The Mediterranean Diet in Pregnancy: Implications for Maternal Brain Morphometry in a Secondary Analysis of the IMPACT BCN Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Maternal Brain MR Acquisition and Processing
2.3. Dietary Questionnaires and Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Maternal Brain MR Results
3.3. Association of Maternal Brain MR and Diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094. [Google Scholar] [CrossRef] [PubMed]
- Staubo, S.C.; Aakre, J.A.; Vemuri, P.; Syrjanen, J.A.; Mielke, M.M.; Geda, Y.E.; Kremers, W.K.; Machulda, M.M.; Knopman, D.S.; Petersen, R.C.; et al. Mediterranean Diet, Micronutrients and Macronutrients, and MRI Measures of Cortical Thickness. Alzheimers Dement. 2017, 13, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Brickman, A.M.; Stern, Y.; Habeck, C.G.; Razlighi, Q.R.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Scarmeas, N. Mediterranean Diet and Brain Structure in a Multiethnic Elderly Cohort. Neurology 2015, 85, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Ballarini, T.; Melo Van Lent, D.; Brunner, J.; Schröder, A.; Wolfsgruber, S.; Altenstein, S.; Brosseron, F.; Buerger, K.; Dechent, P.; Dobisch, L.; et al. Mediterranean Diet, Alzheimer Disease Biomarkers, and Brain Atrophy in Old Age. Neurology 2021, 96, e2920–e2932. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Walters, M.; Sterling, J.; Quinn, C.; McHugh, P.; Andrews, R.E.; Matthews, D.C.; Ganzer, C.; Osorio, R.S.; Isaacson, R.S.; et al. Lifestyle and Vascular Risk Effects on MRI-Based Biomarkers of Alzheimer’s Disease: A Cross-Sectional Study of Middle-Aged Adults from the Broader New York City Area. BMJ Open 2018, 8, e019362. [Google Scholar] [CrossRef]
- Raji, C.A.; Erickson, K.I.; Lopez, O.L.; Kuller, L.H.; Gach, H.M.; Thompson, P.M.; Riverol, M.; Becker, J.T. Regular Fish Consumption and Age-Related Brain Gray Matter Loss. Am. J. Prev. Med. 2014, 47, 444–451. [Google Scholar] [CrossRef]
- Hoekzema, E.; Barba-Müller, E.; Pozzobon, C.; Picado, M.; Lucco, F.; García-García, D.; Soliva, J.C.; Tobeña, A.; Desco, M.; Crone, E.A.; et al. Pregnancy Leads to Long-Lasting Changes in Human Brain Structure. Nat. Publ. Group 2016, 20, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Tolar-Peterson, T.; Reynolds, A.; Wall, C.; Reeder, N.; Rico Mendez, G. The Effects of Nutritional Interventions on the Cognitive Development of Preschool-Age Children: A Systematic Review. Nutrients 2022, 14, 532. [Google Scholar] [CrossRef]
- Carmona, S.; Martínez-García, M.; Paternina-Die, M.; Barba-Müller, E.; Wierenga, L.M.; Alemán-Gómez, Y.; Pretus, C.; Marcos-Vidal, L.; Beumala, L.; Cortizo, R.; et al. Pregnancy and Adolescence Entail Similar Neuroanatomical Adaptations: A Comparative Analysis of Cerebral Morphometric Changes. Hum. Brain Mapp. 2019, 40, 2143–2152. [Google Scholar] [CrossRef]
- Sastry, P.S. Lipids of Nervous Tissue: Composition and Metabolism. Prog. Lipid Res. 1985, 24, 69–176. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-Chain Omega-3 Fatty Acids and the Brain: A Review of the Independent and Shared Effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- Janssen, C.I.F.; Zerbi, V.; Mutsaers, M.P.C.; de Jong, B.S.W.; Wiesmann, M.; Arnoldussen, I.A.C.; Geenen, B.; Heerschap, A.; Muskiet, F.A.J.; Jouni, Z.E.; et al. Impact of Dietary N-3 Polyunsaturated Fatty Acids on Cognition, Motor Skills and Hippocampal Neurogenesis in Developing C57BL/6J Mice. J. Nutr. Biochem. 2015, 26, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Kevala, K.; Kim, J.; Moon, H.-S.; Jun, S.B.; Lovinger, D.; Kim, H.-Y. Docosahexaenoic Acid Promotes Hippocampal Neuronal Development and Synaptic Function. J. Neurochem. 2009, 111, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Zomeño, M.D.; Martínez-González, M.A.; Salas-Salvadó, J.; Corella, D.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Tinahones, F.J.; Miranda, J.L.; et al. Validity of the Energy-Restricted Mediterranean Diet Adherence Screener. Clin. Nutr. 2021, 40, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-P.; Huang, S.-Y.; Chiu, T.-H.; Huang, K.-C.; Huang, C.-L.; Chang, H.-C.; Pariante, C.M. Omega-3 Fatty Acids for Major Depressive Disorder During Pregnancy: Results From a Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Psychiatry 2008, 69, 644–651. [Google Scholar] [CrossRef]
- Tressou, J.; Buaud, B.; Simon, N.; Pasteau, S.; Guesnet, P. Very Low Inadequate Dietary Intakes of Essential N-3 Polyunsaturated Fatty Acids (PUFA) in Pregnant and Lactating French Women: The INCA2 Survey. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 3–10. [Google Scholar] [CrossRef]
- Al Wattar, H.B.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F.J.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-Style Diet in Pregnant Women with Metabolic Risk Factors (ESTEEM): A Pragmatic Multicentre Randomised Trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; García De La Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean Diet with Additional Extra Virgin Olive Oil and Pistachios Reduces the Incidence of Gestational Diabetes Mellitus (GDM): A Randomized Controlled Trial: The St. Carlos GDM Prevention Study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; García de la Torre, N.; Duran, A.; Fuentes, M.; Bordiú, E.; del Valle, L.; Familiar, C.; Valerio, J.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean Diet with an Enhanced Consumption of Extra Virgin Olive Oil and Pistachios Improves Pregnancy Outcomes in Women Without Gestational Diabetes Mellitus: A Sub-Analysis of the St. Carlos Gestational Diabetes Mellitus Prevention Study. Ann. Nutr. Metab. 2019, 74, 69–79. [Google Scholar] [CrossRef]
- House, J.S.; Mendez, M.; Maguire, R.L.; Gonzalez-Nahm, S.; Huang, Z.; Daniels, J.; Murphy, S.K.; Fuemmeler, B.F.; Wright, F.A.; Hoyo, C. Periconceptional Maternal Mediterranean Diet Is Associated With Favorable Offspring Behaviors and Altered CpG Methylation of Imprinted Genes. Front. Cell Dev. Biol. 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Crovetto, F.; Crispi, F.; Casas, R.; Martín-Asuero, A.; Borràs, R.; Vieta, E.; Estruch, R.; Gratacós, E.; Paules, C.; IMPACT BCN Trial Investigators; et al. Effects of Mediterranean Diet or Mindfulness-Based Stress Reduction on Prevention of Small-for-Gestational Age Birth Weights in Newborns Born to At-Risk Pregnant Individuals: The IMPACT BCN Randomized Clinical Trial. JAMA 2021, 326, 2150. [Google Scholar] [CrossRef] [PubMed]
- Nakaki, A.; Crovetto, F.; Urru, A.; Piella, G.; Borras, R.; Comte, V.; Vellvé, K.; Paules, C.; Segalés, L.; Dacal, M.; et al. Effects of Mediterranean Diet or Mindfulness-Based Stress Reduction on Fetal and Neonatal Brain Development A Secondary Analysis of a Randomized Clinical Trial (IMPACT BCN). Am. J. Obstet. Gyneocol. MFM 2023, 5, 101188. [Google Scholar] [CrossRef] [PubMed]
- Crovetto, F.; Nakaki, A.; Arranz, A.; Borras, R.; Vellvé, K.; Paules, C.; Boutet, M.L.; Castro-Barquero, S.; Freitas, T.; Casas, R.; et al. Effect of a Mediterranean Diet or Mindfulness-Based Stress Reduction During Pregnancy on Child Neurodevelopment: A Prespecified Analysis of the IMPACT BCN Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2330255. [Google Scholar] [CrossRef] [PubMed]
- Casas, I.; Nakaki, A.; Pascal, R.; Castro-Barquero, S.; Youssef, L.; Genero, M.; Benitez, L.; Larroya, M.; Boutet, M.L.; Casu, G.; et al. Effects of a Mediterranean Diet Intervention on Maternal Stress, Well-Being, and Sleep Quality throughout Gestation—The IMPACT-BCN Trial. Nutrients 2023, 15, 2362. [Google Scholar] [CrossRef] [PubMed]
- Crowley, T. The Investigation and Management of the Small–for–Gestational–Age Fetus. 2014, pp. 1–34. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_31.pdf (accessed on 11 November 2021).
- Crovetto, F.; Crispi, F.; Borras, R.; Paules, C.; Casas, R.; Martín-Asuero, A.; Arranz, A.; Vieta, E.; Estruch, R.; Gratacós, E. Mediterranean Diet, Mindfulness-Based Stress Reduction and Usual Care during Pregnancy for Reducing Fetal Growth Restriction and Adverse Perinatal Outcomes: IMPACT BCN (Improving Mothers for a Better PrenAtal Care Trial BarCeloNa): A Study Protocol for a Randomized Controlled Trial. Trials 2021, 22, 362. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Rosas, H.D.; Fischl, B. Highly Accurate Inverse Consistent Registration: A Robust Approach. NeuroImage 2010, 53, 1181–1196. [Google Scholar] [CrossRef]
- Ségonne, F.; Dale, A.M.; Busa, E.; Glessner, M.; Salat, D.; Hahn, H.K.; Fischl, B. A Hybrid Approach to the Skull Stripping Problem in MRI. NeuroImage 2004, 22, 1060–1075. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B. Automatically Parcellating the Human Cerebral Cortex. Cereb. Cortex 2004, 14, 11–22. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; Van Der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Van Der Kouwe, A.J.W.; Makris, N.; Ségonne, F.; Quinn, B.T.; Dale, A.M. Sequence-Independent Segmentation of Magnetic Resonance Images. NeuroImage 2004, 23, S69–S84. [Google Scholar] [CrossRef]
- Sled, J.G.; Zijdenbos, A.P.; Evans, A.C. A Nonparametric Method for Automatic Correction of Intensity Nonuniformity in MRI Data. IEEE Trans. Med. Imaging 1998, 17, 87–97. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef]
- Buckner, R.L.; Head, D.; Parker, J.; Fotenos, A.F.; Marcus, D.; Morris, J.C.; Snyder, A.Z. A Unified Approach for Morphometric and Functional Data Analysis in Young, Old, and Demented Adults Using Automated Atlas-Based Head Size Normalization: Reliability and Validation against Manual Measurement of Total Intracranial Volume. NeuroImage 2004, 23, 724–738. [Google Scholar] [CrossRef]
- Juton, C.; Castro-Barquero, S.; Casas, R.; Freitas, T.; Ruiz-León, A.M.; Crovetto, F.; Domenech, M.; Crispi, F.; Vieta, E.; Gratacós, E.; et al. Reliability and Concurrent and Construct Validity of a Food Frequency Questionnaire for Pregnant Women at High Risk to Develop Fetal Growth Restriction. Nutrients 2021, 13, 1629. [Google Scholar] [CrossRef]
- Alahmadi, A.A.S. Investigating the Sub-Regions of the Superior Parietal Cortex Using Functional Magnetic Resonance Imaging Connectivity. Insights Imaging 2021, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Fan, L.; Xu, J.; Li, C.; Liu, Y.; Fox, P.T.; Eickhoff, S.B.; Yu, C.; Jiang, T. Convergent Functional Architecture of the Superior Parietal Lobule Unraveled with Multimodal Neuroimaging Approaches: Parcellation of Superior Parietal Lobule. Hum. Brain Mapp. 2015, 36, 238–257. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The Precuneus: A Review of Its Functional Anatomy and Behavioural Correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef]
- Hoscheidt, S.; Sanderlin, A.H.; Baker, L.D.; Jung, Y.; Lockhart, S.; Kellar, D.; Whitlow, C.T.; Hanson, A.J.; Friedman, S.; Register, T.; et al. Mediterranean and Western Diet Effects on Alzheimer’s Disease Biomarkers, Cerebral Perfusion, and Cognition in Mid-life: A Randomized Trial. Alzheimer’s Dement. 2022, 18, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Murray, J.; Tsui, W.H.; Li, Y.; Davies, M.; Williams, S.; Pirraglia, E.; Spector, N.; Glodzik, L.; McHugh, P.; et al. Mediterranean Diet and Magnetic Resonance Imaging-Assessed Brain Atrophy in Cognitively Normal Individuals at Risk for Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2014, 1, 23–32. [Google Scholar] [CrossRef]
- Arjmand, G.; Abbas-Zadeh, M.; Eftekhari, M.H. Effect of MIND Diet Intervention on Cognitive Performance and Brain Structure in Healthy Obese Women: A Randomized Controlled Trial. Sci. Rep. 2022, 12, 2871. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive Oil Intake and Risk of Cardiovascular Disease and Mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bulló, M.; Martínez-González, M.Á.; Ros, E.; Corella, D.; Estruch, R.; Fitó, M.; Arós, F.; Wärnberg, J.; Fiol, M.; et al. Frequency of Nut Consumption and Mortality Risk in the PREDIMED Nutrition Intervention Trial. BMC Med. 2013, 11, 164. [Google Scholar] [CrossRef]
- Ni, J.; Nishi, S.K.; Babio, N.; Ros, E.; Basterra-Gortari, F.J.; Corella, D.; Castañer, O.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Higher versus Lower Nut Consumption and Changes in Cognitive Performance over Two Years in a Population at Risk of Cognitive Decline: A Cohort Study. Am. J. Clin. Nutr. 2023, 118, 360–368. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padrós, N.; Cofán, M.; Serra-Mir, M.; Pérez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Doménech, M.; et al. Effect of a 2-Year Diet Intervention with Walnuts on Cognitive Decline. The Walnuts And Healthy Aging (WAHA) Study: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Advice about Eating Fish for Women Who Are or Might Become Pregnant, Breastfeeding Mothers, and Young Children; FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Corella, D.; Castañer, O.; Lamuela-Raventos, R.-M.; Salas-Salvadó, J.; Martínez-González, M.-A.; Ros, E.; Estruch, R. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvención Con DIeta MEDiterránea (PREDIMED) Randomized Controlled Trial1–3. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef]
- Faust, T.E.; Gunner, G.; Schafer, D.P. Mechanisms Governing Activity-Dependent Synaptic Pruning in the Developing Mammalian CNS. Nat. Rev. Neurosci. 2021, 22, 657–673. [Google Scholar] [CrossRef]
- Micheli, L.; Bertini, L.; Bonato, A.; Villanova, N.; Caruso, C.; Caruso, M.; Bernini, R.; Tirone, F. Role of Hydroxytyrosol and Oleuropein in the Prevention of Aging and Related Disorders: Focus on Neurodegeneration, Skeletal Muscle Dysfunction and Gut Microbiota. Nutrients 2023, 15, 1767. [Google Scholar] [CrossRef]
- Yeste, N.; Valent, D.; Arroyo, L.; Vázquez-Gómez, M.; García-Contreras, C.; Pumarola, M.; González-Bulnes, A.; Bassols, A. Polyphenols and IUGR Pregnancies: Effects of the Antioxidant Hydroxytyrosol on Brain Neurochemistry and Development in a Porcine Model. Antioxidants 2021, 10, 884. [Google Scholar] [CrossRef]

| Characteristics | Mediterranean Diet | Usual Care | p Value |
|---|---|---|---|
| n = 34 | n = 37 | ||
| Age (years) | 38.3 (3.3) | 37.0 (4.7) | 0.17 |
| Race and ethnicity | 0.33 | ||
| African American | 1 (2.9%) | 0 (0.0%) | |
| Asian | 1 (2.9%) | 1 (2.7%) | |
| Latin | 4 (11.8%) | 1 (2.7%) | |
| White | 28 (82.4%) | 35 (94.6%) | |
| Education level | 0.95 | ||
| No/primary | 1 (2.9%) | 1 (2.7%) | |
| Secondary/university | 33 (97.1%) | 36 (97.3%) | |
| Socio-economic status I | 0.29 | ||
| Low | 1 (2.9%) | 1 (2.7%) | |
| Medium | 12 (35.3%) | 7 (18.9%) | |
| High | 21 (61.8%) | 29 (78.4%) | |
| BMI before pregnancy (kg/m2) | 24.0 (5.0) | 22.8 (2.8) | 0.19 |
| Previous medical condition | |||
| Thyroid disorders | 5 (14.7%) | 1 (2.7%) | 0.07 |
| Autoimmune diseases | 2 (5.9%) | 8 (21.6%) | 0.06 |
| Diabetes mellitus | 6 (17.6%) | 2 (5.4%) | 0.10 |
| Chronic hypertension | 4 (11.8%) | 1 (2.7%) | 0.14 |
| Psychiatric disorders | 1 (2.9%) | 3 (8.1%) | 0.35 |
| Nulliparous | 18 (52.9%) | 22 (59.5%) | 0.58 |
| Assisted reproductive technologies | 13 (38.2%) | 9 (24.3%) | 0.21 |
| During pregnancy | |||
| Cigarette smoking | 5 (14.7%) | 5 (13.5%) | 0.89 |
| Alcohol intake | 6 (17.6%) | 7 (18.9%) | 0.89 |
| Drug consumption | 2 (5.9%) | 0 (0.0%) | 0.14 |
| Sports practice | 11 (32.4%) | 10 (27.0%) | 0.85 |
| Gestational age at recruitment (weeks) | 20.8 (0.7) | 20.7 (0.7) | 0.43 |
| Mediterranean diet adherence score | 8.1 (2.6) | 7.8 (2.5) | 0.61 |
| Pregnancy outcomes | |||
| Gestational diabetes | 4 (11.8%) | 2 (5.4%) | 0.34 |
| Gestational hypertension | 1 (2.9%) | 1 (2.7%) | 0.95 |
| Preeclampsia | 2 (5.9%) | 3 (8.1%) | 0.71 |
| Preterm birth | 0 (0.0%) | 0 (0.0%) | 0.72 |
| Area | Improvements in the Mediterranean Diet | Adjusted Mean Difference (95% CI) | p Value | |
|---|---|---|---|---|
| High | Low | |||
| n = 30 | n = 41 | |||
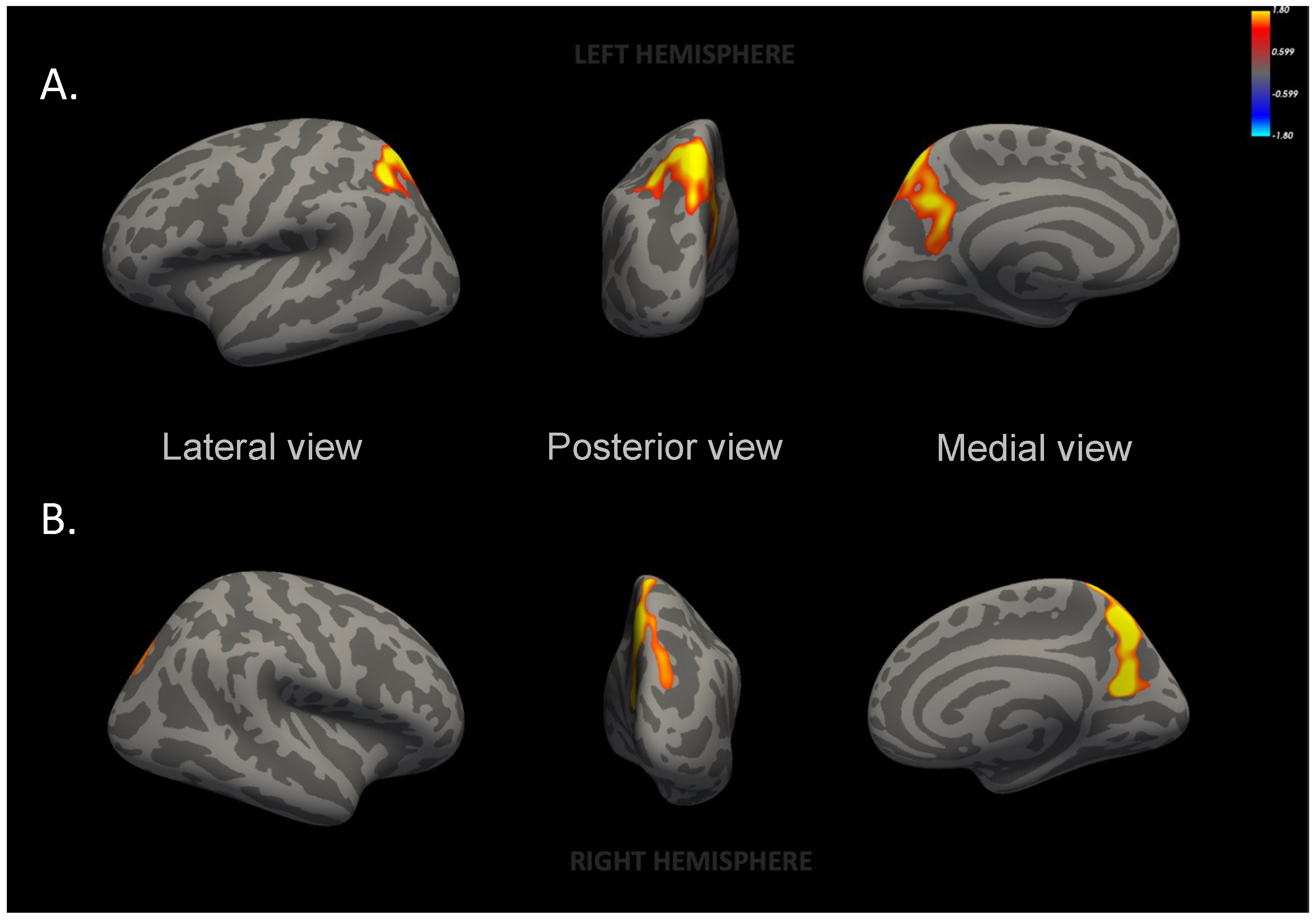
| Left superior parietal | 5221 (95.6) | 4992 (87.3) | 228.9 (−25.0–482.8) | 0.07 |
| Right precuneus | 3691 (62.2) | 3513 (56.8) | 177.2 (12.0–342.4) | 0.03 |
| Left Superior Parietal Area | Right Precuneus Area | |||
|---|---|---|---|---|
| Adjusted Mean Difference (95% CI) | p Value | Adjusted Mean Difference (95% CI) | p Value | |
| Dietary 17-Item Questionnaire I | ||||
| Extra virgin olive oil | 191.2 (−190.7–573.1) | 0.32 | 208.4 (−39.0–455.8) | 0.09 |
| Vegetables | −12.0 (−273.4–249.4) | 0.93 | −46.3 (−217.7–125.0) | 0.59 |
| Fruits | 104.3 (−178.8–387.5) | 0.46 | 99.7 (−85.4–284.9) | 0.28 |
| Sofrito | −33.3 (−284.5–217.8) | 0.79 | −25.8 (−190.8–139.2) | 0.75 |
| Wholegrain cereals, bread, pasta | 223.1 (−31.0–477.2) | 0.08 | −5.1 (−176.0–165.9) | 0.95 |
| Refined cereals, bread, pasta | 207.4 (−57.4–472.1) | 0.12 | 125.4 (−49.1–299.8) | 0.15 |
| Legumes | 108.9 (−153.7–371.4) | 0.41 | 48.3 (−124.7–221.3) | 0.57 |
| Fish/seafood | −68.5 (−317.9–180.9) | 0.58 | −85.3 (−248.1–77.5) | 0.29 |
| Fatty fish | 286.0 (−4.3–576.3) | 0.05 | 115.6 (−78.8–309.9) | 0.23 |
| Red meat | −16.5 (−333.4–300.4) | 0.92 | −34.7 (−242.8–173.3) | 0.73 |
| Processed meat | 121.8 (−177.4–421.1) | 0.42 | 17.2 (−180.4–214.8) | 0.86 |
| Chicken, turkey, rabbit, lean pork | −33.7 (−293.6–226.1) | 0.80 | −93.8 (−263.0–75.3) | 0.27 |
| Carbonated and/or sugar-sweetened beverages | 38.7 (−234.5–311.9) | 0.78 | 38.6 (−140.7–218.0) | 0.66 |
| Nuts, including walnuts, almonds, peanuts | 375.7 (78.9–672.4) | 0.01 | 210.8 (13.2–408.4) | 0.03 |
| Pastries such as cookies, custard pastries or cake | −7.0 (−266.8–252.9) | 0.96 | 1.3 (−169.4–172.1) | 0.98 |
| Dairy products, including calcium-fortified vegetable milk | 154.1 (−100.1–408.3) | 0.23 | −45.3 (−213.9–123.2) | 0.59 |
| Butter, margarine or cream | 73.7 (−188.9–336.3) | 0.58 | 28.0 (−144.8–200.7) | 0.74 |
| Biomarkers II (μmol/g creatinine) | β (95%CI) | p value | β (95%CI) | p value |
| Oleic acid | −1.2 (−52.3–49.9) | 0.96 | −19.7 (−52.5–13.0) | 0.24 |
| Alpha-linolenic acid | −113.6 (−1161.6–934.5) | 0.83 | −83.0 (−768.3–602.3) | 0.81 |
| Alpha-linoleic acid | −1.3 (−39.4–36.9) | 0.94 | 3.8 (−21.2–28.7) | 0.77 |
| Hydroxytyrosol | 119.3 (39–199.6) | 0.006 | 74.3 (21.3–127.4) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakaki, A.; Gomez, Y.; Castro-Barquero, S.; Conti, A.; Vellvé, K.; Casas, I.; Genero, M.; Youssef, L.; Segalés, L.; Benitez, L.; et al. The Mediterranean Diet in Pregnancy: Implications for Maternal Brain Morphometry in a Secondary Analysis of the IMPACT BCN Randomized Clinical Trial. Nutrients 2024, 16, 1604. https://doi.org/10.3390/nu16111604
Nakaki A, Gomez Y, Castro-Barquero S, Conti A, Vellvé K, Casas I, Genero M, Youssef L, Segalés L, Benitez L, et al. The Mediterranean Diet in Pregnancy: Implications for Maternal Brain Morphometry in a Secondary Analysis of the IMPACT BCN Randomized Clinical Trial. Nutrients. 2024; 16(11):1604. https://doi.org/10.3390/nu16111604
Chicago/Turabian StyleNakaki, Ayako, Yvan Gomez, Sara Castro-Barquero, Allegra Conti, Kilian Vellvé, Irene Casas, Mariona Genero, Lina Youssef, Laura Segalés, Leticia Benitez, and et al. 2024. "The Mediterranean Diet in Pregnancy: Implications for Maternal Brain Morphometry in a Secondary Analysis of the IMPACT BCN Randomized Clinical Trial" Nutrients 16, no. 11: 1604. https://doi.org/10.3390/nu16111604
APA StyleNakaki, A., Gomez, Y., Castro-Barquero, S., Conti, A., Vellvé, K., Casas, I., Genero, M., Youssef, L., Segalés, L., Benitez, L., Casas, R., Vieta, E., Bargallo, N., Toschi, N., Estruch, R., Crispi, F., Gratacos, E., & Crovetto, F. (2024). The Mediterranean Diet in Pregnancy: Implications for Maternal Brain Morphometry in a Secondary Analysis of the IMPACT BCN Randomized Clinical Trial. Nutrients, 16(11), 1604. https://doi.org/10.3390/nu16111604









