Metabolic and Low-Grade Inflammation Risk in Young Adults with a History of Extrauterine Growth Restriction
Highlights
- Young adults with a history of extrauterine growth restriction (EUGR) showed a higher percentage of hypertension and insulin resistance compared to the reference healthy group.
- The EUGR group, despite showing lower body weights for its members, shows a different adipokines profile, with higher levels of leptin and lower levels of adiponectin and resistin compared to the healthy reference group.
- Young adults with a history of EUGR could present altered metabolic conditions that may increase future risks of metabolic and cardiovascular diseases.
- Further research is needed to establish preventive strategies in the follow-up of patients with EUGR.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical History, Physical Examination, and Blood Pressure Measurements
2.3. Biochemical Parameters, Proinflammatory Biomarkers and Adipokines
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Born Too Soon: Decade of Action on Preterm Birth; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Figueras-Aloy, J.; Palet-Trujols, C.; Matas-Barceló, I.; Botet-Mussons, F.; Carbonell-Estrany, X. Extrauterine growth restriction in very preterm infant: Etiology, diagnosis, and 2-year follow-up. Eur. J. Pediatr. 2020, 179, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynecol. Obstet. 2021, 152, 3–57. [Google Scholar] [CrossRef] [PubMed]
- Albu, A.R.; Anca, A.F.; Horhoianu, V.; Horhoianu, I.A. Predictive factors for intrauterine growth restriction. J. Med. Life 2014, 7, 165–171. [Google Scholar] [PubMed]
- Kerkhof, G.F.; Willemsen, R.H.; Leunissen, R.W.J.; Breukhoven, P.E.; Hokken-Koelega, A.C.S. Health Profile of Young Adults Born Preterm: Negative Effects of Rapid Weight Gain in Early Life. J. Clin. Endocrinol. Metab. 2012, 97, 4498–4506. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, E.L.S.S.; de Lima Macêna, M.; Bueno, N.B.; de Oliveira, A.C.M.; Mello, C.S. Premature birth, low birth weight, small for gestational age and chronic non-communicable diseases in adult life: A systematic review with meta-analysis. Early Hum. Dev. 2020, 149, 105154. [Google Scholar] [CrossRef] [PubMed]
- Gounaris, A.K.; Sokou, R.; Gounari, E.A.; Panagiotounakou, P.; Grivea, I.N. Extrauterine Growth Restriction and Optimal Growth of Very Preterm Neonates: State of the Art. Nutrients 2023, 15, 3231. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Cormack, B.; Goldberg, D.; Nasser, R.; Alshaikh, B.; Eliasziw, M.; Hay, W.W.; Hoyos, A.; Anderson, D.; Bloomfield, F.; et al. Extrauterine growth restriction’ and ‘postnatal growth failure’ are misnomers for preterm infants. J. Perinatol. 2020, 40, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.J.; Tancredi, D.J.; Bertino, E.; Lee, H.C.; Profit, J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rotteveel, J.; van Weissenbruch, M.M.; Twisk, J.W.R.; de Waal, H.A.D.-V. Infant and Childhood Growth Patterns, Insulin Sensitivity, and Blood Pressure in Prematurely Born Young Adults. Pediatrics 2008, 122, 313–321. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Rigo, J. Extrauterine growth restriction in very-low-birthweight infants. Acta Paediatr. 2004, 93, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.E.; Richardson, D.K.; Schmid, C.H.; Ausman, L.M.; Dwyer, J.T. Intersite Differences in Weight Growth Velocity of Extremely Premature Infants. Pediatrics 2002, 110, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.H.; Thomas, P.; Peabody, J. Extrauterine Growth Restriction Remains a Serious Problem in Prematurely Born Neonates. Pediatrics 2003, 111, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo Renau, M.; Aldecoa-Bilbao, V.; Balcells Esponera, C.; del Rey Hurtado de Mendoza, B.; Iriondo Sanz, M.; Iglesias-Platas, I. Applying Methods for Postnatal Growth Assessment in the Clinical Setting: Evaluation in a Longitudinal Cohort of Very Preterm Infants. Nutrients 2019, 11, 2772. [Google Scholar] [CrossRef] [PubMed]
- Zozaya, C.; Díaz, C.; de Pipaón, M.S. How Should We Define Postnatal Growth Restriction in Preterm Infants? Neonatology 2018, 114, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Nobile, S.; Di Sipio Morgia, C.; Vento, G. Perinatal Origins of Adult Disease and Opportunities for Health Promotion: A Narrative Review. J. Pers. Med. 2022, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alisi, A.; Panera, N.; Agostoni, C. Low birth weight and catch-up-growth associated with metabolic syndrome: A ten year systematic review. Pediatr. Endocrinol. Rev. 2008, 6, 241–247. [Google Scholar] [PubMed]
- Briana, D.D.; Malamitsi-Puchner, A. Intrauterine growth restriction and adult disease: The role of adipocytokines. Eur. J. Endocrinol. 2009, 160, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jiménez, M.; Gómez-García, F.; Gil-Campos, M.; Pérez-Navero, J. Comorbidities in childhood associated with extrauterine growth restriction in preterm infants: A scoping review. Eur. J. Pediatr. 2020, 179, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Espejo, M.; Gil-Campos, M.; Mesa, M.D.; García-Rodríguez, C.E.; Muñoz-Villanueva, M.C.; Pérez-Navero, J.L. Alterations in the antioxidant defense system in prepubertal children with a history of extrauterine growth restriction. Eur. J. Nutr. 2014, 53, 607–615. [Google Scholar] [CrossRef]
- Ortiz Espejo, M.; Gil Campos, M.; Muñoz Villanueva, M.C.; Pérez Navero, J.L. Alteraciones metabólicas en prepúberes con retraso del crecimiento extrauterino. An. Pediatr. (Engl. Ed.) 2012, 77, 247–253. [Google Scholar] [CrossRef]
- Frithioff-Bøjsøe, C.; Lund, M.A.; Lausten-Thomsen, U.; Hedley, P.L.; Pedersen, O.; Christiansen, M.; Baker, J.L.; Hansen, T.; Holm, J.-C. Leptin, adiponectin, and their ratio as markers of insulin resistance and cardiometabolic risk in childhood obesity. Pediatr. Diabetes 2020, 21, 194–202. [Google Scholar] [CrossRef]
- Kopec, G.; Shekhawat, P.S.; Mhanna, M.J. Prevalence of diabetes and obesity in association with prematurity and growth restriction. Diabetes Metab. Syndr. Obes. 2017, 10, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.M.; Katz, S.L.; Leeson, P.; Thébaud, B.; Nuyt, A.-M. Preterm birth: Risk factor for early-onset chronic diseases. Can. Med. Assoc. J. 2016, 188, 736–746. [Google Scholar] [CrossRef]
- Moreno-Mendez, E.; Quintero-Fabian, S.; Fernandez-Mejia, C.; Lazo-De-La-Vega-Monroy, M.L. Early-life programming of adipose tissue. Nutr. Res. Rev. 2020, 33, 244–259. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, M.D.; Gil-Campos, M.; Flores-Rojas, K.; Muñoz-Villanueva, M.C.; Aguilera-García, C.M.; Torre-Aguilar, M.J.D.L.; Pérez-Navero, J.L. Plasma adipokines profile in prepubertal children with a history of prematurity or extrauterine growth restriction. Nutrients 2020, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Díaz, M.D.; Pérez-Navero, J.L.; Flores-Rojas, K.; Olza-Meneses, J.; Muñoz-Villanueva, M.C.; Aguilera-García, C.M.; Gil-Campos, M. Prematurity with Extrauterine Growth Restriction Increases the Risk of Higher Levels of Glucose, Low-Grade of Inflammation and Hypertension in Prepubertal Children. Front. Pediatr. 2020, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Díaz, M.D.; Gil-Campos, M.; Flores-Rojas, K.; Muñoz-Villanueva, M.C.; Mesa, M.D.; de la Torre-Aguilar, M.J.; Gil, Á.; Pérez-Navero, J.L. Impaired Antioxidant Defence Status Is Associated with Metabolic-Inflammatory Risk Factors in Preterm Children with Extrauterine Growth Restriction: The BIORICA Cohort Study. Front. Nutr. 2021, 8, 793862. [Google Scholar] [CrossRef] [PubMed]
- Sánchez González, E.; Carrascosa Lezcano, A.; Fernández García, J.M.; Ferrández Longás, A.; López de Lara, D.; López-Siguero, J.P. Estudios españoles de crecimiento: Situación actual, utilidad y recomendaciones de uso. An. Pediatr. (Engl. Ed.) 2011, 74, 193-e1–193-e16. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. Body Mass Index (BMI). 2023. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed on 12 December 2023).
- WHO. World Health Organization. BMI-for-Age (5–19 Years). 2007. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years (accessed on 10 May 2024).
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- García Cuartero, B.; Lacalle, C.G.; Rey, C.C.; Villar, M.A.; Martínez, E.D. Índice HOMA y QUICKI, insulina y péptido C en niños sanos. Puntos de corte de riesgo cardiovascular. An. Pediatr. (Engl. Ed.) 2007, 66, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Rogero Blanco, M.E.; Albañil Ballesteros, M.R.; Sánchez Martin, M.; Rabanal Basalo, A.; Olivas Domínguez, A.; García Lacalle, C. Prevalencia de resistencia a insulina en una población de jóvenes adultos. Relación con el estado ponderal. Endocrinol. Nutr. 2012, 59, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Masoodian, S.M.; Omidifar, A.; Moradkhani, S.; Asiabanha, M.; Khoshmirsafa, M. HOMA-IR mean values in healthy individuals: A population-based study in iranian subjects. J. Diabetes Metab. Disord. 2022, 22, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Finken, M.J.J.; Dekker, F.W.; De Zegher, F.; Wit, J.M. Long-term height gain of prematurely born children with neonatal growth restraint: Parallellism with the growth pattern of short children born small for gestational age. Pediatrics 2006, 118, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Tauber, M. Final height and intrauterine growth retardation. Ann. Endocrinol. 2017, 78, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Comité Nacional de Endocrinología. Restricción del crecimiento intrauterino: Perspectiva endocrinológica. Resumen. Arch. Argent Pediatr. 2017, 115, s63–s67. [Google Scholar] [CrossRef] [PubMed]
- Evensen, K.A.I.; Steinshamn, S.; Tjønna, A.E.; Stølen, T.; Høydal, M.A.; Wisløff, U.; Brubakk, A.-M.; Vik, T. Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood. Early Hum. Dev. 2009, 85, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- López-Jiménez, F.; Cortés-Bergoderi, M. Obesidad y corazón. Rev. Esp. Cardiol. 2011, 64, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, A.; Brunelli, V.; Prefumo, F.; Frusca, T.; Lees, C.C. Early onset fetal growth restriction. Matern. Health Neonatol. Perinatol. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Skilton, M.R.; Crispi, F. Human fetal growth restriction: A cardiovascular journey through to adolescence. J. Dev. Orig. Health Dis. 2016, 7, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Batsis, J.A.; Coutinho, T.; Somers, V.K.; Hodge, D.O.; Carter, R.E.; Sochor, O.; Kragelund, C.; Kanaya, A.M.; Zeller, M.; et al. Normal-Weight Central Obesity and Mortality Risk in Older Adults with Coronary Artery Disease. Mayo Clin. Proc. 2016, 91, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.A.; Smith, G.D.; Ben-Shlomo, Y.; Litchfield, P. Low Birth Weight Is Associated with Higher Adult Total Cholesterol Concentration in Men. Circulation 2004, 110, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Owen, C.G.; Whincup, P.H.; Cook, D.G.; Colman, S.; Collins, R. Birth Weight and Subsequent Cholesterol Levels. JAMA 2004, 292, 2755. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, P.M.L.; Hardy, R.J.; Kuh, D.J.; Langenberg, C.; Wadsworth, M.E.J. Birth Weight and Lipids in a National Birth Cohort Study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, P.M.L.; Cassidy, A.; Swaminathan, R.; Falchi, M.; Spector, T.D.; MacGregor, A.J. Intrauterine, Environmental, and Genetic Influences in the Relationship Between Birth Weight and Lipids in a Female Twin Cohort. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2373–2379. [Google Scholar] [CrossRef] [PubMed]
- González-Barranco, J.; Rıos-Torres, J.M.; Castillo-Martınez, L.; López-Alvarenga, J.C.; Aguilar-Salinas, C.A.; Bouchard, C.; Deprès, J.P.; Tremblay, A. Effect of malnutrition during the first year of life on adult plasma insulin and glucose tolerance. Metabolism 2003, 52, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, S.; Montelisciani, L.; Giussani, M.; Lieti, G.; Patti, I.; Orlando, A.; Antolini, L.; Parati, G. Role of Insulin Resistance as a Mediator of the Relationship between Body Weight, Waist Circumference, and Systolic Blood Pressure in a Pediatric Population. Metabolites 2023, 13, 327. [Google Scholar] [PubMed]
- Cândido, A.P.C.; Geloneze, B.; Calixto, A.; Vasques, A.C.J.; Freitas, R.N.D.; Freitas, S.N.D.; Machado-Coelho, G.L.L. Adiponectin, HOMA-Adiponectin, HOMA-IR in Children and Adolescents: Ouro Preto Study. Indian J. Pediatr. 2021, 88, 336–344. [Google Scholar] [CrossRef]
- Deusdará, R.; de Moura Souza, A.; Szklo, M. Association between Obesity, Overweight, Elevated Waist Circumference, and Insulin Resistance Markers among Brazilian Adolescent Students. Nutrients 2022, 14, 3487. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, P.; Antolini, L.; Street, M.E.; Giussani, M.; Galbiati, S.; Valsecchi, M.G.; Stella, A.; Zuccotti, G.V.; Bernasconi, S.; Genovesi, S. Adiponectin and Hypertension in Normal-Weight and Obese Children. Am. J. Hypertens. 2013, 26, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Klünder-Klünder, M.; Flores-Huerta, S.; García-Macedo, R.; Peralta-Romero, J.; Cruz, M. Adiponectin in eutrophic and obese children as a biomarker to predict metabolic syndrome and each of its components. BMC Public Health 2013, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Saarikoski, L.A.; Juonala, M.; Huupponen, R.; Viikari, J.S.; Lehtimäki, T.; Jokinen, E.; Hutri-Kähönen, N.; Taittonen, L.; Laitinen, T.; Raitakari, O.T. Low serum adiponectin levels in childhood and adolescence predict increased intima-media thickness in adulthood. The Cardiovascular Risk in Young Finns Study. Ann. Med. 2017, 49, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pérez, A.; Blanco-Vaca, F. Adiponectina: Un nuevo nexo entre obesidad, resistencia a la insulina y enfermedad cardiovascular. Med. Clin. 2005, 124, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.U.; Ha, K.H.; Han, S.J.; Kim, H.J.; Kim, D.J. The Association of Adiponectin and Visceral Fat with Insulin Resistance and β-Cell Dysfunction. J. Korean Med. Sci. 2018, 34, e7. [Google Scholar] [CrossRef] [PubMed]
- Zamojska, J.; Niewiadomska-Jarosik, K.; Wosiak, A.; Gruca, M.; Smolewska, E. Serum Adipocytokines Profile in Children Born Small and Appropriate for Gestational Age—A Comparative Study. Nutrients 2023, 15, 868. [Google Scholar] [CrossRef]
- Aknc, A.; Karakurt, C.; Gurbuz, S.; Elkran, O.; Nalbantoglu, O.; Kocak, G.; Guldur, T.; Yologlu, S. Association of cardiac changes with serum adiponectin and resistin levels in obese and overweight children. J. Cardiovasc. Med. 2013, 14, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Giapros, V.; Vavva, E.; Siomou, E.; Kolios, G.; Tsabouri, S.; Cholevas, V.; Bairaktari, E.; Tzoufi, M.; Challa, A. Low-birth-weight, but not catch-up growth, correlates with insulin resistance and resistin level in SGA infants at 12 months. J. Matern. -Fetal Neonatal Med. 2017, 30, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Kojima-Ishii, K.; Toda, N.; Okubo, K.; Tocan, V.; Ohyama, N.; Makimura, M.; Matsuo, T.; Ochiai, M.; Ohga, S.; Ihara, K. Metabolic and immunological assessment of small-for-gestational-age children during one-year treatment with growth hormone: The clinical impact of apolipoproteins. Endocr. J. 2018, 65, 449–459. [Google Scholar] [CrossRef]
- Briana, D.D.; Boutsikou, M.; Baka, S.; Gourgiotis, D.; Marmarinos, A.; Hassiakos, D.; Malamitsi-Puchner, A. Perinatal Changes of Plasma Resistin Concentrations in Pregnancies with Normal and Restricted Fetal Growth. Neonatology 2008, 93, 153–157. [Google Scholar] [CrossRef]
- Prencipe, G.; Minnone, G.; Strippoli, R.; De Pasquale, L.; Petrini, S.; Caiello, I.; Manni, L.; De Benedetti, F.; Bracci-Laudiero, L. Nerve Growth Factor Downregulates Inflammatory Response in Human Monocytes through TrkA. J. Immunol. 2014, 192, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Q.; Cai, D.; Guo, H.; Fang, J.; Cui, H.; Gou, L.; Deng, J.; Wang, Z.; Zuo, Z. Resistin, a Novel Host Defense Peptide of Innate Immunity. Front. Immunol. 2021, 12, 699807. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Olsen, I.E. How Fast Should the Preterm Infant Grow? Curr. Pediatr. Rep. 2013, 1, 240–246. [Google Scholar]
- Kakatsaki, I.; Papanikolaou, S.; Roumeliotaki, T.; Anagnostatou, N.H.; Lygerou, I.; Hatzidaki, E. The Prevalence of Small for Gestational Age and Extrauterine Growth Restriction among Extremely and Very Preterm Neonates, Using Different Growth Curves, and Its Association with Clinical and Nutritional Factors. Nutrients 2023, 15, 3290. [Google Scholar] [PubMed]
- Clark, R.H.; Olsen, I.E.; Spitzer, A.R. Assessment of Neonatal Growth in Prematurely Born Infants. Clin. Perinatol. 2014, 41, 295–307. [Google Scholar] [CrossRef] [PubMed]
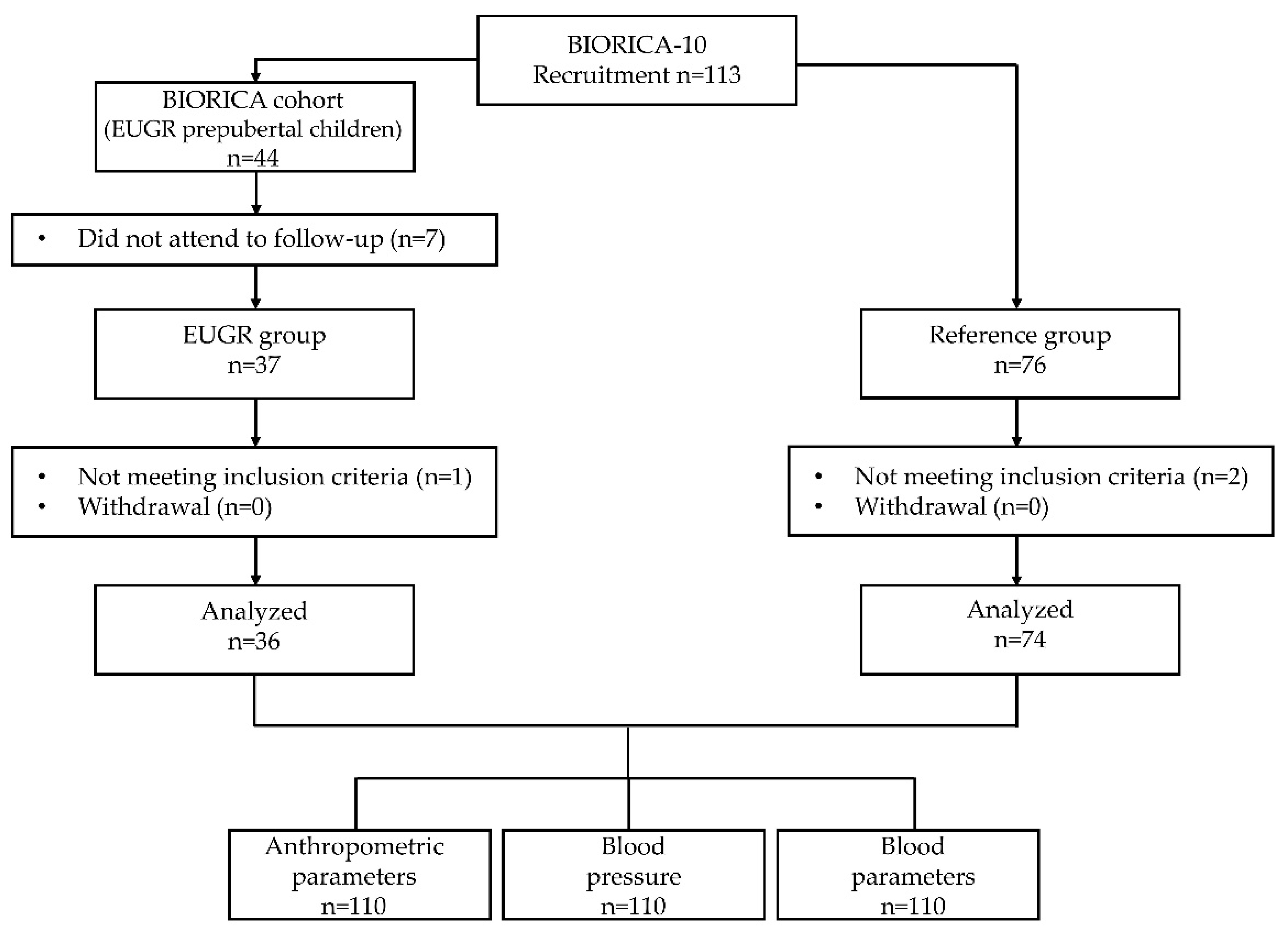
| General Variables | EUGR Group (n = 36) | Control Group (n = 74) | p Value |
|---|---|---|---|
| Sex (M/F) | 25/11 | 35/39 | 0.029 a |
| Age (years) | 17 (6) | 21(2) | <0.001 b |
| Weight (kg) | 53.4 ± 2.2 | 64.4 ± 2.1 | 0.008 c |
| Height (cm) | 161.4 ± 1.5 | 168.7 ± 0.9 | <0.001 c |
| BMI (kg/m2) | 21.8 ± 0.4 | 22.4 ± 0.6 | 0.480 c |
| NW/OW/UW (%) | 52.8/19.4/27.8 | 78.1/20.5/1.4 | <0.001 a |
| WC (cm) | 76.4 ± 1.7 | 68.3 ± 1.1 | 0.001 c |
| Fat Mass (kg) | 11.9 ± 1.1 | 14.2 ± 0.7 | 0.121 c |
| Fat mass (%) | 19.6 ± 1.2 | 22.7 ± 0.7 | 0.054 c |
| FMI (kg/m2) | 4.7 ± 0.4 | 5.0 ± 0.2 | 0.532 c |
| Lean mass (kg) | 45.2 ± 1.5 | 50.1 ± 0.9 | 0.016 c |
| Lean mass (%) | 80.4 ± 1.2 | 77.3 ± 0.7 | 0.054 c |
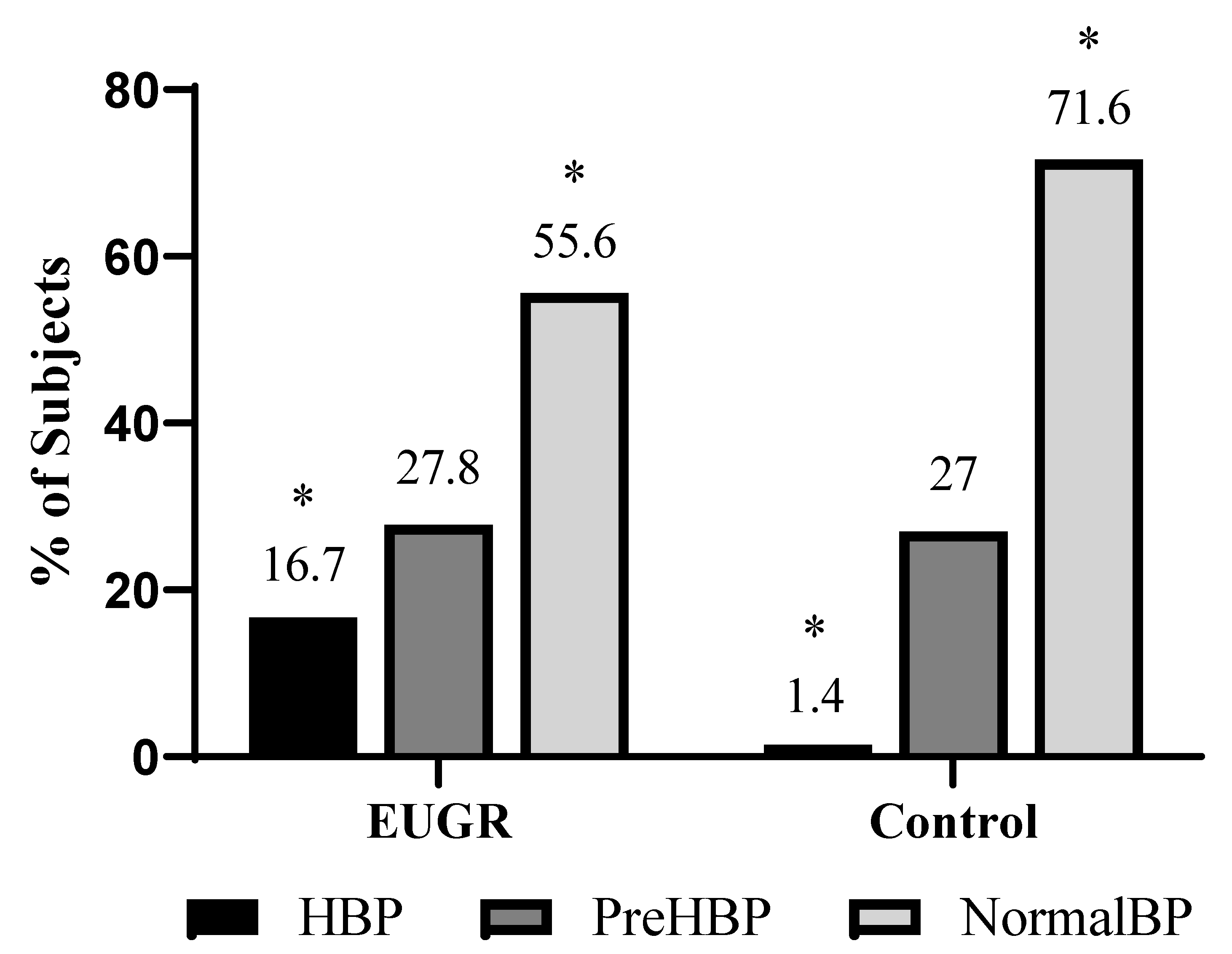
| SBP (mmHg) | 122.2 ± 2.08 | 115.9 ± 1.2 | 0.022 c |
| DBP (mmHg) | 67.6 ± 1.8 | 67.5 ± 1.07 | 0.968 c |
| HR (lpm) | 78.4 ± 3.5 | 70.8 ± 2.06 | 0.097 c |
| EUGR Group (n = 36) | Control Group (n = 74) | p Value a | |
|---|---|---|---|
| Glycemic metabolism and insulin sensitivity | |||
| Glucose (mg/dL) | 86.2 ± 1.7 | 85.2 ± 1.04 | 0.681 |
| Insulin (µU/mL) | 9.6 ± 0.7 | 7.2 ± 0.4 | 0.012 |
| HOMA-IR | 2.0 ± 0.2 | 1.5 ± 0.09 | 0.025 |
| Lipid parameters | |||
| Triacylglyerols (mg/dL) | 59.6 ± 6.9 | 77.2 ± 4.1 | 0.053 |
| Cholesterol (mg/dL) | 150.3 ± 5.7 | 165.9 ± 3.4 | 0.040 |
| HDLc (mg/dL) | 57.2 ± 2.4 | 57.9 ± 1.5 | 0.827 |
| LDLc (mg/dL) | 81.07 ± 4.7 | 92.6 ± 2.8 | 0.064 |
| Apo A (mg/dL) | 128.2 ± 4.09 | 131.2 ± 2.4 | 0.576 |
| Apo B (mg/dL) | 58.3 ± 3.6 | 63.8 ± 2.1 | 0.240 |
| Adipokines and inflammatory parameters | |||
| Adiponectin (mg/L) | 16.3 ± 2.6 | 25.7 ± 1.5 | 0.006 |
| Resistin (μg/L) | 12.6 ± 2.3 | 33.7 ± 1.4 | <0.001 |
| Leptin (μg/L) | 4.6 ± 0.5 | 3.0 ± 0.3 | 0.023 |
| PAI-1 (μg/L) | 15.9 ± 1.9 | 10.8 ± 1.2 | 0.050 |
| NGF (pg/mL) | 3.2 ± 0.3 | 2.4 ± 0.2 | 0.016 |
| MCP-1 (pg/mL) | 94.06 ± 8.9 | 106.7 ± 5.4 | 0.281 |
| TNF-α (pg/mL) | 1.7 ± 0.2 | 2.5 ± 0.1 | 0.002 |
| IL-8 (pg/mL) b | 0.5 ± 2.09 | 6.3 ± 1.3 | 0.046 |
| CRP (mg/L) | 0.6 ± 0.5 | 1.4 ± 0.3 | 0.239 |
| OR | IC (95%) | p Value a | |
|---|---|---|---|
| Height | 0.936 | (0.9–0.98) | 0.002 |
| Weight | 0.933 | (0.9–0.97) | <0.001 |
| WC | 1.059 | (1.02–1.11) | 0.007 |
| SBP | 3.125 | (1.11–8.8) | 0.030 |
| HOMA-IR | 1.839 | (1.19–2.84) | 0.006 |
| Adiponectin | 0.871 | (0.82–0.93) | <0.001 |
| Resistin | 0.754 | (0.67–0.85) | <0.001 |
| Leptin | 1.144 | (1.01–1.3) | 0.037 |
| NGF | 2.041 | (1.38–3.03) | <0.001 |
| TNF-α | 0.222 | (0.09–0.49) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomino-Fernández, L.; Pastor-Villaescusa, B.; Velasco, I.; Rico, M.d.l.C.; Roa, J.; Gil, Á.; Gil-Campos, M. Metabolic and Low-Grade Inflammation Risk in Young Adults with a History of Extrauterine Growth Restriction. Nutrients 2024, 16, 1608. https://doi.org/10.3390/nu16111608
Palomino-Fernández L, Pastor-Villaescusa B, Velasco I, Rico MdlC, Roa J, Gil Á, Gil-Campos M. Metabolic and Low-Grade Inflammation Risk in Young Adults with a History of Extrauterine Growth Restriction. Nutrients. 2024; 16(11):1608. https://doi.org/10.3390/nu16111608
Chicago/Turabian StylePalomino-Fernández, Laura, Belén Pastor-Villaescusa, Inmaculada Velasco, María de la Cruz Rico, Juan Roa, Ángel Gil, and Mercedes Gil-Campos. 2024. "Metabolic and Low-Grade Inflammation Risk in Young Adults with a History of Extrauterine Growth Restriction" Nutrients 16, no. 11: 1608. https://doi.org/10.3390/nu16111608
APA StylePalomino-Fernández, L., Pastor-Villaescusa, B., Velasco, I., Rico, M. d. l. C., Roa, J., Gil, Á., & Gil-Campos, M. (2024). Metabolic and Low-Grade Inflammation Risk in Young Adults with a History of Extrauterine Growth Restriction. Nutrients, 16(11), 1608. https://doi.org/10.3390/nu16111608








