Antidiabetic Effect of Passiflora ligularis Leaves in High Fat-Diet/Streptozotocin-Induced Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Extract and Fraction of Passiflora ligularis
2.3. Chemical Characterization and Quantification of Flavonoids in the Extract and Ethanolic Fraction of P. ligularis Leaves
2.4. Induction of T2DM and Experimental Design
- Vehicle group: Diabetic control mice receiving the vehicle (0.5% CMC w/v and 0.5% Tween 80 w/v).
- Metformin group: Diabetic mice receiving metformin (250 mg/kg) as a positive control.
- Aqueous extract group: Diabetic mice receiving the aqueous extract of leaves of P. ligularis (500 mg/kg).
- Ethanolic fraction group: Diabetic mice receiving the ethanolic fraction of P. ligularis (250 mg/kg).
2.5. Oral Glucose Tolerance Test
2.6. Insulin Resistance Index (HOMA-IR)
2.7. Histopathological Examination
2.8. Biochemical Parameters
2.8.1. Analysis of Oxidative Stress Parameters
2.8.2. Serum Lipid Profile
2.9. Statistical Analysis
3. Results
3.1. Chemical Characterization
3.2. Effect of P. ligularis on Blood Glucose Levels
3.3. Oral Glucose Tolerance Test (OGTT)
3.4. Insulin Resistance Index (HOMA-IR)
3.5. Histopathological Examination
3.6. Analysis of Oxidative Stress Parameters
3.7. Effect of P. ligularis on Serum Lipid Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Kotwas, A.; Karakiewicz, B.; Zabielska, P.; Wieder-Huszla, S.; Jurczak, A. Epidemiological Factors for Type 2 Diabetes Mellitus: Evidence from the Global Burden of Disease. Arch. Public Health 2021, 79, 110. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Sanches, J.M.; Zhao, L.N.; Salehi, A.; Wollheim, C.B.; Kaldis, P. Pathophysiology of Type 2 Diabetes and the Impact of Altered Metabolic Interorgan Crosstalk. FEBS J. 2023, 290, 620–648. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; del Cañizo-Gómez, F.J. Update on the Treatment of Type 2 Diabetes Mellitus. World J. Diabetes 2016, 7, 354. [Google Scholar] [CrossRef] [PubMed]
- Surya, S.; Salam, A.D.; Tomy, D.V.; Carla, B.; Kumar, R.A.; Sunil, C. Diabetes Mellitus and Medicinal Plants-a Review. Asian Pac. J. Trop. Dis. 2014, 4, 337–347. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxid. Med. Cell Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential. of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Salaj, N.; Kladar, N.; Srđenović Čonić, B.; Jeremić, K.; Hitl, M.; Gavarić, N.; Božin, B. Traditional Multi-Herbal Formula in Diabetes Therapy—Antihyperglycemic and Antioxidant Potential. Arab. J. Chem. 2021, 14, 103347. [Google Scholar] [CrossRef]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora Edulis: An Insight Into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, V.L.; Silva, C.G.; Campana, P.R.V. Flavonoids of Passiflora: Isolation, Structure Elucidation, and Biotechnological Application. Stud. Nat. Prod. Chem. 2021, 71, 263–310. [Google Scholar]
- Miroddi, M.; Calapai, G.; Navarra, M.; Minciullo, P.L.; Gangemi, S. Passiflora incarnata L.: Ethnopharmacology, Clinical Application, Safety and Evaluation of Clinical Trials. J. Ethnopharmacol. 2013, 150, 791–804. [Google Scholar] [CrossRef]
- Saravanan, S.; Parimelazhagan, T. In Vitro Antioxidant, Antimicrobial and Anti-Diabetic Properties of Polyphenols of Passiflora Ligularis Juss. Fruit Pulp. Food Sci. Hum. Wellness 2014, 3, 56–64. [Google Scholar] [CrossRef]
- Aragón Novoa, D.M.; Ospina Giraldo, L.F.; Ramos Rodríguez, F.A.; Castellanos Hernández, L.; Costa Modesti, G.; Barreto Silva, F.R.M. Passiflora Ligularis Juss. (Granadilla): Farmacológicos de Una Estudios Químicos y Planta Con Potencial Terapéutico, 1st ed.; Aragón Novoa, D.M., Ed.; Universidad Nacional de Colombia—Sede Bogotá: Bogotá, Colombia, 2021; ISBN 9789587946420. [Google Scholar]
- Sepúlveda, P.M.; Echeverrry, S.; Costa, G.; Aragón, M. Passiflora Ligularis Leaf Ultrasound-Assisted Extraction in the Optimization of Flavonoid Content and Enhancement of Hypoglycemic Activity. J. Appl. Pharm. Sci. 2020, 10, 086–094. [Google Scholar] [CrossRef]
- Echeverry, S.M.; Rey, D.; Valderrama, I.H.; de Araujo, B.V.; Aragón, D.M. Development of a Self-Emulsifying Drug Delivery System (SEDDS) to Improve the Hypoglycemic Activity of Passiflora Ligularis Leaves Extract. J. Drug Deliv. Sci. Technol. 2021, 64, 102604. [Google Scholar] [CrossRef]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental Animal Models for Diabetes and Its Related Complications—A Review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef] [PubMed]
- Rey, D.; Miranda Sulis, P.; Alves Fernandes, T.; Gonçalves, R.; Silva Frederico, M.J.; Costa, G.M.; Aragon, M.; Ospina, L.F.; Mena Barreto Silva, F.R. Astragalin Augments Basal Calcium Influx and Insulin Secretion in Rat Pancreatic Islets. Cell Calcium 2019, 80, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Rey, D.; Fernandes, T.A.; Sulis, P.M.; Gonçalves, R.; Sepúlveda, R.M.; Silva Frederico, M.J.; Aragon, M.; Ospina, L.F.; Costa, G.M.; Silva, F.R.M.B. Cellular Target of Isoquercetin from Passiflora Ligularis Juss for Glucose Uptake in Rat Soleus Muscle. Chem. Biol. Interact. 2020, 330, 109198. [Google Scholar] [CrossRef]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szucs, G.; Attieh, Z.; Murlasits, Z.; Török, S.; Pósa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 9051426. [Google Scholar] [CrossRef]
- Dash, S.; Pattnaik, G.; Kar, B.; Sahoo, N.; Bhattacharya, S. An Approach towards Method Development to Investigate the Anti-Diabetic Activity on Experimental Animals. Curr. Trends Biotechnol. Pharm. 2021, 15, 330–348. [Google Scholar]
- Saliu, T.P.; Kumrungsee, T.; Miyata, K.; Tominaga, H.; Yazawa, N.; Hashimoto, K.; Kamesawa, M.; Yanaka, N. Comparative Study on Molecular Mechanism of Diabetic Myopathy in Two Different Types of Streptozotocin-Induced Diabetic Models. Life Sci. 2022, 288, 120183. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; Wang, Y.; Wang, K.; Ji, B.P.; Zhou, F. Stability of a Type 2 Diabetes Rat Model Induced by High-Fat Diet Feeding with Low-Dose Streptozotocin Injection. J. Zhejiang Univ. Sci. B 2018, 19, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.J.A.; De Celle, T.; Debets, J.J.M.; Brouns, A.E.; Callahan, M.F.; Smith, T.L. Effects of Anesthetics on Systemic Hemodynamics in Mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, S.M.; Valderrama, I.H.; Costa, G.M.; Ospina-Giraldo, L.F.; Aragón, D.M. Development and Optimization of Microparticles Containing a Hypoglycemic Fraction of Calyces from Physalis Peruviana. J. Appl. Pharm. Sci. 2018, 8, 10–18. [Google Scholar] [CrossRef][Green Version]
- Abdelhameed, R.F.A.; Ibrahim, A.K.; Elfaky, M.A.; Habib, E.S.; Mahamed, M.I.; Mehanna, E.T.; Darwish, K.M.; Khodeer, D.M.; Ahmed, S.A.; Elhady, S.S. Antioxidant and Anti-Inflammatory Activity of Cynanchum acutum L. Isolated Flavonoids Using Experimentally Induced Type 2 Diabetes Mellitus: Biological and In Silico Investigation for NF-ΚB Pathway/MiR-146a Expression Modulation. Antioxidants 2021, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ebrahimpour, P.; Liu, Y.; Yang, C.; Alonso, L.C. Pancreatic Islet Embedding for Paraffin Sections. J. Vis. Exp. 2018, 2018, 57931. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, A.S.D.; Attanayake, A.P.; Kalansuriya, P. Biochemical Characterization of High Fat Diet Fed and Low Dose Streptozotocin Induced Diabetic Wistar Rat Model. J. Pharmacol. Toxicol. Methods 2022, 113, 107144. [Google Scholar] [CrossRef]
- Hori, M. An Automatic Chromatographic Method for the Separation of Flavonoid Compounds. Bull. Chem. Soc. Jpn. 1969, 42, 2333–2336. [Google Scholar] [CrossRef]
- Rosler, K.-H.; Goodwin, R.S. A General Use of Amberlite XAD-2 Resin for the Purification of Flavonoids from Aqueous Fractions. J. Nat. Prod. 1984, 47, 188. [Google Scholar] [CrossRef]
- Queiroz, E.A.M.; Paim, R.T.T.; Lira, S.M.; da Silva, J.Y.G.; Lima, C.L.S.; Holanda, M.O.; Benjamin, S.R.; Vieira, Í.G.P.; Guedes, M.I.F. Antihyperglycemic Effect of Passiflora Glandulosa Cav. Fruit Rinds Flour in Streptozotocin-Induced Diabetic Mice. Asian Pac. J. Trop. Med. 2018, 11, 510–517. [Google Scholar] [CrossRef]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the Glucose Tolerance Test in Mice. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Rajan, M.; de Souza Araújo, A.A.; Narain, N. Potential of Passion (Passiflora Spp.) Fruit in Control of Type II Diabetes. Curr. Res. Diabetes Obes. J. 2018, 7, CRDOJ.MS.ID.555712. [Google Scholar] [CrossRef]
- Asir, P.J.; Hemmalakshmi, S.; Priyanga, S.; Devaki, K. Antidiabetic Activity of Aqueous and Ethanolic Extracts of Passiflora foetida L. in Alloxan Induced Diabetes Rats. J. Pharm. Res. 2014, 3, 1627–1641. [Google Scholar]
- Meneses, C.; Monzón Daza, G.; Modesti Costa, G.; Aragón Novoa, M.; Ramos, R.F.; Castellanos Hernández, L. Evaluación de La Actividad Antiinflamatoria Del Extracto Acuoso, La Fracción Butanólica y Compuestos Identificados En Las Hojas de Passiflora Ligularis Juss. In Passiflora Ligularis Juss. (Granadilla): Estudios Químicos y Farmacológicos de una Planta con Potencial Terapéutico; Aragon, M., Ed.; Universidad Nacional de Colombia—Sede Bogotá: Bogotá, Colombia, 2021; pp. 41–54. ISBN 9789587946420. [Google Scholar]
- Zhang, L.; Zhang, S.-T.; Yin, Y.-C.; Xing, S.; Li, W.-N.; Fu, X.-Q. Hypoglycemic Effect and Mechanism of Isoquercitrin as an Inhibitor of Dipeptidyl Peptidase-4 in Type 2 Diabetic Mice. RSC Adv. 2018, 8, 14967–14974. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, K.; Sravanthi, K.; Shaker, I.; Ponnulakshmi, R.; Selvaraj, J. Role of Chrysin on Expression of Insulin Signaling Molecules. J. Ayurveda Integr. Med. 2015, 6, 248. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.; Elkfury, J.L.; Jornada, M.N.; Foletto, K.C.; Bertoluci, M.C. Validation of HOMA-IR in a Model of Insulin-Resistance Induced by a High-Fat Diet in Wistar Rats. Arch. Endocrinol. Metab. 2016, 60, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.d.A.E.; Milenkovic, D.; Borges, T.K.d.S.; Rosa, A.J.d.M.; Morand, C.; Oliveira, L.d.L.d.; Costa, A.M. Acute Effects of the Consumption of Passiflora Setacea Juice on Metabolic Risk Factors and Gene Expression Profile in Humans. Nutrients 2020, 12, 1104. [Google Scholar] [CrossRef]
- Sarto, D.A.Q.S.; de Siqueira, A.H.D.; de Almeida Magalhaes, F.M.; de Paula Caproni, K.; Martins, Â.M.; Santos, G.B.; da Silva, D.B.; Boas, B.M.V.; Garcia, J.A.D. Dry Extract of Passiflora incarnata L. Leaves as A Cardiac And Hepatic Oxidative Stress Protector In Ldlr-/-Mice Fed High-Fat Diet. Braz. Arch. Biol. Technol. 2018, 61, e18180147. [Google Scholar] [CrossRef]
- Huang, X.-L.; He, Y.; Ji, L.-L.; Wang, K.-Y.; Wang, Y.-L.; Chen, D.-F.; Geng, Y.; Ouyang, P.; Lai, W.-M. Hepatoprotective Potential of Isoquercitrin against Type 2 Diabetes-Induced Hepatic Injury in Rats. Oncotarget 2017, 8, 101545. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, R.; Dhaouefi, Z.; Toumia, I.B.; Lahmar, A.; Sioud, F.; Bouhajeb, R.; Bellalah, A.; Chekir-Ghedira, L. Erica Multiflora Extract Rich in Quercetin-3-O-Glucoside and Kaempferol-3-O-Glucoside Alleviates High Fat and Fructose Diet-Induced Fatty Liver Disease by Modulating Metabolic and Inflammatory Pathways in Wistar Rats. J. Nutr. Biochem. 2020, 86, 108490. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Shirakawa, J.; Okuyama, T.; Kyohara, M.; Yamazaki, S.; Togashi, Y.; Terauchi, Y. Effects of Metformin on Compensatory Pancreatic β-Cell Hyperplasia in Mice Fed a High-Fat Diet. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E367–E380. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of Oxidative Stress during Diabetes Mellitus. J. Biomark. 2013, 2013, 378790. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Catechin Treatment Ameliorates Diabetes and Its Complications in Streptozotocin-Induced Diabetic Rats. Dose-Response 2017, 15, 1559325817691158. [Google Scholar] [CrossRef] [PubMed]
- Oldoni, T.L.C.; Merlin, N.; Bicas, T.C.; Prasniewski, A.; Carpes, S.T.; Ascari, J.; de Alencar, S.M.; Massarioli, A.P.; Bagatini, M.D.; Morales, R.; et al. Antihyperglycemic Activity of Crude Extract and Isolation of Phenolic Compounds with Antioxidant Activity from Moringa Oleifera Lam. Leaves Grown in Southern Brazil. Food Res. Int. 2021, 141, 110082. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J. Clinical Review 124: Diabetic Dyslipidemia—Causes and Consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.; Nguyen, T.T.; Zimmerman, B.R. Hyperlipidemia and Diabetes Mellitus. Mayo Clin. Proc. 1998, 73, 969–976. [Google Scholar] [CrossRef]
- Panchanathan, S.; Rajendran, J. Evidence of Anti-Hyperglycemic and Anti-Oxidant Effect of Passiflora Edulis Flavicarpa (Sims.) in Streptozotocin Induced Diabetic Rats. Not. Sci. Biol. 2015, 7, 383–389. [Google Scholar] [CrossRef]
- Sunny, A.; Perumal, V.; Chandy, V. Leaves of Passiflora Edulis. World J. Pharm. Res. 2020, 9, 1513–1522. [Google Scholar]
- Angel-Isaza, J.; Carmona-Hernandez, J.C.; González-Correa, C.H.; Narváez-Solarte, W.V. Potential Hypoglycemic and Antilipidemic Activity of Polyphenols from Passiflora Ligularis (Granadilla). Molecules 2023, 28, 3551. [Google Scholar] [CrossRef] [PubMed]
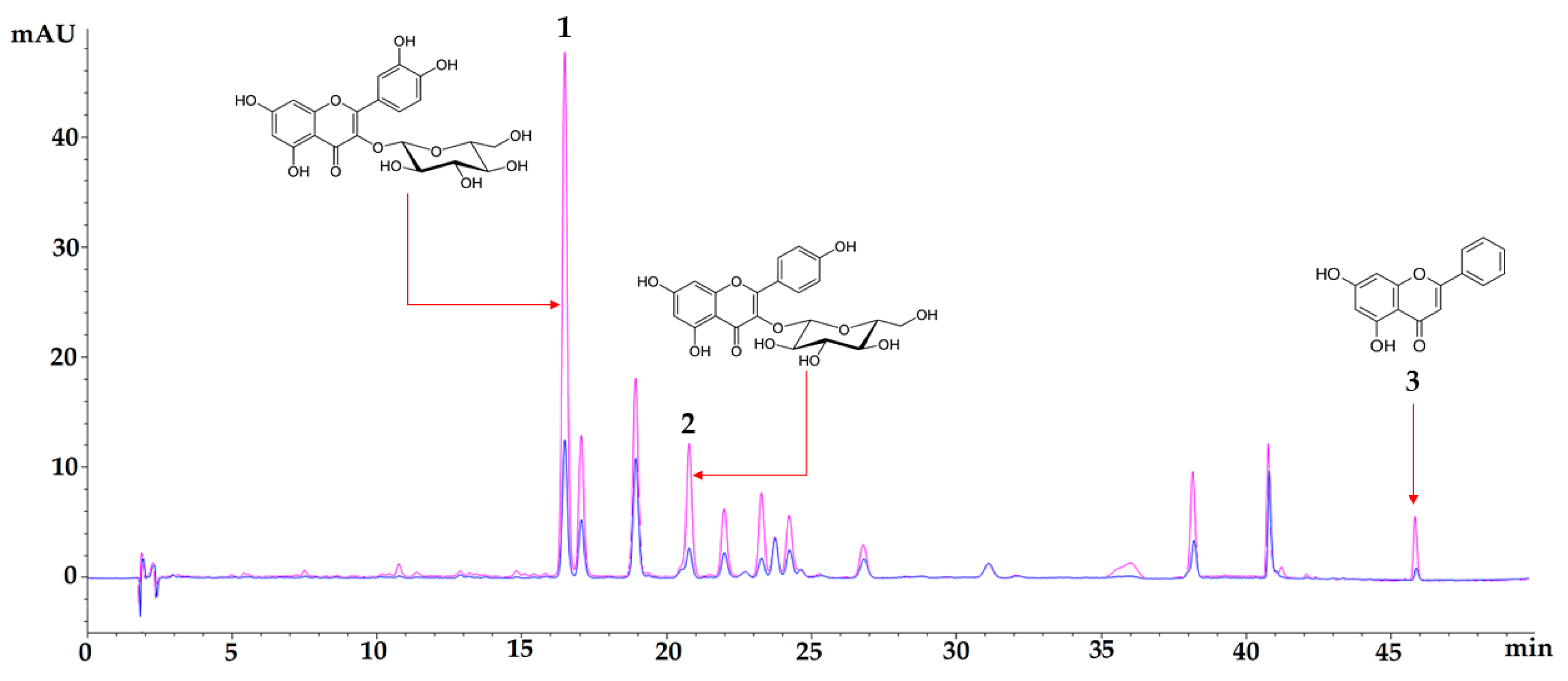
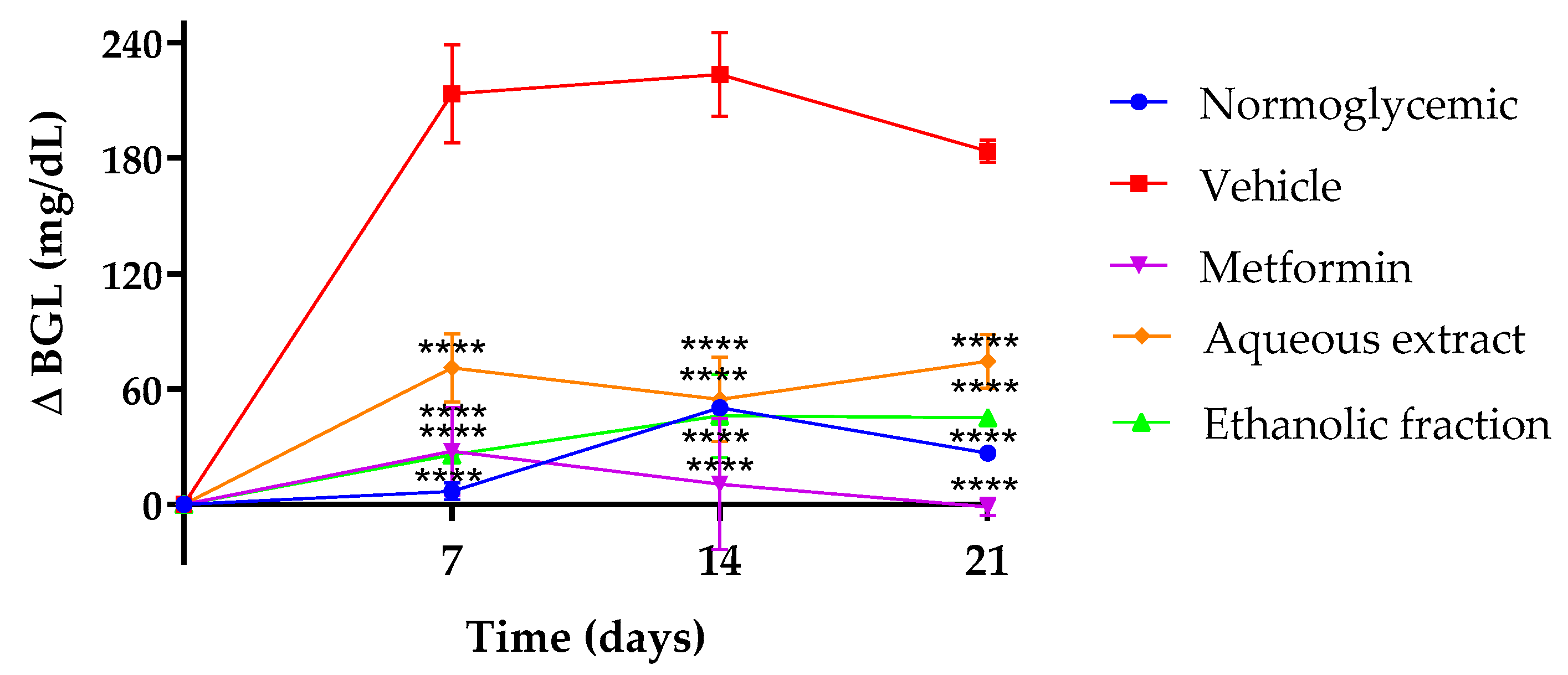
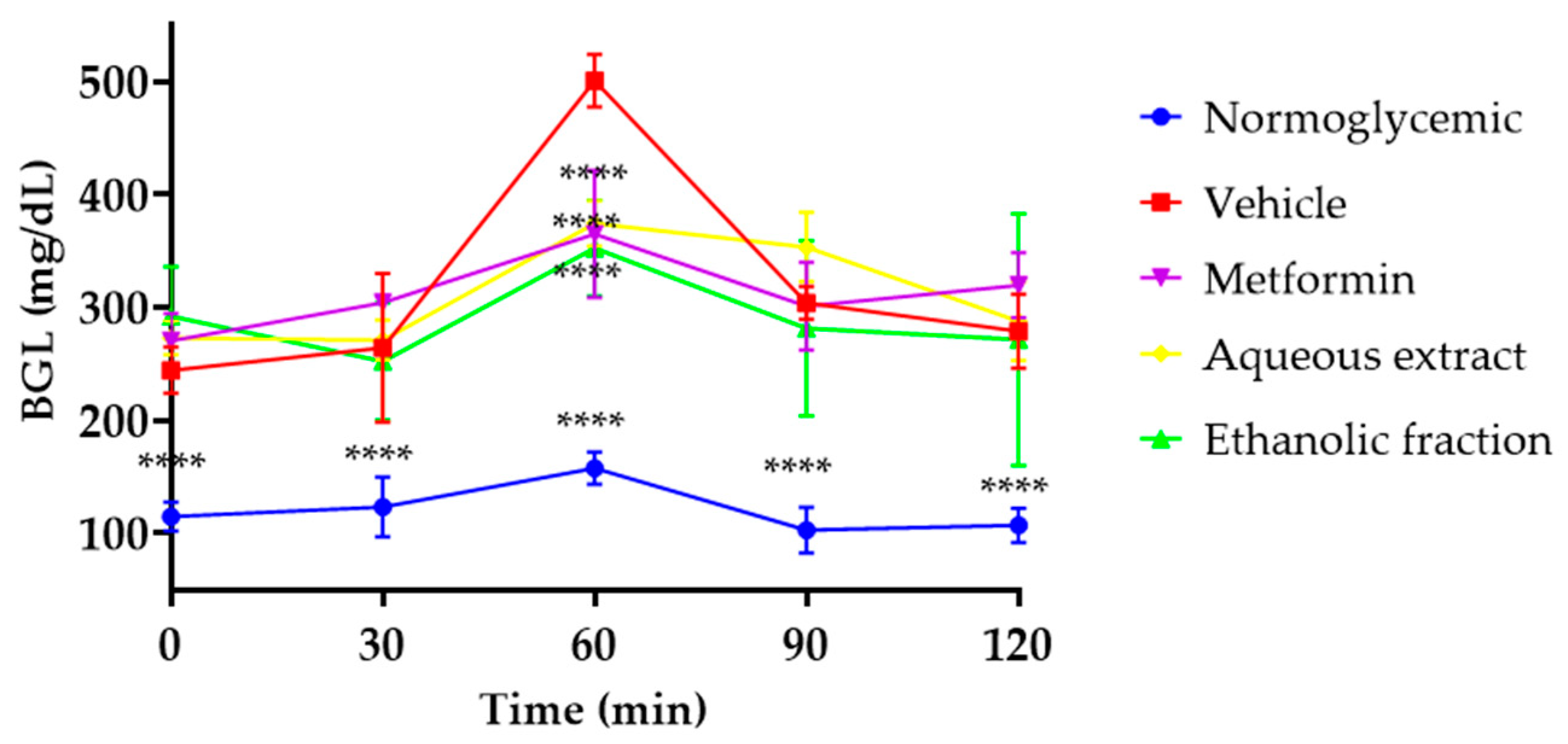


| TREATMENT | Fasting Glucose mg/dL | Insulin Levels (µUI/mL) | HOMA-IR |
|---|---|---|---|
| Normoglycemic | 105.600 ± 7.579 **** | 2.267 ± 0.332 **** | 0.591 ± 0.086 **** |
| vehicle | 434.000 ± 26.069 | 23.178 ± 2.527 | 24.830 ± 2.421 |
| Metformin 250 mg/kg | 347.286 ± 19.499 **** | 15.877 ± 1.752 **** | 13.610 ± 1.343 **** |
| Aqueous extract 500 mg/kg | 333.800 ± 15.766 **** | 12.493 ± 1.407 **** | 10.294 ± 1.037 **** |
| Ethanol fraction 250 mg/kg | 292.600 ± 27.207 **** | 12.986 ± 1.217 **** | 9.379 ± 0.786 **** |
| TREATMENT | Triglyceride (TG) mg/dL | Total Cholesterol mg/dL | LDL-C mg/dL | HDL-C mg/dL |
|---|---|---|---|---|
| Normoglycemic | 96.870 ± 2.230 | 140.280 ± 15.345 | 88.820 ± 25.778 | 37.843 ± 1.926 |
| vehicle | 225.506 ± 13.345 | 252.076 ± 5.479 | 174.658 ± 17.526 | 28.923 ± 0.081 |
| Metformin 250 mg/kg | 169.606 ± 3.615 **** | 159.775 ± 4.805 **** | 98.438 ± 13.633 *** | 36.727 ± 4.824 ** |
| Aqueous extract 500 mg/kg | 159.450 ± 7.070 **** | 207.410 ± 17.580 * | 131.464 ± 25.953 * | 34.263 ± 2.206 * |
| Extract fraction 250 mg/kg | 157.160 ± 0.430 **** | 162.700 ± 13.790 **** | 117.336 ± 2.449 ** | 36.237 ± 1.980 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey, D.P.; Echeverry, S.M.; Valderrama, I.H.; Rodriguez, I.A.; Ospina, L.F.; Mena Barreto Silva, F.R.; Aragón, M. Antidiabetic Effect of Passiflora ligularis Leaves in High Fat-Diet/Streptozotocin-Induced Diabetic Mice. Nutrients 2024, 16, 1669. https://doi.org/10.3390/nu16111669
Rey DP, Echeverry SM, Valderrama IH, Rodriguez IA, Ospina LF, Mena Barreto Silva FR, Aragón M. Antidiabetic Effect of Passiflora ligularis Leaves in High Fat-Diet/Streptozotocin-Induced Diabetic Mice. Nutrients. 2024; 16(11):1669. https://doi.org/10.3390/nu16111669
Chicago/Turabian StyleRey, Diana P., Sandra M. Echeverry, Ivonne H. Valderrama, Ingrid A. Rodriguez, Luis F. Ospina, Fatima Regina Mena Barreto Silva, and Marcela Aragón. 2024. "Antidiabetic Effect of Passiflora ligularis Leaves in High Fat-Diet/Streptozotocin-Induced Diabetic Mice" Nutrients 16, no. 11: 1669. https://doi.org/10.3390/nu16111669
APA StyleRey, D. P., Echeverry, S. M., Valderrama, I. H., Rodriguez, I. A., Ospina, L. F., Mena Barreto Silva, F. R., & Aragón, M. (2024). Antidiabetic Effect of Passiflora ligularis Leaves in High Fat-Diet/Streptozotocin-Induced Diabetic Mice. Nutrients, 16(11), 1669. https://doi.org/10.3390/nu16111669







