of Flavan-3-ols on Vascular Health
4.1. Antioxidant Activity
4.2. Anti-Inflammatory Regulation
4.3. Flavan-3-ol Microbial-Derived Metabolites and Cardiovascular Health
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Crowe-White, K.M.; Evans, L.W.; Kuhnle, G.G.C.; Milenkovic, D.; Stote, K.; Wallace, T.; Handu, D.; Senkus, K.E. Flavan-3-ols and Cardiometabolic Health: First Ever Dietary Bioactive Guideline. Adv. Nutr. 2022, 13, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Risk Factors Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [CrossRef] [PubMed]
- GBD 2017 Diet Collaborators Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [CrossRef] [PubMed]
- Figueiredo, C.S.; Roseira, E.S.; Viana, T.T.; Silveira, M.A.D.; de Melo, R.M.V.; Fernandez, M.G.; Lemos, L.M.G.; Passos, L.C.S. Inflammation in Coronary Atherosclerosis: Insights into Pathogenesis and Therapeutic Potential of Anti-Inflammatory Drugs. Pharmaceuticals 2023, 16, 1242. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Marventano, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kroeger, C.M.; Cassidy, S.; Mitra, S.; Ribeiro, R.V.; Jose, S.; Masedunskas, A.; Senior, A.M.; Fontana, L. Vegetarian Dietary Patterns and Cardiometabolic Risk in People with or at High Risk of Cardiovascular Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2325658. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, N.B.; Lambert, M.N.T.; Jeppesen, P.B. The Effect of Plant Derived Bioactive Compounds on Inflammation: A Systematic Review and Meta-Analysis. Mol. Nutr. Food Res. 2020, 64, e2000473. [Google Scholar] [CrossRef]
- Grosso, G. Effects of Polyphenol-Rich Foods on Human Health. Nutrients 2018, 10, 1089. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The effect of dietary polyphenols on vascular health and hypertension: Current evidence and mechanisms of action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.; Santos, C.N. Worldwide (poly)phenol intake: Assessment methods and identified gaps. Eur. J. Nutr. 2017, 56, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Raman, G.; Shams-White, M.; Avendano, E.E.; Chen, F.; Novotny, J.A.; Cassidy, A. Dietary intakes of flavan-3-ols and cardiovascular health: A field synopsis using evidence mapping of randomized trials and prospective cohort studies. Syst. Rev. 2018, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, dairy foods, and cardiovascular and metabolic health: A review of emerging biologic pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, e2001019. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L. Dietary fibre modifies gut microbiota: What’s the role of (poly)phenols? Int. J. Food Sci. Nutr. 2020, 71, 783–784. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Steiling, H.; Williamson, G.; Lean, M.E.J.; Crozier, A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol. Nutr. Food Res. 2010, 54, 323–334. [Google Scholar] [CrossRef]
- Baky, M.H.; Elshahed, M.; Wessjohann, L.; Farag, M.A. Interactions between dietary flavonoids and the gut microbiome: A comprehensive review. Br. J. Nutr. 2022, 128, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Di Pede, G.; Mena, P.; Bresciani, L.; Achour, M.; Lamuela-Raventós, R.M.; Estruch, R.; Landberg, R.; Kulling, S.E.; Wishart, D.; Rodriguez-Mateos, A.; et al. Revisiting the bioavailability of flavan-3-ols in humans: A systematic review and comprehensive data analysis. Mol. Asp. Med. 2023, 89, 101146. [Google Scholar] [CrossRef]
- Anhê, F.F.; Choi, B.S.Y.; Dyck, J.R.B.; Schertzer, J.D.; Marette, A. Host-Microbe Interplay in the Cardiometabolic Benefits of Dietary Polyphenols. Trends Endocrinol. Metab. 2019, 30, 384–395. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 1245. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018, 8, 635. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; -Or-Rashid, M.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Li, X.S.; Haghikia, A.; Li, L.; Wilcox, J.; Romano, K.A.; Buffa, J.A.; Witkowski, M.; Demuth, I.; König, M.; et al. Atlas of gut microbe-derived products from aromatic amino acids and risk of cardiovascular morbidity and mortality. Eur. Heart J. 2023, 44, 3085–3096. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.Á.; Ramos, S. Impact of dietary flavanols on microbiota, immunity and inflammation in metabolic diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- García-Cordero, J.; Martinez, A.; Blanco-Valverde, C.; Pino, A.; Puertas-Martín, V.; San Román, R.; de Pascual-Teresa, S. Regular consumption of cocoa and red berries as a strategy to improve cardiovascular biomarkers via modulation of microbiota metabolism in healthy aging adults. Nutrients 2023, 15, 2299. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.-H.; Lin, S.-Y.; Chen, L.-L.; Ouyang, K.-H.; Wang, W.-J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Chronopoulos, A.K.; Singh, I.; Francis, M.A.; Moriarty, H.; Pike, M.J.; Turner, A.H.; Mann, N.J.; Sinclair, A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003, 77, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.-K.; Milbury, P.; Paul, S.M.; Blumberg, J.; et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef]
- Fraga, C.G.; Actis-Goretta, L.; Ottaviani, J.I.; Carrasquedo, F.; Lotito, S.B.; Lazarus, S.; Schmitz, H.H.; Keen, C.L. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin. Dev. Immunol. 2005, 12, 11–17. [Google Scholar] [CrossRef]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Wang-Polagruto, J.F.; Villablanca, A.C.; Polagruto, J.A.; Lee, L.; Holt, R.R.; Schrader, H.R.; Ensunsa, J.L.; Steinberg, F.M.; Schmitz, H.H.; Keen, C.L. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. S2), S177–S186, discussion S206. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schömig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.N. Short-Term Consumption of a Dark Chocolate Containing Flavanols is Followed by a Significant Decrease in Normotensive Population. Pak. J. Nutr. 2008, 7, 773–781. [Google Scholar] [CrossRef]
- Crews, W.D.; Harrison, D.W.; Wright, J.W. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: Clinical findings from a sample of healthy, cognitively intact older adults. Am. J. Clin. Nutr. 2008, 87, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.; Coates, A.M.; Buckley, J.D.; Howe, P.R.C. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int. J. Obes. 2008, 32, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef]
- Muniyappa, R.; Hall, G.; Kolodziej, T.L.; Karne, R.J.; Crandon, S.K.; Quon, M.J. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am. J. Clin. Nutr. 2008, 88, 1685–1696. [Google Scholar] [CrossRef]
- Shiina, Y.; Funabashi, N.; Lee, K.; Murayama, T.; Nakamura, K.; Wakatsuki, Y.; Daimon, M.; Komuro, I. Acute effect of oral flavonoid-rich dark chocolate intake on coronary circulation, as compared with non-flavonoid white chocolate, by transthoracic Doppler echocardiography in healthy adults. Int. J. Cardiol. 2009, 131, 424–429. [Google Scholar] [CrossRef]
- Heiss, C.; Jahn, S.; Taylor, M.; Real, W.M.; Angeli, F.S.; Wong, M.L.; Amabile, N.; Prasad, M.; Rassaf, T.; Ottaviani, J.I.; et al. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J. Am. Coll. Cardiol. 2010, 56, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Njike, V.Y.; Faridi, Z.; Shuval, K.; Dutta, S.; Kay, C.D.; West, S.G.; Kris-Etherton, P.M.; Katz, D.L. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int. J. Cardiol. 2011, 149, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Tsang, C.; Ostertag, L.M.; Fyfe, L.; Al-Dujaili, E.A.S. Differential effect of polyphenol-rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: A randomized clinical trial. Food Funct. 2012, 3, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Sudano, I.; Wolfrum, M.; Thomas, R.; Enseleit, F.; Périat, D.; Kaiser, P.; Hirt, A.; Hermann, M.; Serafini, M.; et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur. Heart J. 2012, 33, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Mogollon, J.A.; Bujold, E.; Lemieux, S.; Bourdages, M.; Blanchet, C.; Bazinet, L.; Couillard, C.; Noël, M.; Dodin, S. Blood pressure and endothelial function in healthy, pregnant women after acute and daily consumption of flavanol-rich chocolate: A pilot, randomized controlled trial. Nutr. J. 2013, 12, 41. [Google Scholar] [CrossRef]
- Neufingerl, N.; Zebregs, Y.E.M.P.; Schuring, E.A.H.; Trautwein, E.A. Effect of cocoa and theobromine consumption on serum HDL-cholesterol concentrations: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Esser, D.; Mars, M.; Oosterink, E.; Stalmach, A.; Müller, M.; Afman, L.A. Dark chocolate consumption improves leukocyte adhesion factors and vascular function in overweight men. FASEB J. 2014, 28, 1464–1473. [Google Scholar] [CrossRef]
- Ibero-Baraibar, I.; Abete, I.; Navas-Carretero, S.; Massis-Zaid, A.; Martinez, J.A.; Zulet, M.A. Oxidised LDL levels decreases after the consumption of ready-to-eat meals supplemented with cocoa extract within a hypocaloric diet. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 416–422. [Google Scholar] [CrossRef]
- Nickols-Richardson, S.M.; Piehowski, K.E.; Metzgar, C.J.; Miller, D.L.; Preston, A.G. Changes in body weight, blood pressure and selected metabolic biomarkers with an energy-restricted diet including twice daily sweet snacks and once daily sugar-free beverage. Nutr. Res. Pract. 2014, 8, 695–704. [Google Scholar] [CrossRef]
- Sarriá, B.; Martínez-López, S.; Sierra-Cinos, J.L.; García-Diz, L.; Mateos, R.; Bravo, L. Regular consumption of a cocoa product improves the cardiometabolic profile in healthy and moderately hypercholesterolaemic adults. Br. J. Nutr. 2014, 111, 122–134. [Google Scholar] [CrossRef] [PubMed]
- West, S.G.; McIntyre, M.D.; Piotrowski, M.J.; Poupin, N.; Miller, D.L.; Preston, A.G.; Wagner, P.; Groves, L.F.; Skulas-Ray, A.C. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br. J. Nutr. 2014, 111, 653–661. [Google Scholar] [CrossRef]
- Heiss, C.; Sansone, R.; Karimi, H.; Krabbe, M.; Schuler, D.; Rodriguez-Mateos, A.; Kraemer, T.; Cortese-Krott, M.M.; Kuhnle, G.G.C.; Spencer, J.P.E.; et al. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: A randomized, controlled, double-masked trial. Age 2015, 37, 9794. [Google Scholar] [CrossRef]
- Koli, R.; Köhler, K.; Tonteri, E.; Peltonen, J.; Tikkanen, H.; Fogelholm, M. Dark chocolate and reduced snack consumption in mildly hypertensive adults: An intervention study. Nutr. J. 2015, 14, 84. [Google Scholar] [CrossRef]
- Massee, L.A.; Ried, K.; Pase, M.; Travica, N.; Yoganathan, J.; Scholey, A.; Macpherson, H.; Kennedy, G.; Sali, A.; Pipingas, A. The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: A randomized, controlled trial. Front. Pharmacol. 2015, 6, 93. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study--a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Balz, M.; Kimball, J.; Ensunsa, J.L.; Fong, R.; Momma, T.Y.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. Safety and efficacy of cocoa flavanol intake in healthy adults: A randomized, controlled, double-masked trial. Am. J. Clin. Nutr. 2015, 102, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Rassaf, T.; Rammos, C.; Hendgen-Cotta, U.B.; Heiss, C.; Kleophas, W.; Dellanna, F.; Floege, J.; Hetzel, G.R.; Kelm, M. Vasculoprotective Effects of Dietary Cocoa Flavanols in Patients on Hemodialysis: A Double-Blind, Randomized, Placebo-Controlled Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Rull, G.; Mohd-Zain, Z.N.; Shiel, J.; Lundberg, M.H.; Collier, D.J.; Johnston, A.; Warner, T.D.; Corder, R. Effects of high flavanol dark chocolate on cardiovascular function and platelet aggregation. Vascul. Pharmacol. 2015, 71, 70–78. [Google Scholar] [CrossRef]
- Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G.G.C.; Spencer, J.P.E.; Schroeter, H.; Merx, M.W.; et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: A randomised, controlled, double-masked trial: The Flaviola Health Study. Br. J. Nutr. 2015, 114, 1246–1255. [Google Scholar] [CrossRef]
- Njike, V.Y.; Hamburg, N.; Kellogg, M.; Annapureddy, A.; Vita, J. Dose and response to cocoa (DARC): A randomized double-blind controlled trial. Clin. Trials Regul. Sci. Cardiol. 2016, 23–24, 9–15. [Google Scholar] [CrossRef]
- Garcia-Yu, I.A.; Garcia-Ortiz, L.; Gomez-Marcos, M.A.; Rodriguez-Sanchez, E.; Agudo-Conde, C.; Gonzalez-Sanchez, J.; Maderuelo-Fernandez, J.A.; Recio-Rodriguez, J.I. Effects of Cocoa-Rich Chocolate on Blood Pressure, Cardiovascular Risk Factors, and Arterial Stiffness in Postmenopausal Women: A Randomized Clinical Trial. Nutrients 2020, 12, 1758. [Google Scholar] [CrossRef] [PubMed]
- Nantz, M.P.; Rowe, C.A.; Bukowski, J.F.; Percival, S.S. Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition 2009, 25, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kafeshani, M.; Entezari, M.H.; Karimian, J.; Pourmasoumi, M.; Maracy, M.R.; Amini, M.R.; Hadi, A. A comparative study of the effect of green tea and sour tea on blood pressure and lipid profile in healthy adult men. ARYA Atheroscler. 2017, 13, 109–116. [Google Scholar]
- Takahashi, M.; Miyashita, M.; Suzuki, K.; Bae, S.-R.; Kim, H.-K.; Wakisaka, T.; Matsui, Y.; Takeshita, M.; Yasunaga, K. Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women. Br. J. Nutr. 2014, 112, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Sone, T.; Kuriyama, S.; Nakaya, N.; Hozawa, A.; Shimazu, T.; Nomura, K.; Rikimaru, S.; Tsuji, I. Randomized controlled trial for an effect of catechin-enriched green tea consumption on adiponectin and cardiovascular disease risk factors. Food Nutr. Res. 2011, 55, 8326. [Google Scholar] [CrossRef] [PubMed]
- Diepvens, K.; Kovacs, E.M.R.; Nijs, I.M.T.; Vogels, N.; Westerterp-Plantenga, M.S. Effect of green tea on resting energy expenditure and substrate oxidation during weight loss in overweight females. Br. J. Nutr. 2005, 94, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; George, T.W.; Lodge, J.K.; Rodriguez-Mateos, A.M.; Spencer, J.P.E.; Minihane, A.M.; Rimbach, G. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J. Nutr. 2009, 139, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, R.; Kotani, K.; Ayabe, M.; Tsuzaki, K.; Shimada, J.; Sakane, N.; Takase, H.; Ichikawa, H.; Yonei, Y.; Ishii, K. Minor effects of green tea catechin supplementation on cardiovascular risk markers in active older people: A randomized controlled trial. Geriatr. Gerontol. Int. 2013, 13, 622–629. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M.; Nishimura, M.; Kitaichi, N.; Nesumi, A.; Monobe, M.; Nomura, S.; Horie, Y.; Tachibana, H.; Nishihira, J. A Randomized, Placebo-Controlled Study on the Safety and Efficacy of Daily Ingestion of Green Tea (Camellia sinensis L.) cv. “Yabukita” and “Sunrouge” on Eyestrain and Blood Pressure in Healthy Adults. Nutrients 2018, 10, 569. [Google Scholar] [CrossRef]
- Fukino, Y.; Shimbo, M.; Aoki, N.; Okubo, T.; Iso, H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J. Nutr. Sci. Vitaminol. 2005, 51, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.M.; Coates, A.M.; Buckley, J.D.; Ross, R.; Thielecke, F.; Howe, P.R.C. Can EGCG reduce abdominal fat in obese subjects? J. Am. Coll. Nutr. 2007, 26, 396S–402S. [Google Scholar] [CrossRef]
- Nagao, T.; Hase, T.; Tokimitsu, I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity 2007, 15, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Fukino, Y.; Ikeda, A.; Maruyama, K.; Aoki, N.; Okubo, T.; Iso, H. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur. J. Clin. Nutr. 2008, 62, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Tsai, T.-H.; Kao, Y.-H.; Hwang, K.-C.; Tseng, T.-Y.; Chou, P. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2008, 27, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Du, M.; Sanchez, K.; Leyva, M.J.; Betts, N.M.; Blevins, S.; Wu, M.; Aston, C.E.; Lyons, T.J. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition 2011, 27, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Lane, J.; Holyoak, C.; Nicol, B.; Mayes, A.E.; Dadd, T. Health effects of green tea catechins in overweight and obese men: A randomised controlled cross-over trial. Br. J. Nutr. 2011, 106, 1880–1889. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Liao, Y.-L.; Lin, S.-C.; Tsai, T.-H.; Huang, C.-J.; Chou, P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern. Med. Rev. 2011, 16, 157–163. [Google Scholar]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol. Trace Elem. Res. 2012, 149, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Huang, C.-J.; Huang, L.-H.; Chen, I.-J.; Chiu, J.-P.; Hsu, C.-H. Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: A randomized, double-blinded, and placebo-controlled trial. PLoS ONE 2014, 9, e91163. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C. Supplementation of the Pure Flavonoids Epicatechin and Quercetin Affects Some Biomarkers of Endothelial Dysfunction and Inflammation in (Pre)Hypertensive Adults: A Randomized Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-J.; Liu, C.-Y.; Chiu, J.-P.; Hsu, C.-H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Salmeán, G.; Meaney, E.; Lanaspa, M.A.; Cicerchi, C.; Johnson, R.J.; Dugar, S.; Taub, P.; Ramírez-Sánchez, I.; Villarreal, F.; Schreiner, G.; et al. A randomized, placebo-controlled, double-blind study on the effects of (-)-epicatechin on the triglyceride/HDLc ratio and cardiometabolic profile of subjects with hypertriglyceridemia: Unique in vitro effects. Int. J. Cardiol. 2016, 223, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.H.; Hsu, C.H. Does supplementation with green tea extract improve acne in post-adolescent women? A randomized, double-blind, and placebo-controlled clinical trial. Complement. Ther. Med. 2016, 25, 159–163. [Google Scholar] [CrossRef]
- Saarenhovi, M.; Salo, P.; Scheinin, M.; Lehto, J.; Lovró, Z.; Tiihonen, K.; Lehtinen, M.J.; Junnila, J.; Hasselwander, O.; Tarpila, A.; et al. The effect of an apple polyphenol extract rich in epicatechin and flavan-3-ol oligomers on brachial artery flow-mediated vasodilatory function in volunteers with elevated blood pressure. Nutr. J. 2017, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Kirch, N.; Berk, L.; Liegl, Y.; Adelsbach, M.; Zimmermann, B.F.; Stehle, P.; Stoffel-Wagner, B.; Ludwig, N.; Schieber, A.; Helfrich, H.-P.; et al. A nutritive dose of pure (-)-epicatechin does not beneficially affect increased cardiometabolic risk factors in overweight-to-obese adults-a randomized, placebo-controlled, double-blind crossover study. Am. J. Clin. Nutr. 2018, 107, 948–956. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The role of inflammation in cardiovascular disease. Int. J. Mol. Sci. 2022, 23, 2906. [Google Scholar] [CrossRef]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Brånén, L.; Hovgaard, L.; Nitulescu, M.; Bengtsson, E.; Nilsson, J.; Jovinge, S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Meth. Enzymol. 1990, 186, 343–355. [Google Scholar]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Martín, M.A.; Ramos, S.; Bravo, L.; Goya, L. Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on CaCO2 cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Velalar, C.N.; Ruan, R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radic. Biol. Med. 2008, 44, 1032–1041. [Google Scholar] [CrossRef]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar]
- Naderi, G.A.; Asgary, S.; Sarraf-Zadegan, N.; Shirvany, H. Anti-oxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol. Cell. Biochem. 2003, 246, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Ruskovska, T.; Maksimova, V.; Milenkovic, D. Polyphenols in human nutrition: From the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability—An overview and perspective. Br. J. Nutr. 2020, 123, 241–254. [Google Scholar] [CrossRef]
- Ruskovska, T.; Massaro, M.; Carluccio, M.A.; Arola-Arnal, A.; Muguerza, B.; Vanden Berghe, W.; Declerck, K.; Bravo, F.I.; Calabriso, N.; Combet, E.; et al. Systematic bioinformatic analysis of nutrigenomic data of flavanols in cell models of cardiometabolic disease. Food Funct. 2020, 11, 5040–5064. [Google Scholar] [CrossRef]
- Corral-Jara, K.F.; Nuthikattu, S.; Rutledge, J.; Villablanca, A.; Morand, C.; Schroeter, H.; Milenkovic, D. Integrated Multi-Omic Analyses of the Genomic Modifications by Gut Microbiome-Derived Metabolites of Epicatechin, 5-(4′-Hydroxyphenyl)-γ-Valerolactone, in TNFalpha-Stimulated Primary Human Brain Microvascular Endothelial Cells. Front. Neurosci. 2021, 15, 622640. [Google Scholar] [CrossRef]
- Oleaga, C.; Ciudad, C.J.; Izquierdo-Pulido, M.; Noé, V. Cocoa flavanol metabolites activate HNF-3β, Sp1, and NFY-mediated transcription of apolipoprotein AI in human cells. Mol. Nutr. Food Res. 2013, 57, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Márquez Campos, E.; Stehle, P.; Simon, M.-C. Microbial Metabolites of Flavan-3-Ols and Their Biological Activity. Nutrients 2019, 11, 2260. [Google Scholar] [CrossRef]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef]
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4531–4538. [Google Scholar] [CrossRef]
- Kutschera, M.; Engst, W.; Blaut, M.; Braune, A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 2011, 111, 165–175. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, E.J.J.; Westerhuis, J.A.; Grün, C.H.; Jacobs, D.M.; Eilers, P.H.C.; Mulder, T.P.; Foltz, M.; Garczarek, U.; Kemperman, R.; Vaughan, E.E.; et al. Population-based nutrikinetic modeling of polyphenol exposure. Metabolomics 2014, 10, 1059–1073. [Google Scholar] [CrossRef]
- Trošt, K.; Ulaszewska, M.M.; Stanstrup, J.; Albanese, D.; De Filippo, C.; Tuohy, K.M.; Natella, F.; Scaccini, C.; Mattivi, F. Host: Microbiome co-metabolic processing of dietary polyphenols—An acute, single blinded, cross-over study with different doses of apple polyphenols in healthy subjects. Food Res. Int. 2018, 112, 108–128. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X.; Jiang, M.; Zhang, H.; Wang, Y.; Zhang, Y.; Seviour, R.; Kong, Y. In vitro co-metabolism of epigallocatechin-3-gallate (EGCG) by the mucin-degrading bacterium Akkermansia muciniphila. PLoS ONE 2021, 16, e0260757. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Jena, P.K.; Liu, H.-X.; Hu, Y.; Nagar, N.; Bronner, D.N.; Settles, M.L.; Bäumler, A.J.; Wan, Y.-J.Y. Obesity treatment by epigallocatechin-3-gallate-regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. 2018, 32, fj201800370R. [Google Scholar] [CrossRef]
- Patial, V.; Katoch, S.; Chhimwal, J.; Dadhich, G.; Sharma, V.; Rana, A.; Joshi, R.; Padwad, Y. Catechins prevent obesity-induced kidney damage by modulating PPARγ/CD36 pathway and gut-kidney axis in rats. Life Sci. 2023, 316, 121437. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhut, K.; Högger, P. Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol). Free Radic. Biol. Med. 2012, 53, 305–313. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Effects of Metabolites Produced from (-)-Epigallocatechin Gallate by Rat Intestinal Bacteria on Angiotensin I-Converting Enzyme Activity and Blood Pressure in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2015, 63, 8262–8266. [Google Scholar] [CrossRef]
- Lee, C.C.; Kim, J.H.; Kim, J.S.; Oh, Y.S.; Han, S.M.; Park, J.H.Y.; Lee, K.W.; Lee, C.Y. 5-(3′,4′-Dihydroxyphenyl-γ-valerolactone), a Major Microbial Metabolite of Proanthocyanidin, Attenuates THP-1 Monocyte-Endothelial Adhesion. Int. J. Mol. Sci. 2017, 18, 1363. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Llorach, R.; Lamuela-Raventós, R.M.; Jáuregui, O.; Estruch, R.; Izquierdo-Pulido, M.; Andrés-Lacueva, C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7258–7267. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-W.; Wang, N.; Li, W.; Xu, W.; Wu, S. Biotransformation of 4,5-O-dicaffeoylquinic acid methyl ester by human intestinal flora and evaluation on their inhibition of NO production and antioxidant activity of the products. Food Chem. Toxicol. 2013, 55, 297–303. [Google Scholar] [CrossRef]
- di Gesso, J.L.; Kerr, J.S.; Zhang, Q.; Raheem, S.; Yalamanchili, S.K.; O’Hagan, D.; Kay, C.D.; O’Connell, M.A. Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol. Nutr. Food Res. 2015, 59, 1143–1154. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Wang, H.; Wang, J.-H.; Wang, Q.; Ma, Q.-F.; Chen, Y.-Y. Protocatechuic Acid Inhibits Inflammatory Responses in LPS-Stimulated BV2 Microglia via NF-κB and MAPKs Signaling Pathways. Neurochem. Res. 2015, 40, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Bae, O.-N.; Lim, K.-M.; Noh, J.-Y.; Kang, S.; Chung, K.Y.; Chung, J.-H. Novel antiplatelet activity of protocatechuic acid through the inhibition of high shear stress-induced platelet aggregation. J. Pharmacol. Exp. Ther. 2012, 343, 704–711. [Google Scholar] [CrossRef]
- Semaming, Y.; Kumfu, S.; Pannangpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J. Endocrinol. 2014, 223, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Dua, T.K.; Khanra, R.; Joardar, S.; Nandy, A.; Saha, A.; De Feo, V.; Dewanjee, S. Protocatechuic Acid, a Phenolic from Sansevieria roxburghiana Leaves, Suppresses Diabetic Cardiomyopathy via Stimulating Glucose Metabolism, Ameliorating Oxidative Stress, and Inhibiting Inflammation. Front. Pharmacol. 2017, 8, 251. [Google Scholar] [CrossRef]
- Qian, Y.; Babu, P.V.A.; Symons, J.D.; Jalili, T. Metabolites of flavonoid compounds preserve indices of endothelial cell nitric oxide bioavailability under glucotoxic conditions. Nutr. Diabetes 2017, 7, e286. [Google Scholar] [CrossRef]
| Author, Year, Country | Study Design | Participants (Mean Age) | Duration | Treatment | Polyphenol Constituent (Daily Intake) | Comparison | Main Findings |
|---|---|---|---|---|---|---|---|
| Murphy, 2003, Australia [38] | Double-blind, placebo-controlled | 32 healthy subjects (treatment group: 40 y; placebo group: 47 y) | 28 d | Tablets containing flavanols and procyanidins | 234 mg flavanols and procyanidins | Placebo tablets (≤6 mg flavanols and procyanidins) | No divergences were seen between groups after treatment. |
| Engler, 2004, USA [39] | Double-blind, placebo-controlled | 21 healthy volunteers (38 y) | 2 wk | Dark chocolate bars | 213 mg total procyanidins and 46 mg epicatechin | Placebo dark chocolate bars (traces of procyanidins and epicatechin) | High-flavonoid chocolate intake was associated with a significant amelioration of FMD (p = 0.024). |
| Fraga, 2005, Argentina [40] | Crossover, placebo-controlled | 28 healthy participants (19 y) | 2 × 14 d | Flavanol-containing milk chocolate | 168 mg flavanols (39 mg epicatechin and catechin and 126 mg of procyanidins) | Cocoa butter chocolate (<5 mg/d of flavanols) | FCMC consumption was associated with a significant decrease in DBP (p = 0.01) and the mean BP (p = 0.008). |
| Grassi, 2005, Italy [41] | Crossover, placebo-controlled | 15 normotensive control (34 y) and 20 never-treated, grade I essential hypertensive individuals (43 y) | 2 × 15 d (7 d washout) | Dark chocolate bars | 88 mg flavanols (21.91 mg catechin, 65.97 mg epicatechin, 0.59 mg quercetin, 0.03 mg kaempferol, and 0.31 mg isorhamnetin) | Flavanol-free white chocolate bars | SBP and DBP significantly decreased after dark chocolate consumption compared to white chocolate and baseline values (p < 0.0001). FMD increased after dark chocolate consumption but not after white chocolate consumption (p < 0.0001). |
| Wang-Polagruto, 2006, USA [42] | Double-blind, placebo-controlled | 32 postmenopausal hypercholesterolemic women (high-flavanol group: 57 y; low-flavanol group: 55 y) | 6 wk | High-flavanol cocoa beverage | 446 mg flavanols | Placebo cocoa beverage (43 mg flavanols) | Low-flavanol consumption led to a significant decrease in SBP and DBP (p < 0.05). High-flavanol treatment led to an increase in brachial artery hyperemic blood flow after (p < 0.05) compared to the baseline. |
| Baba, 2007, Japan [43] | Double-blind, placebo-controlled | 25 healthy male subjects (38 y) | 12 wk | Cocoa powder + sugar | 98.02 mg epicatechins, 35.1 mg catechins, 41.08 procyanidin B2, and 24.9 mg procyanidin C1 | Sugar | No significant results were found in SBP and DBP. |
| Taubert, 2007, Germany [44] | Investigator-blinded, placebo-controlled | 44 participants (64 y) | 18 wk | Dark chocolate | 30 mg polyphenols (catechin 1.7 mg, epicatechin 5.1 mg, epicatechin-gallate 0.3 mg, procyanidin dimer 6.8 mg, procyanidin dimer-gallate 1.8 mg, procyanidin tetramer 3.7 mg, procyanidin pentamer 2.6 mg, and flavonols < 0.05 mg) | Polyphenol-free white chocolate | Dark chocolate consumption reduced SBP and DBP (p < 0.001). |
| Abdullah A, 2008, Saudi Arabia [45] | Placebo-controlled | 89 healthy female (21 y) | 15 d | (i) Dark chocolate | 1.89 mg gallic acid, 1.08 mg ECG, 3.99 mg catechin, caffeine 16.99 mg, 4.01 mg EC, epigallocatechin 3-gallate 1.40 mg, and epicatechin 3-gallate 0.14 mg | (ii) White chocolate or no chocolate; (iii) no chocolate | Dark chocolate intake was associated with decreased SBP and DBP (p < 0.05). |
| Crews, 2008, USA [46] | Double-blind, placebo-controlled | 90 healthy participants (≥60 y) | 6 wk | Dark chocolate bar and artificially sweetened cocoa beverage | 754.71 mg total proanthocyanidins/g | Placebo bar (0.20 mg/g proanthocyanin) and beverage (40.87 mg/g proanthocyanin) | The dark chocolate and cocoa groups displayed an increased pulse rate at the midpoint and at the end of treatment (p = 0.007). |
| Davison, 2008, Australia [47] | Double-blind, placebo-controlled | 49 participants (41 y) | 12 wk | High-flavanol cocoa drink | 902 mg flavanols | Low-flavanol cocoa drink (36 mg/d flavanols) | High-flavanol cocoa intake increased FMD (p < 0.01) and reduced DBP and mean arterial BP (p < 0.05). |
| Grassi, 2008, Italy [48] | Placebo-controlled, crossover | 19 essential hypertension participants (44 y) | 2 × 15 d (7 d washout) | Flavanol-rich dark chocolate | 1008 mg total phenols (110.9 mg EC, 36.12 mg catechin, 2.5 mg quercetin, 0.03 mg kaempferol, and 0.2 mg isorhamnetin) | Flavanol-free white chocolate | Flavanol-rich chocolate reduced the SBP, DBP and ambulatory pressure which was inversely correlated with an increase in FMD with FRDC (p < 0.0001). |
| Muniyappa, 2008, USA [49] | Double-blind, placebo-controlled, crossover | 20 participants with mild-to-moderate hypertension (43 y) | 2 × 2 wk (1 wk washout) | Flavanol-rich cocoa drink | 902 mg cocoa polyphenols (174 mg EC, 62 mg catechin, and 676 mg procyanidins) | Flavanol-poor placebo (28 mg cocoa polyphenols: 2 mg epicatechin, 8 mg catechin, and 16 mg procyanidins) | Cocoa treatment for 2 wk was able to increase insulin-stimulated brachial arterial dilatation compared to placebo (p = 0.028). |
| Shiina, 2009, Japan [50] | Single-blind, placebo-controlled | 39 healthy men (29 y) | 2 wk | Flavonoid-rich dark chocolate | Catechin, epicatechin, and procyanidin (550 mg) | White chocolate | Dark chocolate consumption was associated with increased change in CFVR compared to control group (p < 0.01). |
| Heiss, 2010, USA [51] | Double-blind, placebo-controlled, crossover | 16 participants CAD (64 y) | 2 × 30 d (1 wk washout) | Cocoa drink | 750 mg flavanols, monomers–decamers, 130 mg monomers, 118 mg EC, 12 mg catechin, 106 mg dimers, and 516 mg trimers–decamers | Placebo cocoa drink (18 mg flavanols, monomers–decamers, 6 mg monomers, 2 mg EC, 4 mg catechin, 4 mg dimers, and 6 mg trimers–decamers) | High-flavanol intervention led to a significant increase in FMD (p < 0.05) and a decrease in SBP (p = 0.013). |
| Njike, 2011, USA [52] | Double-blind, placebo-controlled crossover | 37 healthy participants (52 y) | 2 × 6 wk (4 wk washout) | (i) Sugar-free cocoa beverage; (ii) sugar-sweetened cocoa beverage | 21 mg catechin, 48 mg epicatechin, 92 mg procyanidin dimer, 98 mg procyanidin trimer, 31 mg procyanidin tetramer, 55 mg procyanidin pentamer and hexamer, and 805 mg total procyanidin | Sugar-sweetened cocoa-free beverage | Cocoa ingestion improved FMD relative to the control group (p < 0.01). |
| Almoosawi, 2012, UK [53] | Single-blind placebo-controlled, crossover | 42 women (21 normal BMI, 13 overweight, and 8 obese) | 2 × 4 wk (2 wk washout) | Dark chocolate | 500 mg total polyphenols, 18.99 mg epicatechin and catechin | Placebo dark chocolate | Decrease in SBP (p = 0.007) and DBP (p = 0.003) after dark chocolate intake. |
| Desideri, 2012, Italy [54] | Double-blind | 90 elderly individuals | 8 wk | (i) High-flavanol cocoa drink; (ii) intermediate-flavanol cocoa drink | (i) 990 mg flavanols; (ii) 520 mg flavanols | Low flavanols drink (45 mg flavanols) | High and intermediate flavanol intake was associated with reduced BP after 8 wk of treatment (p < 0.0001) and compared to the low group (p < 0.05). |
| Flammer, 2012, Switzerland [55] | Double-blind, placebo-controlled | 20 participants with congestive heart failure (64 y) | 4 wk | Flavanol-rich chocolate | 10.8 mg catechin, 36 mg epicatechin | Placebo chocolate | Flavanol-rich chocolate administration led to significant increase in FMD (p = 0.045). No changes were observed for BP and HR. |
| Mogollon, 2013, Canada [56] | Double-blind, placebo-controlled | 42 healthy, pregnant women (29 y) | 12 wk | High-flavanol dark chocolate | 400 mg total flavanols, 64 mg total catechin and epicatechin | Low-flavanol chocolate | Results showed no differences. |
| Neufingerl, 2013, The Netherlands [57] | Double-blind, placebo-controlled | 143 healthy participants (theobromine + cocoa group: 55 y; theobromine group: 53 y; cocoa group: 55 y; placebo group: 55 y) | 4 wk | (i) Cocoa drink; (ii) theobromine drink; (iii) theobromine + cocoa drink | (i) 325 mg flavanols, 150 mg theobromine; (ii) 850 mg theobromine; (iii) 325 mg flavanols, 1000 mg theobromine | Placebo drink | No changes were reported in SBP, DBP, and HR among the groups. |
| Esser, 2014, UK [58] | Double-blind, placebo-controlled, crossover | 41 overweight, middle-aged men (57 y) | 4 wk (4 wk washout) | High-flavanol chocolate | 1078 mg flavanols, 349 mg epicatechins | Normal flavanol chocolate | An increase in FMD by 1% and a decrease in the AIX independent of the type of chocolate consumed. |
| Ibero-Baraibar, 2014, Spain [59] | Double-blind, placebo-controlled | 47 healthy participants with BMI of 30.59 ± 2.33 kg/m2 (57 y) | 4 wk | Cocoa extract | 414.26 mg total flavanols, 153.44 mg epicatechin, 14.56 mg catechin, 99.40 mg dimer B2, 13.44 mg dimer B1, and 133.53 mg oligomeric procyanidins | Control meal | Both groups showed an improvement in SBP and DBP after 4 weeks of intervention. |
| Nickols-Richardson, 2014, USA [60] | Placebo-controlled | 51 overweight/obese postmenopausal women (35 y) | 18 wk | Sugar-free natural cocoa beverage + chocolate snack | 270 mg flavanols | Non-cocoa beverage and non-cocoa snack | Both groups observed a significant reduction in SBP and DBP independent of intervention. |
| Sarriá, 2014, Spain [61] | Placebo-controlled, crossover | 24 normocholesterolemic (M: 28 y; F: 26 y) and 20 moderately hypercholesterolemic (M: 35 y; F: 25 y) volunteers | 2 × 2 wk | Soluble cocoa powder + milk | 416.4 mg polyphenols, 44.1 mg flavanols, 9.3 mg epicatechins, 18 mg catechins, and 16.5 mg procyanidin B2 | Milk | No significant changes were observed in SBP, DBP, and HR between groups. |
| West, 2014, USA [62] | Placebo-controlled, crossover | 30 middle-aged, overweight, and moderately obese adults (51 y) | 2 × 4 wk (2 wk washout) | Dark chocolate + sugar-free cocoa beverage | 814 mg total flavanols | Low-flavanol chocolate bar and cocoa-free beverage with no added sugar (3 mg/d total flavanols) | Increase in brachial artery diameter before hyperemia (p = 0.001) and at peak dilation following cuff release (p = 0.0001). Resting (p = 0.04) and peak (p = 0.03) hyperemic blood flow increased after treatment. Substantial decreases in the AIX were observed only in women (p = 0.01). |
| Heiss, 2015, Germany. [63] | Double-blind, placebo-controlled | 42 healthy participants (22 young (26 y); 20 elderly (60 y) | 14 d | CF drink | 900 mg flavanols, 146 mg monomers, 128 mg (-)-epicatechin, 14 mg (-)-catechin, 4 mg (+)-catechin, and 754 mg dimers–decamers | CF free | Improvement in FMD (p < 0.001), reduced PWV and lowered total peripheral resistance, and increased arteriolar and microvascular vasodilatory capacity, and central DBP in both groups. In elderly group, CF decreased the aortic AIX and office peripheral SBP (p < 0.05). |
| Koli, 2015, Finland [64] | Crossover | 22 participants with mild hypertension (45 y) | 2 × 8 wk (4 wk washout) | Dark chocolate | 602.7 mg flavanols | Snack restriction | No changes were observed after dark chocolate intake. |
| Massee, 2015, Australia [65] | Double-blind, placebo-controlled | 38 healthy participants (24 y) | 4 wk | Cocoa tablet | 250 mg catechin | Placebo tablets (inert cellulose powder) | No differences were observed compared to baseline values. |
| Mastroiacovo, 2015, Italy [66] | Double-blind, placebo-controlled | 90 elderly participants (69 y) | 8 wk | (i) High flavanol drink; (ii) intermediate-flavanol drink | (i) 993 mg total flavanols, 185 mg epicatechin, 62 mg catechin, 182 mg dimers, 141 mg trimers, 126 mg tetramers, 297 mg pentamers–decamers; (ii) 520 mg total flavanols, 95 mg epicatechin, 35 mg catechin, 96 mg dimers, 72 mg trimers, 64 mg tetramers, and 158 mg pentamers–decamers | Low flavanol drink containing 48 mg total flavanols, 5 mg epicatechin, 8 mg catechin, 10 mg dimers, 4 mg trimers, 2 mg tetramers, and 17 mg pentamers–decamers | SBP was significantly reduced in HF and IF after treatment (p < 0.0001) and compared to the LF group (p < 0.0001). |
| Ottaviani, 2015, USA [67] | Double-blind, placebo-controlled | 59 healthy adults (41 y) | 12 wk | Cocoa extract capsules | Up to 2000 mg cocoa flavanols (248 mg flavanol monomers, 1752 mg procyanidins) | Placebo capsules, cocoa-flavanol-free | Treatment was not associated with significant changes in BP or platelet function. |
| Rassaf, 2016, Germany [68] | Double-blind, placebo-controlled | 49 participants with ESRD (65 y) | 30 days | Cocola-flavanol-rich beverage | 900 mg total cocoa flavanols | Placebo beverage | A significant increase in FMD (p < 0.001), a reduction in DBP (p = 0.03), and an increase in HR (p = 0.01) compared to the placebo group. |
| Rull, 2015, UK [69] | Double-blind placebo-controlled, crossover | 32 healthy participants (55 years) | 2 × 6 wk | High-flavanol dark chocolate | 1064 mg total flavanols, 223 mg monomers, 192 mg procyanidin dimers, and 649 mg procyanidin trimers to decamers | Low-flavanol dark chocolate (88 mg total flavanols, 23 mg monomers, 24 mg procyanidin dimers, and 41 mg procyanidin trimers to decamers) | The LFDC group showed an increase in 24 h (p = 0.008) and daily HR (p = 0.001) compared to pre-study values. |
| Sansone, 2015, Germany [70] | Double-blind, placebo-controlled | 100 healthy individuals (47 y) | 1 mo | Cocoa-flavanol drink | 900 mg total flavanols, 146 mg monomers, 128 mg (-)-epicatechin, 14 mg (-)-catechin, 4 mg (+)-catechin, and 754 mg dimers–decamers | Cocoa-flavanol-free drink | The CF group showed an increase in FMD and a decrease in office and central SBP and DBP. PWV and the AIX decreased in the CF group compared to the control group. |
| Njike, 2016, USA [71] | Double-blind, placebo-controlled | 101 adults with stage 1 hypertension (140–159/90–99 mmHg) on no more than one BP medication (53 y) | 2 × 8 wk (4 wk washout) | (i) 10 g cocoa powder; (ii) 5 g cocoa powder | (i) 261.8 mg, flavanols, 45.8 mg epicatechin; (ii) 130.9 mg flavonols, 22.9 mg epicatechin | Placebo chocolate | Treatment reduced BP after subgroup analysis (24 h SBP, p = 0.038, 24 h DBP, p = 0.023). It reduced BP (p = 0.009) and improved endothelial function (p = 0.031) in participants on beta blockers. Participants on diuretics daily consumption showed significant increase in 24 h ambulatory SBP (p = 0.022). |
| Garcia-Yu, 2020, Spain [72] | Controlled | 137 postmenopausal women (intervention group 57 y; control group 57 y) | 6 mo | Chocolate (99% cocoa) | 0.58 mg protocatechuic acid, 1.76 mg procyanidin dimer (B3), 10.4 mg catechin, 14.4 mg procyanidin dimer (B2), 26.1 mg epicatechin,8.53 mg procyanidin trimer (C1), 3.54 mg procyanidin A hexoside, 0.02 mg quercetin glucoside, and 0.03 mg quercetin arabinoside | No intervention | No notable changes were observed between groups for SBP, DBP, or brachial–ankle PWV. For PP, in contrast to the increase observed in the control group, there was a decrease in the intervention group (p = 0.048). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godos, J.; Romano, G.L.; Laudani, S.; Gozzo, L.; Guerrera, I.; Dominguez Azpíroz, I.; Martínez Diaz, R.; Quiles, J.L.; Battino, M.; Drago, F.; et al. Flavan-3-ols and Vascular Health: Clinical Evidence and Mechanisms of Action. Nutrients 2024, 16, 2471. https://doi.org/10.3390/nu16152471
Godos J, Romano GL, Laudani S, Gozzo L, Guerrera I, Dominguez Azpíroz I, Martínez Diaz R, Quiles JL, Battino M, Drago F, et al. Flavan-3-ols and Vascular Health: Clinical Evidence and Mechanisms of Action. Nutrients. 2024; 16(15):2471. https://doi.org/10.3390/nu16152471
Chicago/Turabian StyleGodos, Justyna, Giovanni Luca Romano, Samuele Laudani, Lucia Gozzo, Ida Guerrera, Irma Dominguez Azpíroz, Raquel Martínez Diaz, José L. Quiles, Maurizio Battino, Filippo Drago, and et al. 2024. "Flavan-3-ols and Vascular Health: Clinical Evidence and Mechanisms of Action" Nutrients 16, no. 15: 2471. https://doi.org/10.3390/nu16152471
APA StyleGodos, J., Romano, G. L., Laudani, S., Gozzo, L., Guerrera, I., Dominguez Azpíroz, I., Martínez Diaz, R., Quiles, J. L., Battino, M., Drago, F., Giampieri, F., Galvano, F., & Grosso, G. (2024). Flavan-3-ols and Vascular Health: Clinical Evidence and Mechanisms of Action. Nutrients, 16(15), 2471. https://doi.org/10.3390/nu16152471














