Supplementation of Seaweed Extracts to the Diet Reduces Symptoms of Alzheimer’s Disease in the APPswePS1ΔE9 Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. H. elongata and S. fusiforme Extractions
2.1.1. Lipid Extracts of H. elongata and S. fusiforme
2.1.2. Supercritical Fluid Extraction of S. fusiforme
2.2. LXR Reporter Assay
2.2.1. Culture of Cell Lines HEK293, CCF-STTG1, SH-SY5Y, and CHME3
2.2.2. Dual Luciferase Reporter Assay
2.3. Animals
2.4. Animal Diet
2.4.1. Chow Supplemented with H. elongata Lipid Extract
2.4.2. Chow Supplemented with Supercritical Fluid Extract of S. fusiforme
2.5. Cognitive Tests
2.5.1. Object Recognition Task and Object Location Task
2.5.2. Y-Maze Spontaneous Alternation Test
2.6. Tissue sample Preparation
2.7. Triglyceride and Neutral Lipid Quantification
2.8. RNA Isolation and cDNA Synthesis
2.9. Quantitative Real-Time PCR
2.10. Lipoprotein Profile
2.11. Immunohistochemistry
2.11.1. Brain—Paraffin Sections
2.11.2. Liver—Cryosections
2.11.3. Image Analysis
2.12. Quantification of Extracellular Soluble Aβ42
2.13. Cytokine Measurements
2.13.1. THP-1 Cell Culture
2.13.2. Cytokines Measurements
2.14. Cholesterol Efflux
2.14.1. THP-1 Cell Culture
2.14.2. Cholesterol Efflux Assay
2.15. OPC Differentiation
2.15.1. Primary OPC Culture
2.15.2. Immunocytochemistry
2.16. RNA Sequencing and Data Analysis
2.17. Sterol Measurement
2.18. Tau and p-Tau Measurements in Differentiated SH-SY5Y Cells
2.18.1. SH-SY5Y Cell Culture
2.18.2. Protein Isolation from the Forebrain
2.18.3. Western Blot for TAU-5 and AT-8 Measurements
2.19. Statistical Analyses
3. Results
3.1. The Supercritical Fluid Extract of S. fusiforme Activated LXRα and LXRβ
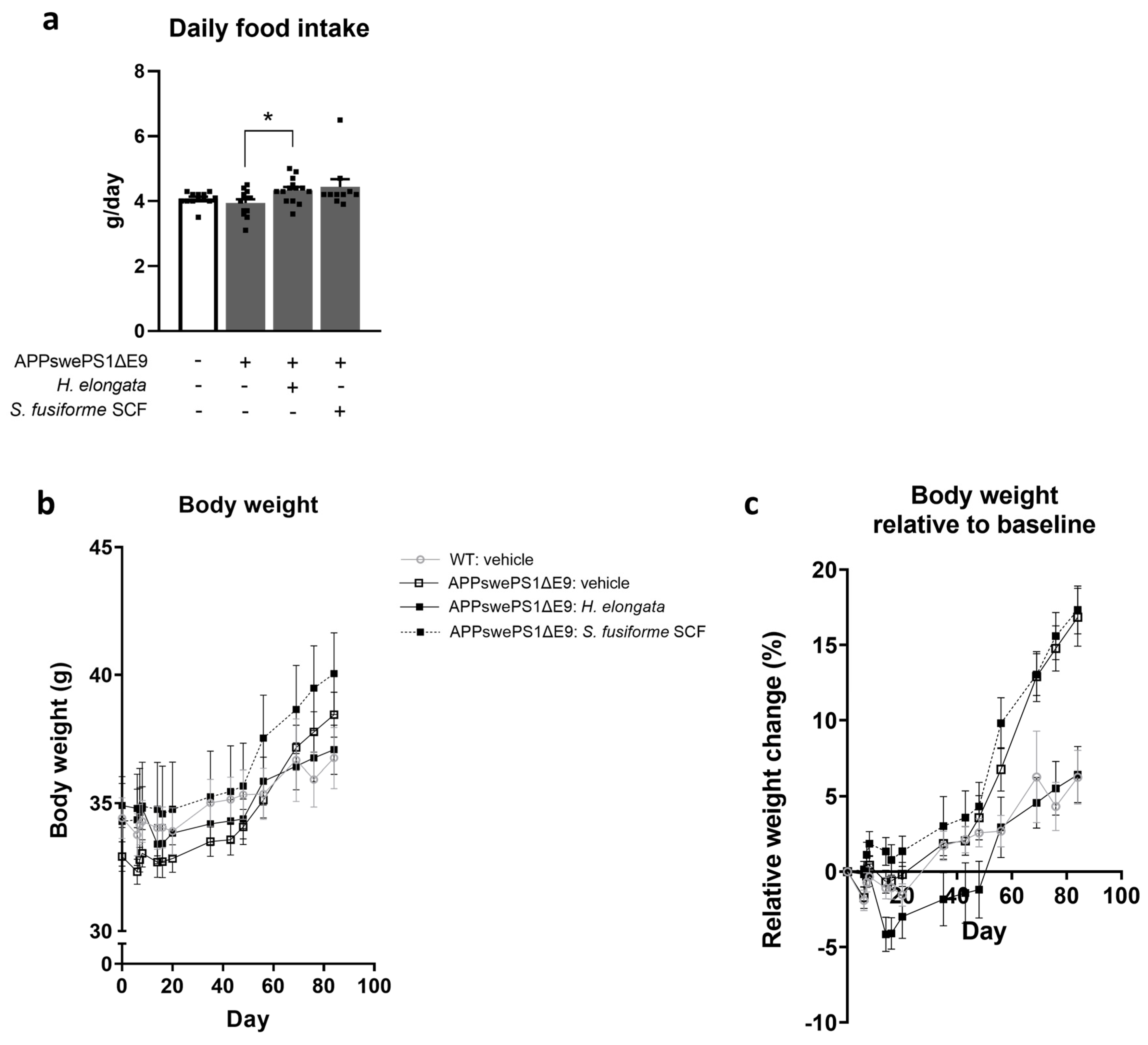
3.2. Food Intake, Body Weight, and Lipid Homeostasis in Liver and Serum upon Supplementation of H. elongata Lipid Extract and S. fusiforme SCF Extract to the Diet
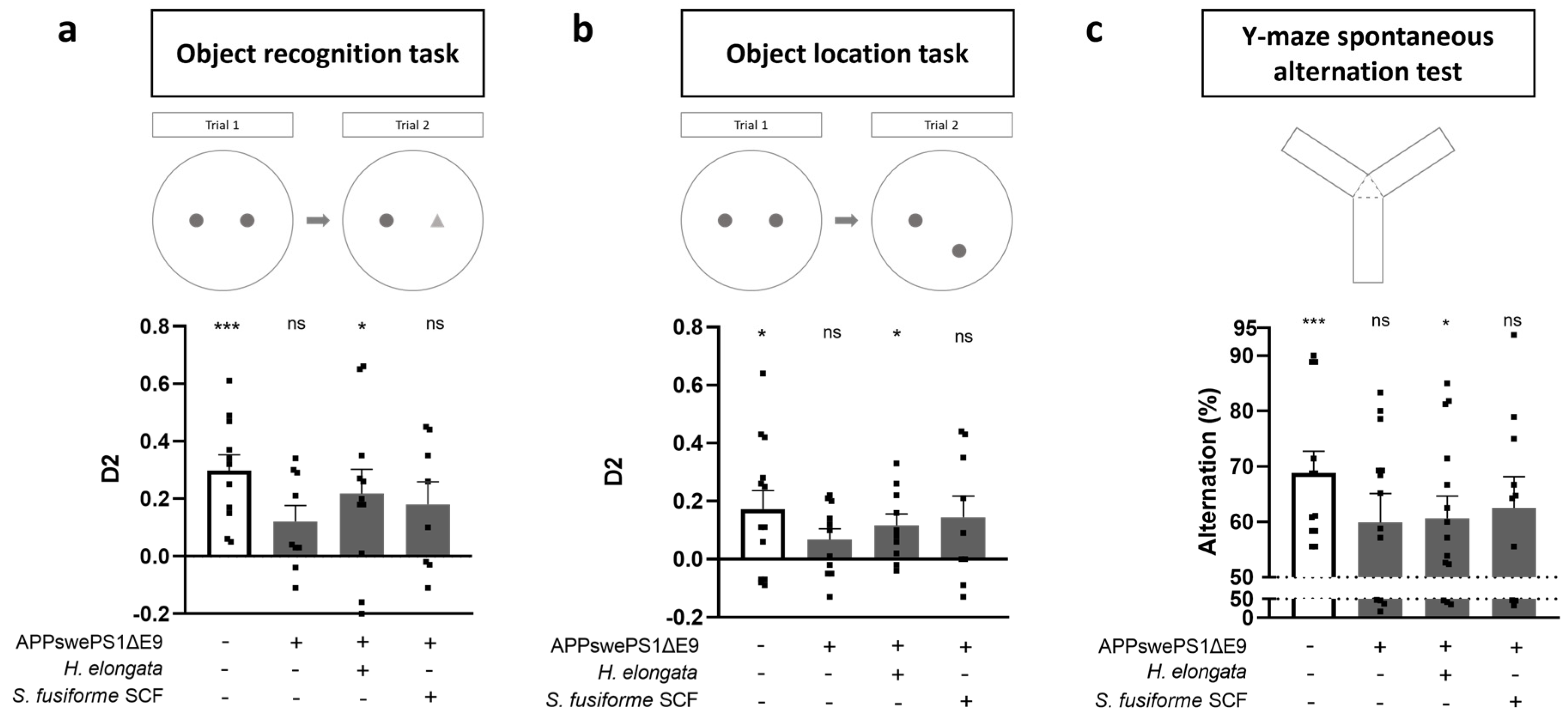
3.3. Prevention of Cognitive Decline in APPswePS1ΔE9 Mice
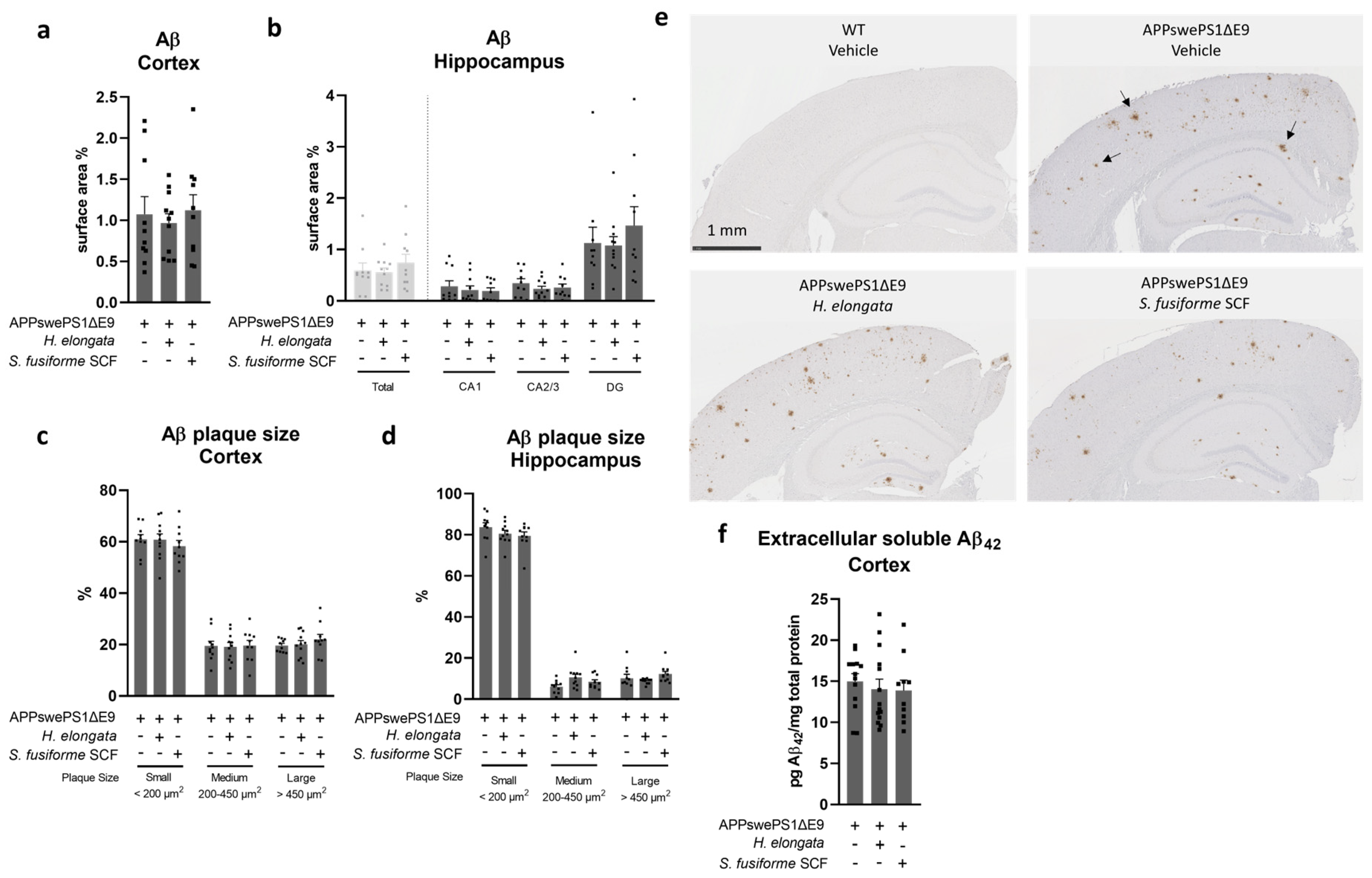
3.4. Aβ Plaque Load in the Cortex and Hippocampus Remained Unaffected by Both Seaweed Extracts
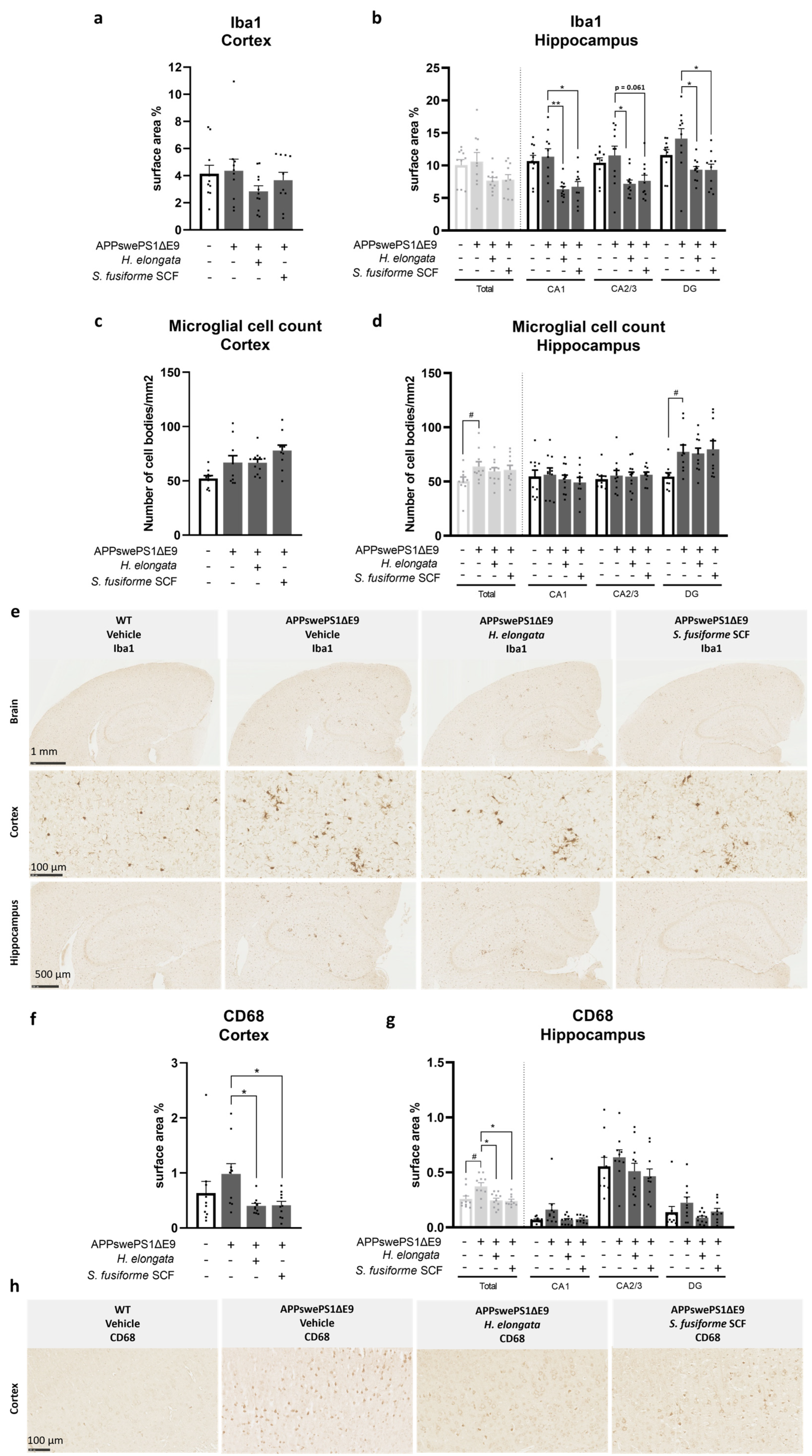
3.5. Microglia Marker Iba1 and Phagocytic Microglia/Macrophage Marker CD68 Decreased by H. elongata and S. fusiforme SCF Extracts
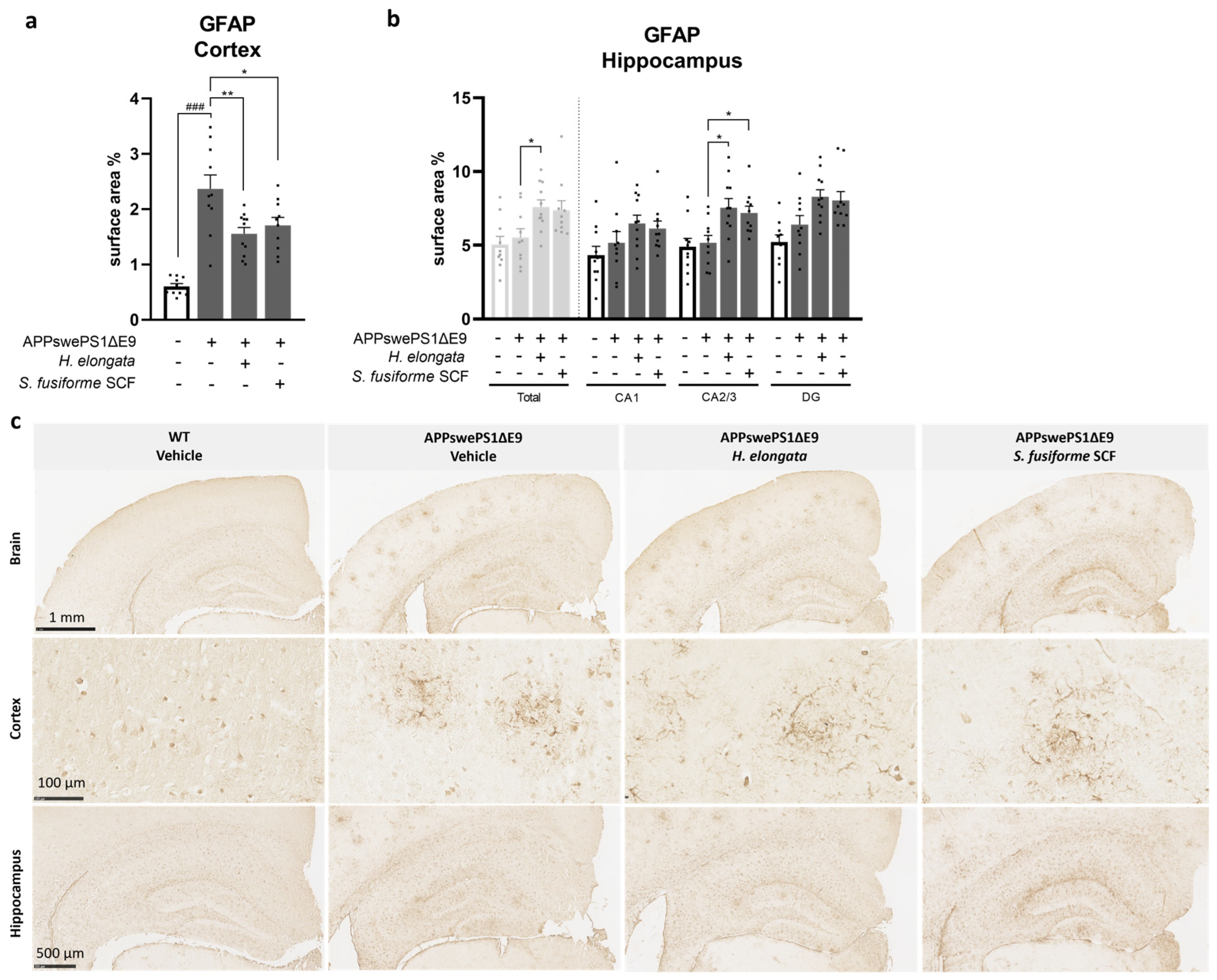
3.6. H. elongata Extract and S. fusiforme SCF Extract Decreased the Astrocytic Marker GFAP in the Cortex of APPswePS1ΔE9 Mice but Increased GFAP in the Hippocampus
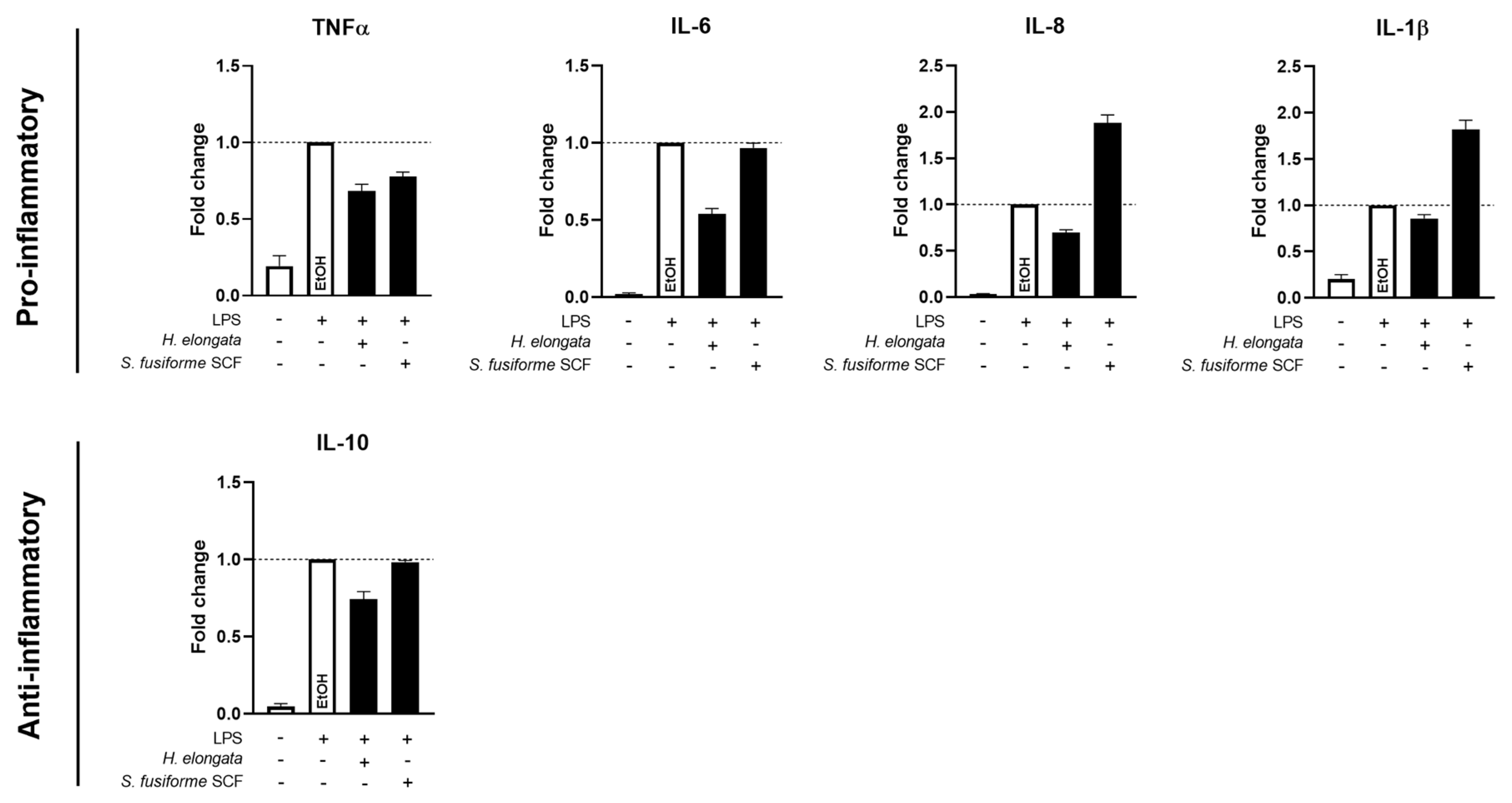
3.7. H. elongata Extract Reduced the Production of Inflammatory Cytokines in THP-1-Derived Macrophages While the S. fusiforme SCF Extract Only Decreased TNFα
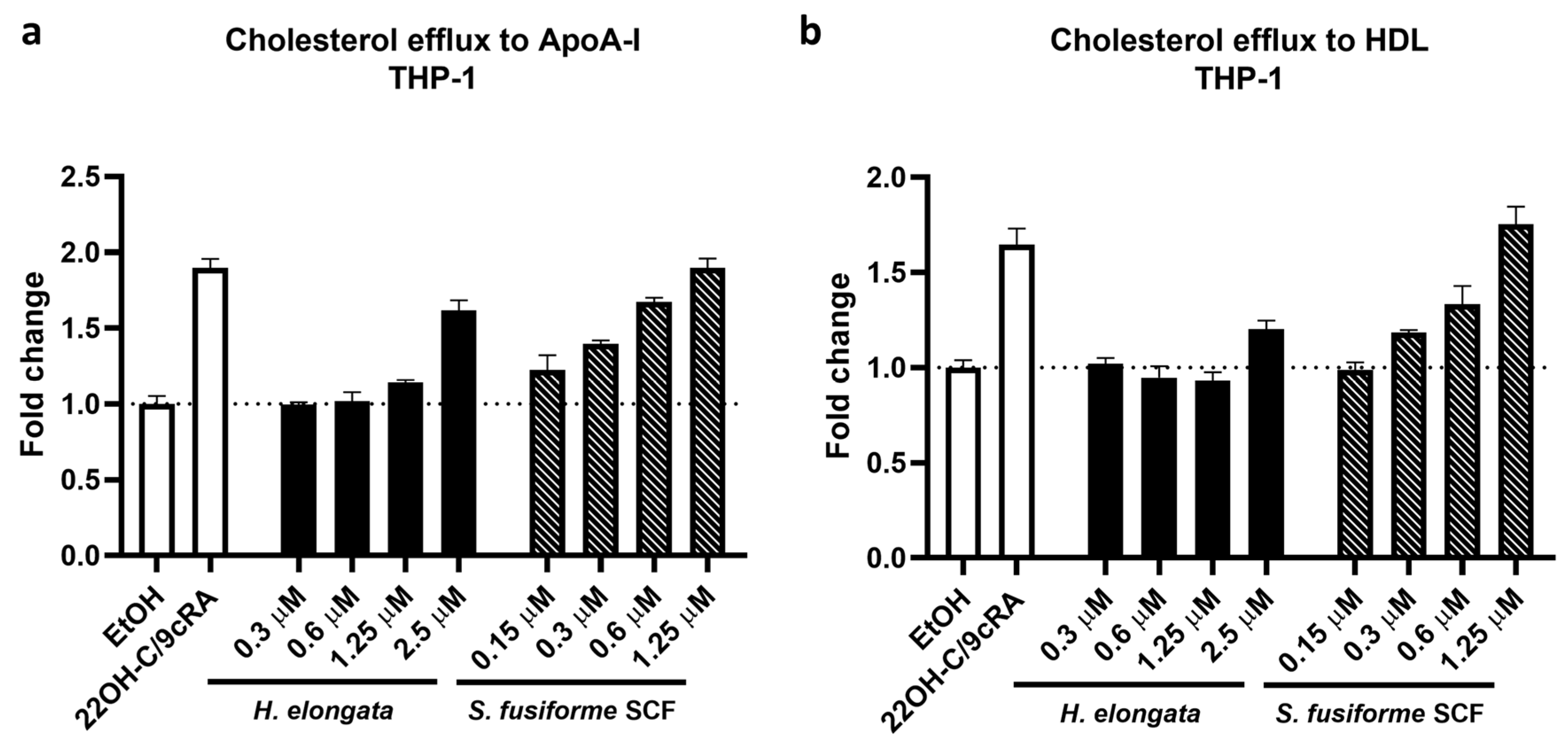
3.8. Promotion of Cholesterol Efflux in THP-1-Derived Macrophages
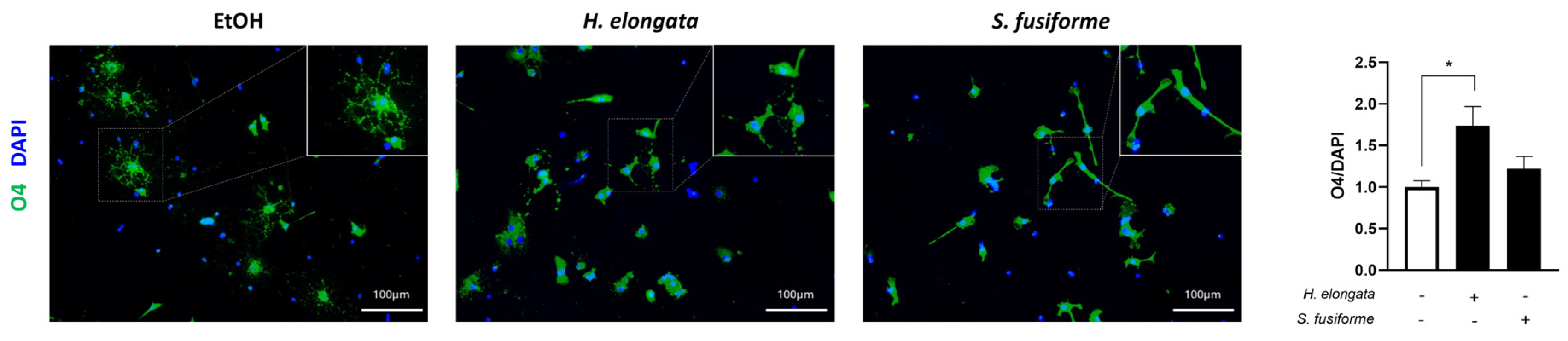
3.9. H. elongata May Promote Early Oligodendrocyte Maturation
3.10. Effects of 1-Week Administration of H. elongata Extract on Hippocampal Transcriptome
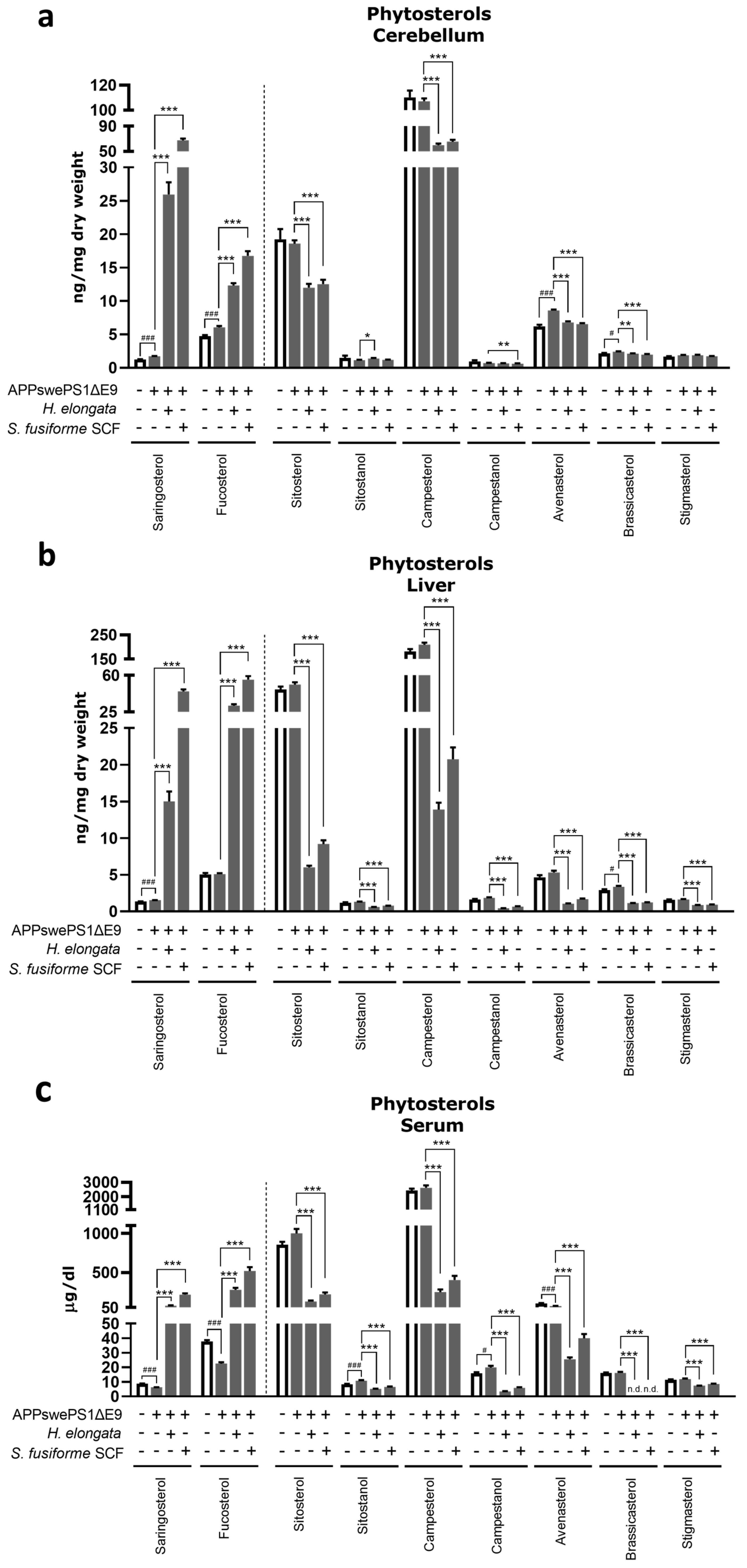
3.11. Phytosterol Concentrations after Diet Supplementation with H. elongata Lipid Extract and S. fusiforme SCF Extract
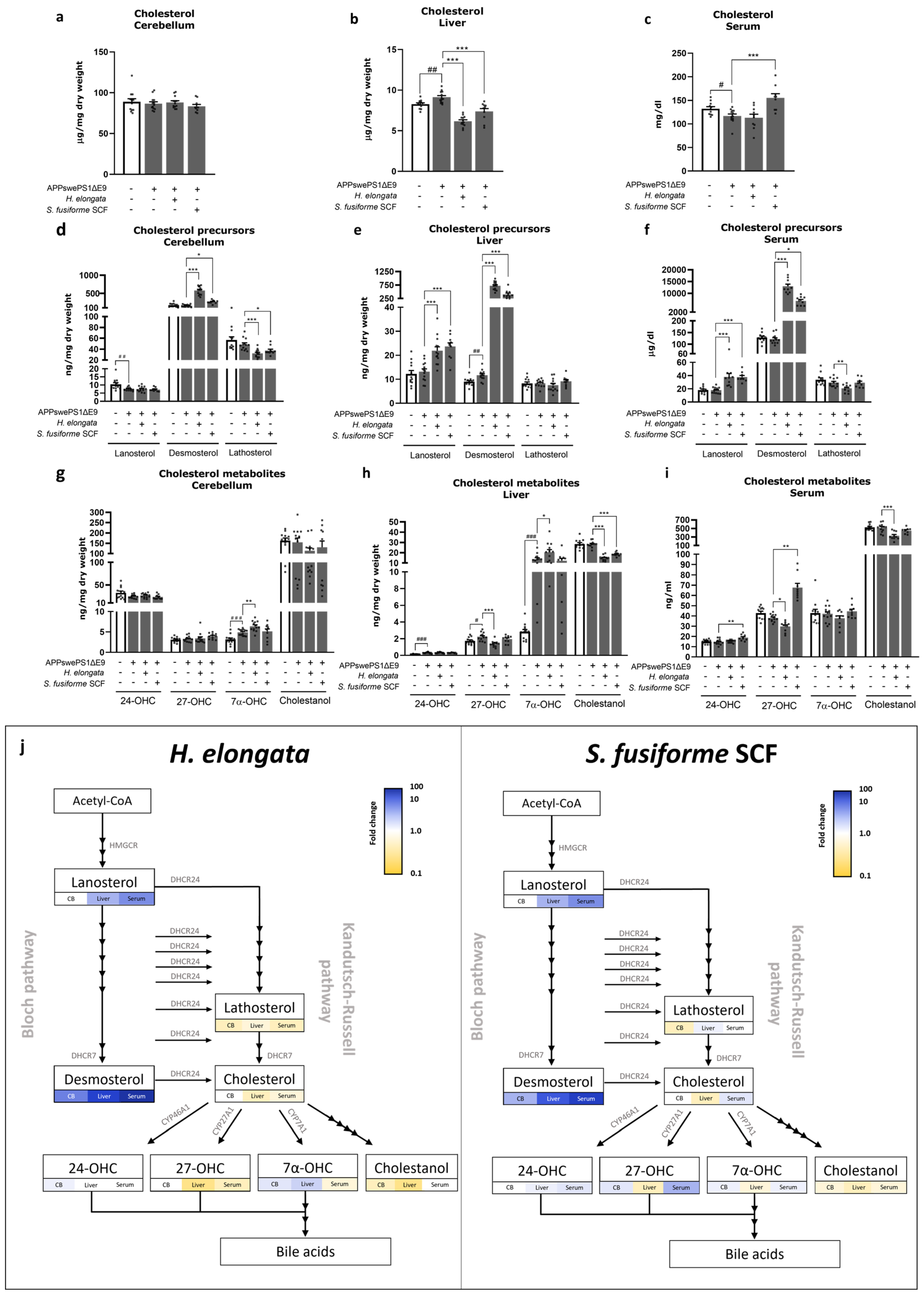
3.12. Cholesterol and Cholesterol Precursors and Metabolites
3.13. The Potential of H. elongata Lipid Extract and S. fusiforme SCF Extract to Suppress Hepatic Inflammation
3.14. H. elongata and S. fusiforme SCF Extracts Reduce AD-Related Tau Pathology In Vitro
4. Discussion
4.1. Prevention of Cognitive Decline by H. elongata Extract, and Possibly by S. fusiforme SCF Extract, with No Impact on Aβ plaque Load
4.2. Effects of H. elongata and S. fusiforme Extracts on Cholesterol Metabolism: Cholesterol-Lowering Effects and a Notable Increase in Endogenous LXR Agonist Desmosterol Possibly through DHCR24 Inhibition
4.3. Human Mutant APPswe and PSENΔE9 Genes Affect Besides the Brain Also the Peripheral System
4.4. Anti-Inflammatory Effects of the Seaweed Extracts on Glial Cells and THP-1 Derived Macrophages: Potential Contribution of Promoted Cholesterol Efflux and Upregulated Desmosterol Concentrations
4.5. H. elongata Extract Modulated Weight Gain in APPswePS1ΔE9 Mice: Implications for Metabolic and Neurodegenerative Conditions
4.6. No Adverse Effects of Seaweed Extracts on Triglyceride Content in Liver or Serum
4.7. A Potential Role for H. elongata in Promoting Myelination
4.8. H. elongata Extract May Reduce the Phosphorylation of Tau and, Thereby, AD-Related Tau Pathology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Jansen, D.; Janssen, C.I.; Vanmierlo, T.; Dederen, P.J.; van Rooij, D.; Zinnhardt, B.; Nobelen, C.L.; Janssen, A.-L.; Hafkemeijer, A.; Mutsaers, M.P.; et al. Cholesterol and synaptic compensatory mechanisms in Alzheimer’s disease mice brain during aging. J. Alzheimers Dis. 2012, 31, 813–826. [Google Scholar] [CrossRef]
- Jones, L.; Holmans, P.A.; Hamshere, M.L.; Harold, D.; Moskvina, V.; Ivanov, D.; Pocklington, A.; Abraham, R.; Hollingworth, P.; Sims, R.; et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS ONE 2010, 5, e13950. [Google Scholar] [CrossRef]
- Kölsch, H.; Heun, R.; Jessen, F.; Popp, J.; Hentschel, F.; Maier, W.; Lütjohann, D. Alterations of cholesterol precursor levels in Alzheimer’s disease. Biochim. Biophys. Acta. 2010, 1801, 945–950. [Google Scholar] [CrossRef]
- Mulder, M. Sterols in the central nervous system. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Goikolea, J.; Parrado-Fernandez, C.; Merino-Serrais, P.; Maioli, S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: Potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 2019, 190, 104–114. [Google Scholar] [CrossRef]
- Vanmierlo, T.; Bloks, V.W.; van Vark-van der Zee, L.; Rutten, K.; Kerksiek, A.; Friedrichs, S.; Sijbrands, E.; Steinbusch, H.W.; Kuipers, F.; Lütjohann, D.; et al. Alterations in brain cholesterol metabolism in the APPSLxPS1mut mouse, a model for Alzheimer’s disease. J. Alzheimers Dis. 2010, 19, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef]
- Kloske, C.M.; Wilcock, D.M. The Important Interface Between Apolipoprotein E and Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2020, 11, 754. [Google Scholar] [CrossRef]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef]
- Zelcer, N.; Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006, 116, 607–614. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, X.; Yan, Y.; Zhang, T. Activation of liver X receptors prevents emotional and cognitive dysfunction by suppressing microglial M1-polarization and restoring synaptic plasticity in the hippocampus of mice. Brain Behav. Immun. 2021, 94, 111–124. [Google Scholar] [CrossRef]
- Moutinho, M.; Landreth, G.E. Therapeutic potential of nuclear receptor agonists in Alzheimer’s disease. J. Lipid Res. 2017, 58, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Lanfranco, M.F.; Ng, C.A.; Rebeck, G.W. ApoE Lipidation as a Therapeutic Target in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6336. [Google Scholar] [CrossRef]
- Mouzat, K.; Chudinova, A.; Polge, A.; Kantar, J.; Camu, W.; Raoul, C.; Lumbroso, S. Regulation of Brain Cholesterol: What Role Do Liver X Receptors Play in Neurodegenerative Diseases? Int. J. Mol. Sci. 2019, 20, 3858. [Google Scholar] [CrossRef] [PubMed]
- Donkin, J.J.; Stukas, S.; Hirsch-Reinshagen, V.; Namjoshi, D.; Wilkinson, A.; May, S.; Chan, J.; Fan, J.; Collins, J.; Wellington, C.L. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J. Biol. Chem. 2010, 285, 34144–34154. [Google Scholar] [CrossRef]
- Jiang, Q.; Lee, C.D.; Mandrekar, S.; Wilkinson, B.; Cramer, P.; Zelcer, N.; Mann, K.; Lamb, B.; Willson, T.M.; Collins, J.L.; et al. ApoE promotes the proteolytic degradation of Abeta. Neuron 2008, 58, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Vanmierlo, T.; Rutten, K.; Dederen, J.; Bloks, V.W.; van Vark-van der Zee, L.C.; Kuipers, F.; Kiliaan, A.; Blokland, A.; Sijbrands, E.J.; Steinbusch, H.; et al. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol. Aging 2011, 32, 1262–1272. [Google Scholar] [CrossRef]
- Riddell, D.R.; Zhou, H.; Comery, T.A.; Kouranova, E.; Lo, C.F.; Warwick, H.K.; Ring, R.H.; Kirksey, Y.; Aschmies, S.; Xu, J.; et al. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer’s disease. Mol. Cell Neurosci. 2007, 34, 621–628. [Google Scholar] [CrossRef]
- Grefhorst, A.; Elzinga, B.M.; Voshol, P.J.; Plo¨sch, T.; Kok, T.; Bloks, V.W.; van der Sluijs, F.H.; Havekes, L.M.; Romijn, J.A.; Verkade, H.J.; et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 2002, 277, 34182–34190. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Liang, G.; Ou, J.; Bashmakov, Y.; Lobaccaro, J.M.; Shimomura, I.; Shan, B.; Brown, M.S.; Goldstein, J.L.; Mangelsdorf, D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes. Dev. 2000, 14, 2819–2830. [Google Scholar] [CrossRef]
- Schultz, J.R.; Tu, H.; Luk, A.; Repa, J.J.; Medina, J.C.; Li, L.; Schwendner, S.; Wang, S.; Thoolen, M.; Mangelsdorf, D.J.; et al. Role of LXRs in control of lipogenesis. Genes. Dev. 2000, 14, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Martens, N.; Schepers, M.; Zhan, N.; Leijten, F.; Voortman, G.; Tiane, A.; Rombaut, B.; Poisquet, J.; van de Sande, N.; Kerksiek, A.; et al. 24(S)-Saringosterol Prevents Cognitive Decline in a Mouse Model for Alzheimer’s Disease. Mar. Drugs. 2021, 19, 190. [Google Scholar] [CrossRef] [PubMed]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Rose, M.; Lewis, J.; Langford, N.; Baxter, M.; Origgi, S.; Barber, M.; MacBain, H.; Thomas, K. Arsenic in seaweed—Forms, concentration and dietary exposure. Food Chem. Toxicol. 2007, 45, 1263–1267. [Google Scholar] [CrossRef]
- Besada, V.; Andrade, J.M.; Schultze, F.; González, J.J. Heavy metals in edible seaweeds commercialised for human consumption. J. Mar. Syst. 2009, 75, 305–313. [Google Scholar] [CrossRef]
- Martens, N.; Zhan, N.; Voortman, G.; Leijten, F.P.; van Rheenen, C.; van Leerdam, S.; Geng, X.; Huybrechts, M.; Liu, H.; Jonker, J.W.; et al. Activation of Liver X Receptors and Peroxisome Proliferator-Activated Receptors by Lipid Extracts of Brown Seaweeds: A Potential Application in Alzheimer’s Disease? Nutrients 2023, 15, 3004. [Google Scholar] [CrossRef]
- Zwarts, I.; van Zutphen, T.; Kruit, J.K.; Liu, W.; Oosterveer, M.H.; Verkade, H.J.; Uhlenhaut, N.H.; Jonker, J.W. Identification of the fructose transporter GLUT5 (SLC2A5) as a novel target of nuclear receptor, L.X.R. Sci. Rep. 2019, 9, 9299. [Google Scholar] [CrossRef]
- Dixon, W.J. Analysis of extreme values. Ann. Math. Stat. 1950, 21, 488–506. [Google Scholar] [CrossRef]
- Dixon, W.J. Ratios involving extreme values. Ann. Math. Stat. 1951, 22, 68–78. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Fedoseienko, A.; Wijers, M.; Wolters, J.C.; Dekker, D.; Smit, M.; Huijkman, N.; Kloosterhuis, N.; Klug, H.; Schepers, A.; van Dijk, K.W.; et al. The COMMD Family Regulates Plasma LDL Levels and Attenuates Atherosclerosis Through Stabilizing the CCC Complex in Endosomal LDLR Trafficking. Circ. Res. 2018, 122, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.E.; Boogert, M.A.v.D.; Rios-Ocampo, W.A.; Jansen, J.C.; Conlon, D.; Chong, P.L.; Levels, J.H.M.; Eilers, R.E.; Sachdev, V.V.; Zelcer, N.; et al. Defective Lipid Droplet-Lysosome Interaction Causes Fatty Liver Disease as Evidenced by Human Mutations in TMEM199 and CCDC115. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Seung, H.S. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Papotti, B.; Borghi, M.O.; Raschi, E.; Zimetti, F.; Bernini, F.; Meroni, P.L.; Ronda, N. Effect of the JAK/STAT Inhibitor Tofacitinib on Macrophage Cholesterol Metabolism. Int. J. Mol. Sci. 2023, 24, 12571. [Google Scholar] [CrossRef] [PubMed]
- Turri, M.; Conti, E.; Pavanello, C.; Gastoldi, F.; Palumbo, M.; Bernini, F.; Aprea, V.; Re, F.; Barbiroli, A.; Emide, D.; et al. Plasma and cerebrospinal fluid cholesterol esterification is hampered in Alzheimer’s disease. Alzheimers Res. Ther. 2023, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Tiane, A.; Schepers, M.; Riemens, R.; Rombaut, B.; Vandormael, P.; Somers, V.; Prickaerts, J.; Hellings, N.; Hove, D.v.D.; Vanmierlo, T. DNA methylation regulates the expression of the negative transcriptional regulators ID2 and ID4 during OPC differentiation. Cell Mol. Life Sci. 2021, 78, 6631–6644. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Lütjohann, D.; Brzezinka, A.; Barth, E.; Abramowski, D.; Staufenbiel, M.; von Bergmann, K.; Beyreuther, K.; Multhaup, G.; Bayer, T.A. Profile of cholesterol-related sterols in aged amyloid precursor protein transgenic mouse brain. J. Lipid Res. 2002, 43, 1078–1085. [Google Scholar] [CrossRef]
- Akkerman, S.; Prickaerts, J.; Steinbusch, H.W.; Blokland, A. Object recognition testing: Statistical considerations. Behav. Brain Res. 2012, 232, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.W.; Akay, L.A.; Davila-Velderrain, J.; von Maydell, D.; Mathys, H.; Davidson, S.M.; Effenberger, A.; Chen, C.Y.; Maner-Smith, K.; Hajjar, I.; et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 2022, 611, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Fitzner, D.; Bosch-Queralt, M.; Weil, M.-T.; Su, M.; Sen, P.; Ruhwedel, T.; Mitkovski, M.; Trendelenburg, G.; Lütjohann, D.; et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018, 359, 684–688. [Google Scholar] [CrossRef]
- Hindinger, C.; Hinton, D.R.; Kirwin, S.J.; Atkinson, R.D.; Burnett, M.E.; Bergmann, C.C.; Stohlman, S.A. Liver X receptor activation decreases the severity of experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2006, 84, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Qin, X.; Wu, L.; Zhang, Y.; Sheng, X.; Yu, Q.; Sheng, H.; Xi, B.; Zhang, J.Z.; Zang, Y.Q. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J. Clin. Investig. 2011, 121, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, S.A.; Spieth, L.; Sun, T.; Hosang, L.; Schlaphoff, L.; Depp, C.; Düking, T.; Winchenbach, J.; Neuber, J.; Ewers, D.; et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci. 2021, 24, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Meffre, D.; Shackleford, G.; Hichor, M.; Gorgievski, V.; Tzavara, E.T.; Trousson, A.; Ghoumari, A.M.; Deboux, C.; Oumesmar, B.N.; Liere, P.; et al. Liver X receptors alpha and beta promote myelination and remyelination in the cerebellum. Proc. Natl. Acad. Sci. USA 2015, 112, 7587–7592. [Google Scholar] [CrossRef] [PubMed]
- Depp, C.; Sun, T.; Sasmita, A.O.; Spieth, L.; Berghoff, S.A.; Nazarenko, T.; Overhoff, K.; Steixner-Kumar, A.A.; Subramanian, S.; Arinrad, S.; et al. Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease. Nature 2023, 618, 349–357. [Google Scholar] [CrossRef]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef]
- van der Kant, R.; Langness, V.F.; Herrera, C.M.; Williams, D.A.; Fong, L.K.; Leestemaker, Y.; Steenvoorden, E.; Rynearson, K.D.; Brouwers, J.F.; Helms, J.B.; et al. Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-β in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell 2019, 24, 363–375.e9. [Google Scholar] [CrossRef]
- Litvinchuk, A.; Suh, J.H.; Guo, J.L.; Lin, K.; Davis, S.S.; Bien-Ly, N.; Tycksen, E.; Tabor, G.T.; Serrano, J.R.; Manis, M.; et al. Amelioration of Tau and ApoE4-linked glial lipid accumulation and neurodegeneration with an LXR agonist. Neuron 2024, 112, 384–403.e8. [Google Scholar] [CrossRef]
- Snowdon, D.A. Aging and Alzheimer’s disease: Lessons from the Nun Study. Gerontologist 1997, 37, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Green, K.N.; Steffan, J.S.; Martinez-Coria, H.; Sun, X.; Schreiber, S.S.; Thompson, L.M.; LaFerla, F.M. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci. 2008, 28, 11500–11510. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Tanaka, K.; Sugano, M.; Vahouny, G.V.; Gallo, L.L. Inhibition of cholesterol absorption in rats by plant sterols. J. Lipid Res. 1988, 29, 1573–1582. [Google Scholar] [CrossRef]
- Yu, L.; York, J.; von Bergmann, K.; Lutjohann, D.; Cohen, J.C.; Hobbs, H.H. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J. Biol. Chem. 2003, 278, 15565–15570. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Sun, L.P.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Direct binding of cholesterol to the purified membrane region of SCAP: Mechanism for a sterol-sensing domain. Mol. Cell. 2004, 15, 259–268. [Google Scholar] [CrossRef]
- Yang, C.; McDonald, J.G.; Patel, A.; Zhang, Y.; Umetani, M.; Xu, F.; Westover, E.J.; Covey, D.F.; Mangelsdorf, D.J.; Cohen, J.C.; et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 2006, 281, 27816–27826. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandak, W.M.; Hylemon, P.B. LXR alpha is the dominant regulator of CYP7A1 transcription. Biochem. Biophys. Res. Commun. 2002, 293, 338–343. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef]
- Uppal, H.; Saini, S.P.; Moschetta, A.; Mu, Y.; Zhou, J.; Gong, H.; Zhai, Y.; Ren, S.; Michalopoulos, G.K.; Mangelsdorf, D.J.; et al. Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology 2007, 45, 422–432. [Google Scholar] [CrossRef]
- Feringa, F.M.; van der Kant, R. Cholesterol and Alzheimer’s Disease; From Risk Genes to Pathological Effects. Front. Aging Neurosci. 2021, 13, 690372. [Google Scholar] [CrossRef] [PubMed]
- Mitsche, M.A.; McDonald, J.G.; Hobbs, H.H.; Cohen, J.C. Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways. Elife 2015, 4, e07999. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Suárez, Y.; Ferruelo, A.J.; Gómez-Coronado, D.; Lasunción, M.A. Inhibition of cholesterol biosynthesis by Delta22-unsaturated phytosterols via competitive inhibition of sterol Delta24-reductase in mammalian cells. Biochem. J. 2002, 366 Pt 1, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Zerenturk, E.J.; Kristiana, I.; Gill, S.; Brown, A.J. The endogenous regulator 24(S),25-epoxycholesterol inhibits cholesterol synthesis at DHCR24 (Seladin-1). Biochim. Biophys. Acta. 2012, 1821, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, T.; Buchebner, M.; Chandak, P.G.; Patankar, J.; Kratzer, A.; Obrowsky, S.; Rechberger, G.N.; Kadam, R.S.; Kompella, U.B.; Kostner, G.M.; et al. Synthetic LXR agonist suppresses endogenous cholesterol biosynthesis and efficiently lowers plasma cholesterol. Curr. Pharm. Biotechnol. 2011, 12, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Daimiel, L.A.; Fernandez-Suarez, M.E.; Rodriguez-Acebes, S.; Crespo, L.; Lasuncion, M.A.; Gomez-Coronado, D.; Martinez-Botas, J. Promoter analysis of the DHCR24 (3β-hydroxysterol Δ(24)-reductase) gene: Characterization of SREBP (sterol-regulatory-element-binding protein)-mediated activation. Biosci. Rep. 2012, 33, 57–69. [Google Scholar] [PubMed]
- Zerenturk, E.J.; Sharpe, L.J.; Brown, A.J. Sterols regulate 3β-hydroxysterol Δ24-reductase (DHCR24) via dual sterol regulatory elements: Cooperative induction of key enzymes in lipid synthesis by Sterol Regulatory Element Binding Proteins. Biochim. Biophys. Acta. 2012, 1821, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Sander, P.; Hamann, H.; Drögemüller, C.; Kashkevich, K.; Schiebel, K.; Leeb, T. Bovine prion protein gene (PRNP) promoter polymorphisms modulate PRNP expression and may be responsible for differences in bovine spongiform encephalopathy susceptibility. J. Biol. Chem. 2005, 280, 37408–37414. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; van den Munckhof, I.C.L.; Schraa, K.; Ter Horst, R.; Koehorst, M.; Van Faassen, M.; Van Der Ley, C.; Doestzada, M.; Zhernakova, D.V.; Kurilshikov, A.; et al. Genetic and Microbial Associations to Plasma and Fecal Bile Acids in Obesity Relate to Plasma Lipids and Liver Fat Content. Cell Rep. 2020, 33, 108212. [Google Scholar] [CrossRef]
- Biallosterski, B.; de Wachter, S.; van Koeveringe, G.; van Kerrebroeck, P.; de Vente, J.; Mulder, M.; Gillespie, J. Changes in bladder innervation in a mouse model of Alzheimer’s disease. J. Chem. Neuroanat. 2010, 39, 204–210. [Google Scholar] [CrossRef]
- Kaur, H.; Seeger, D.; Golovko, S.; Golovko, M.; Combs, C.K. Liver Bile Acid Changes in Mouse Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 7451. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmueller, G.; et al. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimers Dement. 2019, 15, 232–244. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q.; Chen, S.; Xu, C. Functions of amyloid precursor protein in metabolic diseases. Metabolism 2021, 115, 154454. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munafò, A.; Bernardini, R.; Cantarella, G. Role of Microglia and Astrocytes in Alzheimer’s Disease: From Neuroinflammation to Ca(2+) Homeostasis Dysregulation. Cells 2022, 11, 2728. [Google Scholar] [CrossRef]
- Olsthoorn, S.E.M.; Wang, X.; Tillema, B.; Vanmierlo, T.; Kraan, S.; Leenen, P.J.M.; Mulder, M.T. Brown Seaweed Food Supplementation: Effects on Allergy and Inflammation and Its Consequences. Nutrients 2021, 13, 2613. [Google Scholar] [CrossRef]
- Fiala, M.; Liu, Q.N.; Sayre, J.; Pop, V.; Brahmandam, V.; Graves, M.C.; Vinters, H.V. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur. J. Clin. Investig. 2002, 32, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Rodríguez, J.J.; Verkhratsky, A. Neuroglia in neurodegeneration. Brain Res. Rev. 2010, 63, 189–211. [Google Scholar] [CrossRef]
- Heller, C.; Foiani, M.S.; Moore, K.; Convery, R.; Bocchetta, M.; Neason, M.; Cash, D.M.; Thomas, D.; Greaves, C.V.; Woollacott, I.O.; et al. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry. 2020, 91, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Dvorak, F.; Haberer, I.; Sitzer, M.; Foerch, C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc. Dis. 2009, 27, 37–41. [Google Scholar] [CrossRef]
- Shir, D.; Graff-Radford, J.; Hofrenning, E.I.; Lesnick, T.G.; Przybelski, S.A.; Lowe, V.J.; Knopman, D.S.; Petersen, R.C.; Jack, C.R.; Vemuri, P.; et al. Association of plasma glial fibrillary acidic protein (GFAP) with neuroimaging of Alzheimer’s disease and vascular pathology. Alzheimers Dement 2022, 14, e12291. [Google Scholar] [CrossRef] [PubMed]
- Cicognola, C.; Janelidze, S.; Hertze, J.; Zetterberg, H.; Blennow, K.; Mattsson-Carlgren, N.; Hansson, O. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res. Ther. 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Monterey, M.D.; Wei, H.; Wu, X.; Wu, J.Q. The Many Faces of Astrocytes in Alzheimer’s Disease. Front. Neurol. 2021, 12, 619626. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.R.; Li, Y.M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017, 7, 170228. [Google Scholar] [CrossRef] [PubMed]
- Spann, N.J.; Garmire, L.X.; McDonald, J.G.; Myers, D.S.; Milne, S.B.; Shibata, N.; Reichart, D.; Fox, J.N.; Shaked, I.; Heudobler, D.; et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 2012, 151, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Zhou, E.; Müller, C.; Mohammed, Y.; Herceg, S.; Bracher, F.; Rensen, P.C.N.; Wang, Y.; Mirakaj, V.; Giera, M. Inhibition of Δ24-dehydrocholesterol reductase activates pro-resolving lipid mediator biosynthesis and inflammation resolution. Proc. Natl. Acad. Sci. USA 2019, 116, 20623–20634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Ge, X.; Nakashima, H.; Li, R.; van der Zande, H.J.P.; Liu, C.; Li, Z.; Müller, C.; Bracher, F.; Mohammed, Y.; et al. Inhibition of DHCR24 activates LXRα to ameliorate hepatic steatosis and inflammation. EMBO Mol. Med. 2023, 15, e16845. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Majdi, A.; Mahmoudi, J.; Golzari, S.E.J.; Talebi, M. Astrocytic and microglial nicotinic acetylcholine receptors: An overlooked issue in Alzheimer’s disease. J. Neural. Transm. 2016, 123, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Horkowitz, A.P.; Schwartz, A.; Alvarez, C.A.; Herrera, E.B.; Thoman, M.L.; Chatfield, D.A.; Osborn, K.G.; Feuer, R.; George, U.Z.; Phillips, J.A. Acetylcholine Regulates Pulmonary Pathology during Viral Infection and Recovery. Immunotargets Ther. 2020, 9, 333–350. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, X.; Li, P.; Dong, S.; Huang, X.; Ren, X.; Yuan, L. High-fat diet induced discrepant peripheral and central nervous systems insulin resistance in APPswe/PS1dE9 and wild-type C57BL/6J mice. Aging 2020, 13, 1236–1250. [Google Scholar] [CrossRef]
- Müller, L.; Guerra, N.P.; Stenzel, J.; Rühlmann, C.; Lindner, T.; Krause, B.J.; Vollmar, B.; Teipel, S.; Kuhla, A. Long-Term Caloric Restriction Attenuates β-Amyloid Neuropathology and Is Accompanied by Autophagy in APPswe/PS1delta9 Mice. Nutrients 2021, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.V.; Gordon, M.N.; Connor, K.E.; Good, R.A.; Engelman, R.W.; Mason, J.; Morgan, D.G.; Morgan, T.E.; Finch, C.E. Caloric restriction attenuates Aβ-deposition in Alzheimer transgenic models. Neurobiol. Aging 2005, 26, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Halagappa, V.K.M.; Guo, Z.; Pearson, M.; Matsuoka, Y.; Cutler, R.G.; LaFerla, F.M.; Mattson, M.P. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2007, 26, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Repa, J.J.; Gauthier, K.; Mangelsdorf, D.J. Regulation of lipoprotein lipase by the oxysterol receptors, LXRalpha and LXRbeta. J. Biol. Chem. 2001, 276, 43018–43024. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, S.C.; Wilson, L. Inability of tau to properly regulate neuronal microtubule dynamics: A loss-of-function mechanism by which tau might mediate neuronal cell death. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2005, 1739, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Lamela, M.; Anca, J.; Villar, R.; Otero, J.; Calleja, J.M. Hypoglycemic activity of several seaweed extracts. J. Ethnopharmacol. 1989, 27, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.R.; Garcimartín, A.; Bastida, S.; Jiménez-Escrig, A.; Rupérez, P.; Green, B.D.; Rafferty, E.; Sánchez-Muniz, F.J.; Benedí, J. Effects of Undaria pinnatifida, Himanthalia elongata and Porphyra umbilicalis extracts on in vitro α-glucosidase activity and glucose diffusion. Nutr. Hosp. 2014, 29, 1434–1446. [Google Scholar]
- Ilyas, Z.; Ali Redha, A.; Wu, Y.S.; Ozeer, F.Z.; Aluko, R.E. Nutritional and Health Benefits of the Brown Seaweed Himanthalia elongata. Plant Foods Hum. Nutr. 2023, 78, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Segovia, I.; Lerma-García, M.J.; Fuentes, A.; Barat, J.M. Characterization of Spanish powdered seaweeds: Composition, antioxidant capacity and technological properties. Food Res. Int. 2018, 111, 212–219. [Google Scholar] [CrossRef]
- Vanmierlo, T.; Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.-B.; Lütjohann, D.; Mulder, M. Edible seaweed-derived constituents: An undisclosed source of neuroprotective compounds. Neural Regen. Res. 2020, 15, 790–795. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]


| Gene | Gene Name | Primer Sequence |
|---|---|---|
| ACACA | Acetyl-CoA Carboxylase Alpha | F: CTCAACAGCGTACAACACCG R: TGGGGATGTTCCCTCTGTTTG |
| ACTB | Actin Beta | F: TTCTTGGGTATGGAATCCTGTGG R: GTCTTTACGGATGTCAACGTCAC |
| B2M | Beta-2-Microglobulin | F: CATGGCTCGCTCGGTGACC R: AATGTGAGGCGGGTGGAACTG |
| FASN | Fatty Acid Synthase | F: GGCCCCTCTGTTAATTGGCT R: GGGATAACAGCACCTTGGTCA |
| HPRT1 | Hypoxanthine Phosphoribosyltransferase 1 | F: CCTAAGATGAGCGCAAGTTGAA R: CCACAGGACTAGAACACCTGCTAA |
| SCD1 | Stearoyl-CoA Desaturase 1 | F: GGCCTGTACGGGATCATACTG R: GGTCATGTAGTAGAAAATCCCGAAG |
| SDHA | Succinate Dehydrogenase Complex Flavoprotein Subunit A | F: CTTGAATGAGGCTGACTGTG R: ATCACATAAGCTGGTCCTGT |
| SREBF1 | Sterol Regulatory Element Binding Transcription Factor 1 | F: GCCATCGACTACATCCGCTT R: CAGGTCCTTCAGTGATTTGCTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, N.; Zhan, N.; Yam, S.C.; Leijten, F.P.J.; Palumbo, M.; Caspers, M.; Tiane, A.; Friedrichs, S.; Li, Y.; van Vark-van der Zee, L.; et al. Supplementation of Seaweed Extracts to the Diet Reduces Symptoms of Alzheimer’s Disease in the APPswePS1ΔE9 Mouse Model. Nutrients 2024, 16, 1614. https://doi.org/10.3390/nu16111614
Martens N, Zhan N, Yam SC, Leijten FPJ, Palumbo M, Caspers M, Tiane A, Friedrichs S, Li Y, van Vark-van der Zee L, et al. Supplementation of Seaweed Extracts to the Diet Reduces Symptoms of Alzheimer’s Disease in the APPswePS1ΔE9 Mouse Model. Nutrients. 2024; 16(11):1614. https://doi.org/10.3390/nu16111614
Chicago/Turabian StyleMartens, Nikita, Na Zhan, Sammie C. Yam, Frank P. J. Leijten, Marcella Palumbo, Martien Caspers, Assia Tiane, Silvia Friedrichs, Yanlin Li, Leonie van Vark-van der Zee, and et al. 2024. "Supplementation of Seaweed Extracts to the Diet Reduces Symptoms of Alzheimer’s Disease in the APPswePS1ΔE9 Mouse Model" Nutrients 16, no. 11: 1614. https://doi.org/10.3390/nu16111614
APA StyleMartens, N., Zhan, N., Yam, S. C., Leijten, F. P. J., Palumbo, M., Caspers, M., Tiane, A., Friedrichs, S., Li, Y., van Vark-van der Zee, L., Voortman, G., Zimetti, F., Jaarsma, D., Verschuren, L., Jonker, J. W., Kuipers, F., Lütjohann, D., Vanmierlo, T., & Mulder, M. T. (2024). Supplementation of Seaweed Extracts to the Diet Reduces Symptoms of Alzheimer’s Disease in the APPswePS1ΔE9 Mouse Model. Nutrients, 16(11), 1614. https://doi.org/10.3390/nu16111614







