New Value of Acorus tatarinowii/gramineus Leaves as a Dietary Source for Dementia Prevention
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hot Water Extracts of AGR and AGL
2.2. Preparation of Hot Water Extract, Extraction Residue, and Simple Crush Powder of ATL
2.3. Component Analysis of Acorus Preparations
2.4. Mice
2.5. Treatment of Mice
2.6. Behavioral Test
2.7. Histological Analysis of Neuropathology, Brain-Derived Neurotrophic Factor (BDNF) Expression, and Neurogenesis
2.8. Statistical Analysis
3. Results
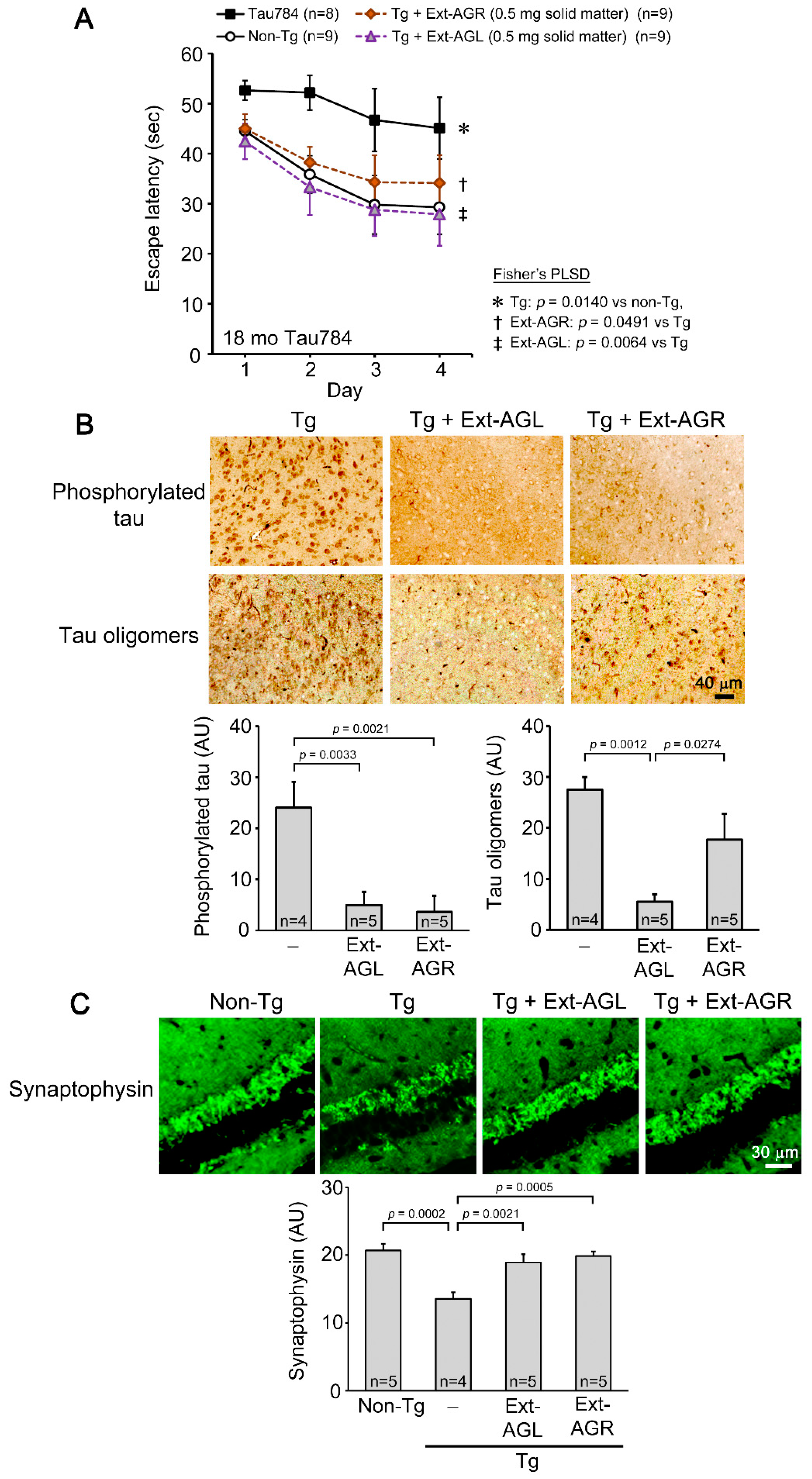
3.1. Comparison of Hot Water Extracts of AGR and AGL in Tau784 Mice
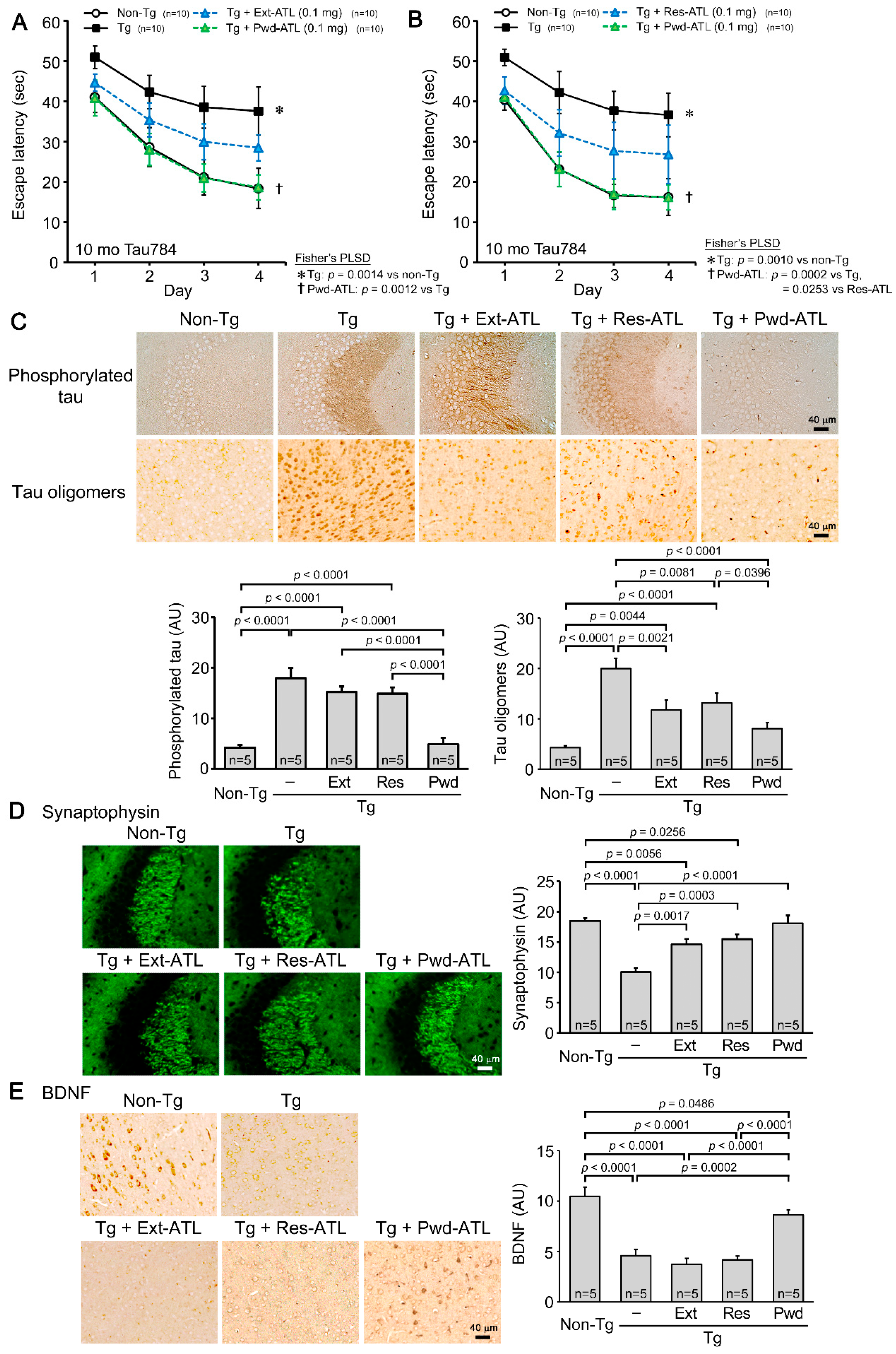
3.2. Comparison of Hot Water Extract, Extraction Residue, and Simple Crush Powder of ATL in Tau784 Mice
3.3. Component Analysis of Acorus Preparations
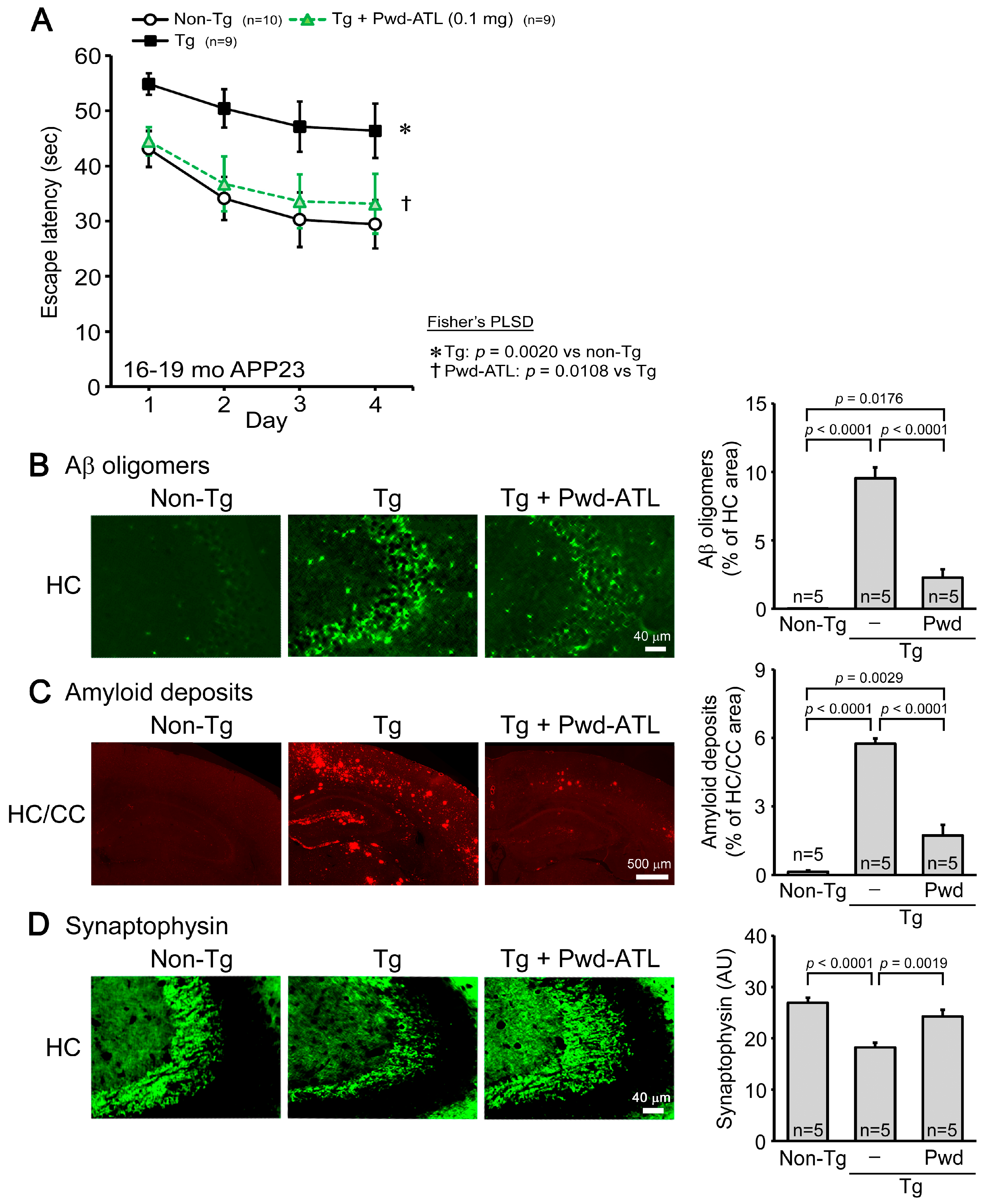
3.4. Effects of ATL Simple Crush Powder on APP23 Mice
3.5. Effects of ATL Simple Crush Powder on Huα-Syn (A53T) Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.L.; Kovacs, G.G. Current Concepts of Mixed Pathologies in Neurodegenerative Diseases. Can. J. Neurol. Sci. 2022, 31, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Selkoe, D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J. Neurochem. 2020, 154, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, B.A.; Limon, A. Synaptic Disruption by Soluble Oligomers in Patients with Alzheimer’s and Parkinson’s Disease. Biomedicines 2022, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Jaunmuktane, Z.; Brandner, S. Invited Review: The role of prion-like mechanisms in neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2020, 46, 522–545. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Uekado, R.; Shigemori, K.; Eguchi, H.; Tomiyama, T. Nasal Rifampicin Halts the Progression of Tauopathy by Inhibiting Tau Oligomer Propagation in Alzheimer Brain Extract-Injected Mice. Biomedicines 2022, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Binette, A.P.; Groot, C.; Smith, R.; Strandberg, O.; Palmqvist, S.; Stomrud, E.; Tideman, P.; Ohlsson, T.; Jogi, J.; et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat. Med. 2022, 28, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Ku, C.F.; Wang, H.Y.; Chan, G.K.; Yao, P.; Lin, H.Q.; Dong, T.T.; Zhang, H.J.; Tsim, K.W. Authentication of Acori Tatarinowii Rhizoma (Shi Chang Pu) and its adulterants by morphological distinction, chemical composition and ITS sequencing. Chin. Med. 2016, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kwak, T.Y.; Bae, M.H.; Shin, H.K.; Choi, B.T. Therapeutic Potential of Active Components from Acorus gramineus and Acorus tatarinowii in Neurological Disorders and Their Application in Korean Medicine. J. Pharmacopunct. 2022, 25, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, H.-P.; Wang, S.; Hu, W.-J.; Li, J.-Y.; Yu, A.-Q.; Bai, Q.-X.; Yang, B.-Y.; Kuang, H.-X. Acorus tatarinowii Schott: A Review of Its Botany, Traditional Uses, Phytochemistry, and Pharmacology. Molecules 2023, 28, 4525. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yang, Y.; Hao, J. Acori Tatarinowii Rhizoma: A comprehensive review of its chemical composition, pharmacology, pharmacokinetics and toxicity. Front. Pharmacol. 2023, 14, 1090526. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Cho, D.Y.; Kim, I.S.; Seol, S.H.; Choi, D.K. Molecular Mechanisms and Therapeutic Potential of α- and β-Asarone in the Treatment of Neurological Disorders. Antioxidants 2022, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Limón, I.D.; Mendieta, L.; Díaz, A.; Chamorro, G.; Espinosa, B.; Zenteno, E.; Guevara, J. Neuroprotective effect of alpha-asarone on spatial memory and nitric oxide levels in rats injected with amyloid-beta (25–35). Neurosci. Lett. 2009, 453, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, D.; Liu, Q.; Zhang, J.; Mu, K.; Gao, X.; Zhang, K.; Li, H.; Wang, Q.; Zheng, Y.; et al. Alpha-asarone Improves Cognitive Function of APP/PS1 Mice and Reducing Aβ42, P-tau and Neuroinflammation, and Promoting Neuron Survival in the Hippocampus. Neuroscience 2021, 458, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Chen, Y.-B.; Chen, D.-F.; Lai, X.-P.; Liu, D.-H.; Deng, R.-D.; Zhou, J.-H.; Zhang, S.-X.; Li, Y.-W.; Lii, H.; et al. β-Asarone inhibits neuronal apoptosis via the CaMKII/CREB/Bcl-2 signaling pathway in an in vitro model and AβPP/PS1 mice. J. Alzheimers Dis. 2013, 33, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Huang, L.; Ning, B.; Wang, N.; Zhang, Q.; Zhu, C.; Fang, Y. β-asarone improves learning and memory and reduces Acetyl Cholinesterase and Beta-amyloid 42 levels in APP/PS1 transgenic mice by regulating Beclin-1-dependent autophagy. Brain Res. 2016, 1652, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Yang, C.; Zhang, Y.; Su, R.Y.; Chen, J.L.; Jiao, M.M.; Chen, H.F.; Zheng, N.; Luo, S.; Chen, Y.B.; et al. Neuroprotective effect of β-asarone against Alzheimer’s disease: Regulation of synaptic plasticity by increased expression of SYP and GluR1. Drug Des. Dev. Ther. 2016, 10, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, N.; Kang, J.; Fang, Y. β-Asarone improves learning and memory in Aβ1-42-induced Alzheimer’s disease rats by regulating PINK1-Parkin-mediated mitophagy. Metab. Brain Dis. 2020, 35, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Yamashita, T.; Kimura, T.; Ohnishi, K.; Takuma, H.; Ozeki, T.; Takashima, A.; Tomiyama, T.; Mori, H. Neurodegenerative disorder FTDP-17-related tau intron 10 +16C → T mutation increases tau exon 10 splicing and causes tauopathy in transgenic mice. Am. J. Pathol. 2013, 183, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Eguchi, H.; Kunori, Y.; Matsumoto, Y.; Taniguchi, T.; Mori, H.; Tomiyama, T. Passive immunotherapy of tauopathy targeting pSer413-tau: A pilot study in mice. Ann. Clin. Transl. Neurol. 2015, 2, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Sturchler-Pierrat, C.; Abramowski, D.; Duke, M.; Wiederhold, K.H.; Mistl, C.; Rothacher, S.; Ledermann, B.; Bürki, K.; Frey, P.; Paganetti, P.A.; et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 1997, 94, 13287–13292. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, D.; D’Hooge, R.; Staufenbiel, M.; Van Ginneken, C.; Van Meir, F.; De Deyn, P.P. Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur. J. Neurosci. 2003, 17, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Sakai, A.; Shigemori, K.; Yokota, A.; Kumagai, T.; Tomiyama, T. Oligomer-Targeting Prevention of Neurodegenerative Dementia by Intranasal Rifampicin and Resveratrol Combination—A Preclinical Study in Model Mice. Front. Neurosci. 2021, 15, 763476. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Stirling, W.; Xu, Y.; Xu, X.; Qui, D.; Mandir, A.S.; Dawson, T.M.; Copeland, N.G.; Jenkins, N.A.; Price, D.L. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 → Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8968–8973. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Hatanaka, Y.; Sakai, A.; Tomiyama, T. Nasal Rifampicin Improves Cognition in a Mouse Model of Dementia with Lewy Bodies by Reducing α-Synuclein Oligomers. Int. J. Mol. Sci. 2021, 22, 8453. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.J.; Kim, N.; Jeon, S.H.; Gee, M.S.; Kim, J.W.; Lee, J.K. Eugenol relieves the pathological manifestations of Alzheimer’s disease in 5×FAD mice. Phytomedicine 2023, 118, 154930. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Shigemori, K.; Uekado, R.; Matsuda, K.; Tomiyama, T. Hawaiian native herb Mamaki prevents dementia by ameliorating neuropathology and repairing neurons in four different mouse models of neurodegenerative diseases. Geroscience 2024, 46, 1971–1987. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Horgusluoglu, E.; Nudelman, K.; Nho, K.; Saykin, A.J. Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Chen, J.; Wang, G.; Li, M.; Zheng, Q.; Li, D. Progress of research into the pharmacological effect and clinical application of the traditional Chinese medicine Rehmanniae Radix. Biomed. Pharmacother. 2023, 168, 115809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, Y.; Wu, P.; Zhao, P.; Zhou, Q.; Zhang, Z.; Wang, Y.; Zhang, X. Polygalae Radix: A review of its traditional uses, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Fitoterapia 2020, 147, 104759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Zhang, Y.; Xie, J. Ziziphi Spinosae Semen: A Natural Herb Resource for Treating Neurological Disorders. Curr. Top. Med. Chem. 2022, 22, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, M.L.; de Vos, W.M. Intrinsic dietary fibers and the gut microbiome: Rediscovering the benefits of the plant cell matrix for human health. Front. Immunol. 2022, 13, 954845. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Zanatta, D.; Syeda, T.; Sánchez-Valle, V.; Irene-Fierro, M.; Torres-Aguilar, P.; Torres-Ramos, M.A.; Shibayama-Salas, M.; Silva-Olivares, A.; Noriega, L.G.; Torres, N.; et al. Dietary Fiber Modulates the Release of Gut Bacterial Products Preventing Cognitive Decline in an Alzheimer’s Mouse Model. Cell. Mol. Neurobiol. 2023, 43, 1595–1618. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Santiago, J.A.; Bernstein, M.; Potashkin, J.A. Diet and lifestyle impact the development and progression of Alzheimer’s dementia. Front. Nutr. 2023, 10, 1213223. [Google Scholar] [CrossRef] [PubMed]
- Charisis, S.; Ntanasi, E.; Yannakoulia, M.; Anastasiou, C.A.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Scarmeas, N. Mediterranean diet and risk for dementia and cognitive decline in a Mediterranean population. J. Am. Geriatr. Soc. 2021, 69, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; Van De Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease—A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Ren, C.; Xing, L.; Guan, L.; Zhang, C.; Sun, X.; Wang, G.; Niu, H.; Qun, S. Ginkgo biloba extract improves cognitive function and increases neurogenesis by reducing Aβ pathology in 5×FAD mice. Am. J. Transl. Res. 2021, 13, 1471–1482. [Google Scholar] [PubMed]
- Wan, W.; Zhang, C.; Danielsen, M.; Li, Q.; Chen, W.; Chan, Y.; Li, Y. EGb761 improves cognitive function and regulates inflammatory responses in the APP/PS1 mouse. Exp. Gerontol. 2016, 81, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhu, Q.; Lu, J. Can We Use Ginkgo biloba Extract to Treat Alzheimer’s Disease? Lessons from Preclinical and Clinical Studies. Cells 2022, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Kartika, H.; Li, Q.X.; Wall, M.M.; Nakamoto, S.T.; Iwaoka, W.T. Major phenolic acids and total antioxidant activity in Mamaki leaves, Pipturus albidus. J. Food Sci. 2007, 72, S696–S701. [Google Scholar] [CrossRef] [PubMed]
- Chellian, R.; Pandy, V.; Mohamed, Z. Pharmacology and toxicology of α- and β-Asarone: A review of preclinical evidence. Phytomedicine 2017, 32, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Uebel, T.; Hermes, L.; Haupenthal, S.; Müller, L.; Esselen, M. α-Asarone, β-asarone, and γ-asarone: Current status of toxicological evaluation. J. Appl. Toxicol. 2021, 41, 1166–1179. [Google Scholar] [CrossRef] [PubMed]

| Components | Pwd-ATR | Pwd-ATL | Ext-ATL |
|---|---|---|---|
| α-Asarone | 39 ppm | 9.8 ppm | <0.1 ppm |
| β-Asarone | 760 ppm | 120 ppm | 0.1 ppm |
| Eugenol | 6.0 ppm | 0.7 ppm | 0.2 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umeda, T.; Sakai, A.; Shigemori, K.; Nakata, K.; Nakajima, R.; Yamana, K.; Tomiyama, T. New Value of Acorus tatarinowii/gramineus Leaves as a Dietary Source for Dementia Prevention. Nutrients 2024, 16, 1589. https://doi.org/10.3390/nu16111589
Umeda T, Sakai A, Shigemori K, Nakata K, Nakajima R, Yamana K, Tomiyama T. New Value of Acorus tatarinowii/gramineus Leaves as a Dietary Source for Dementia Prevention. Nutrients. 2024; 16(11):1589. https://doi.org/10.3390/nu16111589
Chicago/Turabian StyleUmeda, Tomohiro, Ayumi Sakai, Keiko Shigemori, Kunio Nakata, Ryota Nakajima, Kei Yamana, and Takami Tomiyama. 2024. "New Value of Acorus tatarinowii/gramineus Leaves as a Dietary Source for Dementia Prevention" Nutrients 16, no. 11: 1589. https://doi.org/10.3390/nu16111589
APA StyleUmeda, T., Sakai, A., Shigemori, K., Nakata, K., Nakajima, R., Yamana, K., & Tomiyama, T. (2024). New Value of Acorus tatarinowii/gramineus Leaves as a Dietary Source for Dementia Prevention. Nutrients, 16(11), 1589. https://doi.org/10.3390/nu16111589







