Therapeutic Effects of Plant Anthocyanin against Alzheimer’s Disease and Modulate Gut Health, Short-Chain Fatty Acids
Abstract
1. Introduction
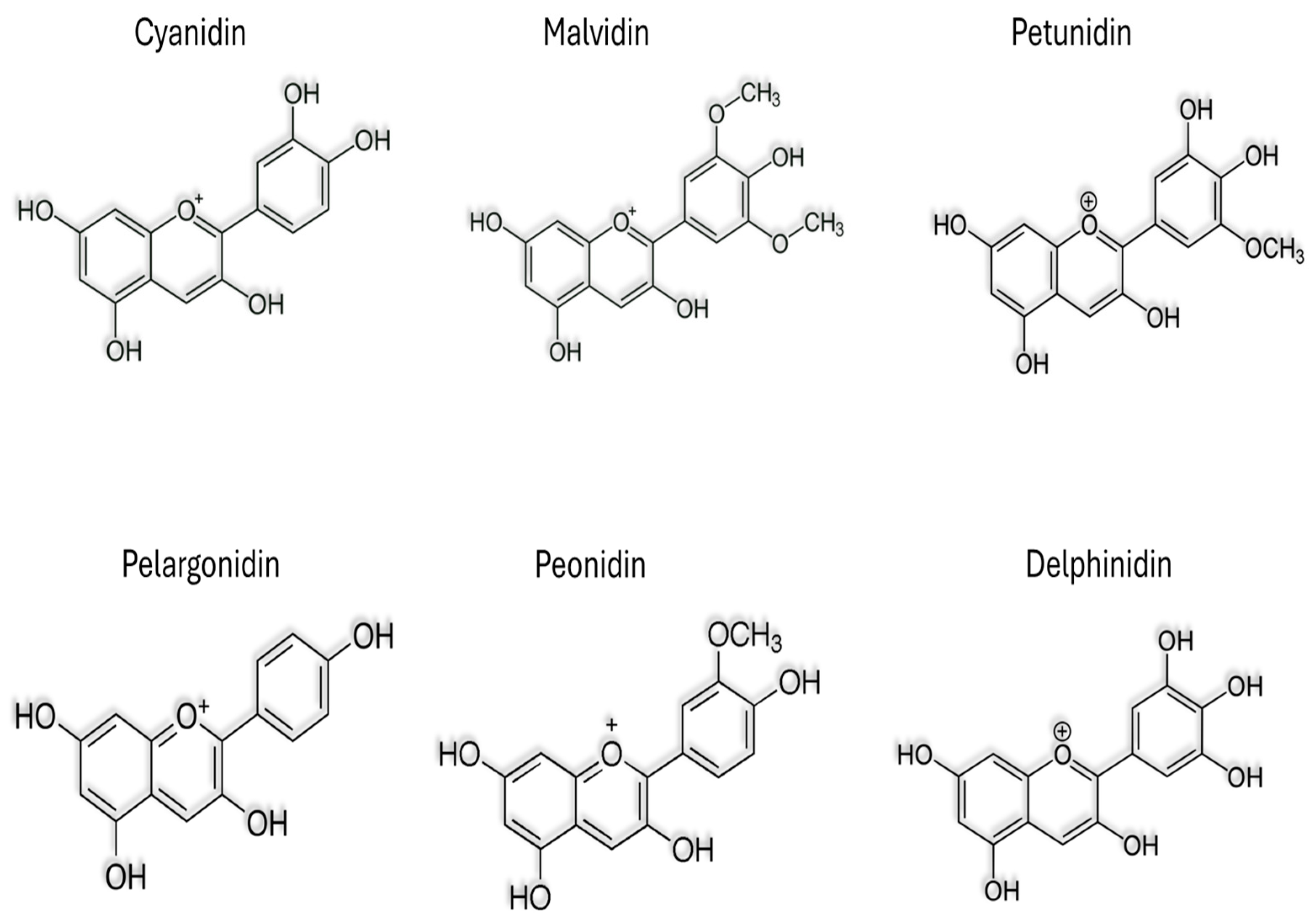
2. Anthocyanins in Plant
3. Anthocyanins on Gut–Brain Axis
4. Anthocyanins on SCFAs
5. Effects of Anthocyanins against Cognitive and Memory Impairments
6. Anthocyanins in Neuroinflammation
7. Anthocyanins in Oxidative Stress
8. Protective Effects of Anthocyanins Neuronal Apoptosis
9. Anthocyanins on Insulin Resistance
10. Effects of Anthocyanins on Neurogenesis
11. Role of Anthocyanins in Amyloid-Beta and Tauopathy
12. Anthocyanins on Proteostasis
13. Effects of Anthocyanins on Epigenetics of Alzheimer’s Disease
14. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef]
- Suresh, S.; Begum, R.F.; Singh S, A.; V, C. Anthocyanin as a therapeutic in Alzheimer’s disease: A systematic review of preclinical evidences. Ageing Res. Rev. 2022, 76, 101595. [Google Scholar] [CrossRef]
- Gao, J.; Wu, Y.; He, D.; Zhu, X.; Li, H.; Liu, H.; Liu, H. Anti-aging effects of Ribes meyeri anthocyanins on neural stem cells and aging mice. Aging 2020, 12, 17738. [Google Scholar] [CrossRef]
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regen. Res. 2014, 9, 1557–1566. [Google Scholar]
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-Loaded PEG-Gold Nanoparticles Enhanced the Neuroprotection of Anthocyanins in an Aβ1–42 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 6490–6506. [Google Scholar] [CrossRef]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef]
- Da Silva, F.L.; Escribano-Bailón, M.T.; Pérez Alonso, J.J.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT-Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Welch, C.R.; Wu, Q.; Simon, J.E. Recent Advances in Anthocyanin Analysis and Characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef]
- Afzal, M.; Redha, A.; AlHasan, R. Anthocyanins Potentially Contribute to Defense against Alzheimer’s Disease. Molecules 2019, 24, 4255. [Google Scholar] [CrossRef]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood–Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef]
- Menconi, J.; Perata, P.; Gonzali, S. In pursuit of purple: Anthocyanin biosynthesis in fruits of the tomato clade. Trends Plant Sci. 2024, 29, 589–604. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Zhang, Y.; Wu, B.; Li, Y.; Tian, L.; Sun, J.; Bai, W. Mechanism of action of anthocyanin on the detoxification of foodborne contaminants—A review of recent literature. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13259. [Google Scholar] [CrossRef]
- Mohammadi, N.; Farrell, M.; O’Sullivan, L.; Langan, A.; Franchin, M.; Azevedo, L.; Granato, D. Effectiveness of anthocyanin-containing foods and nutraceuticals in mitigating oxidative stress, inflammation, and cardiovascular health-related biomarkers: A systematic review of animal and human interventions. Food Funct. 2024, 15, 3274–3299. [Google Scholar] [CrossRef] [PubMed]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Chun, E.M.; Mijan, M.A.; Park, S.H.; Moon, S.-K.; Lim, B.O. Anthocyanins Profiling of Bilberry (Vaccinium myrtillus L.) Extract that Elucidates Antioxidant and Anti-inflammatory Effects. Food Agric. Immunol. 2021, 32, 713–726. [Google Scholar] [CrossRef]
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5570–5615. [Google Scholar] [CrossRef]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-gut microbiota-health axis: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–26. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Rahhal, B.; Qneibi, M.; Jaradat, N.; Hawash, M.; Qadi, M.; Issa, L.; Bdir, S. Multi-biological activity assessment and phytochemical characterization of an aqueous extract of the Cymbopogon citratus grown in Palestine. BMC Complement. Med. Ther. 2024, 24, 27. [Google Scholar] [CrossRef]
- Bitsch, R.; Netzel, M.; Frank, T.; Strass, G.; Bitsch, I. Bioavailability and Biokinetics of Anthocyanins From Red Grape Juice and Red Wine. J. Biomed. Biotechnol. 2004, 2004, 380728. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Somerville, V.X.; Hurst, R.D. The effect of New Zealand blackcurrant on sport performance and related biomarkers: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Hollands, W.; Brett, G.M.; Radreau, P.; Saha, S.; Teucher, B.; Bennett, R.N.; Kroon, P.A. Processing blackcurrants dramatically reduces the content and does not enhance the urinary yield of anthocyanins in human subjects. Food Chem. 2008, 108, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Condurache, N.-N.; Croitoru, C.; Enachi, E.; Bahrim, G.-E.; Stănciuc, N.; Râpeanu, G. Eggplant Peels as a Valuable Source of Anthocyanins: Extraction, Thermal Stability and Biological Activities. Plants 2021, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Li, J.; Shi, C.; Shen, D.; Han, T.; Wu, W.; Lyu, L.; Li, W. Composition and Antioxidant Activity of Anthocyanins and Non-Anthocyanin Flavonoids in Blackberry from Different Growth Stages. Foods 2022, 11, 2902. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Grigoras, C.G.; Destandau, E.; Zubrzycki, S.; Elfakir, C. Sweet cherries anthocyanins: An environmental friendly extraction and purification method. Sep. Purif. Technol. 2012, 100, 51–58. [Google Scholar] [CrossRef]
- Yang, C.; Xue, J.; Qin, Q.; Xia, Y.; Cheng, S.; Jiang, X.; Zhang, S.; Lu, Z.; Qin, X.; Zhang, J.; et al. Prenatal exposure to titanium dioxide nanoparticles induces persistent neurobehavioral impairments in maternal mice that is associated with microbiota-gut-brain axis. Food Chem. Toxicol. 2022, 169, 113402. [Google Scholar] [CrossRef]
- Aburto, M.R.; Cryan, J.F. Gastrointestinal and brain barriers: Unlocking gates of communication across the microbiota–gut–brain axis. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 222–247. [Google Scholar] [CrossRef]
- Doifode, T.; Giridharan, V.V.; Generoso, J.S.; Bhatti, G.; Collodel, A.; Schulz, P.E.; Forlenza, O.V.; Barichello, T. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 2021, 164, 105314. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bruggeman, A.; De Nolf, C.; Vandendriessche, C.; Van Imschoot, G.; Van Wonterghem, E.; Vereecke, L.; Vandenbroucke, R.E. Gut microbiota regulates blood-cerebrospinal fluid barrier function and Aβ pathology. EMBO J. 2023, 42, e111515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Bayazid, A.B.; Kim, J.G.; Azam, S.; Jeong, S.A.; Kim, D.H.; Park, C.W.; Lim, B.O. Sodium butyrate ameliorates neurotoxicity and exerts anti-inflammatory effects in high fat diet-fed mice. Food Chem. Toxicol. 2022, 159, 112743. [Google Scholar] [CrossRef] [PubMed]
- Bayazid, A.B.; Jeong, S.A.; Azam, S.; Oh, S.H.; Lim, B.O. Neuroprotective Effects of Fermented Blueberry and Black Rice against Particulate Matter 2.5 μm-Induced Inflammation In Vitro and In Vivo. Preprints 2023, in press. [Google Scholar]
- Zhang, N.; Jing, P. Red Cabbage Anthocyanins Attenuate Cognitive Impairment By Attenuating Neuroinflammation and Regulating Gut Microbiota in Aging Mice. J. Agric. Food Chem. 2023, 71, 15064–15072. [Google Scholar] [CrossRef] [PubMed]
- Igwe, E.O.; Roodenrys, S.; Probst, Y.C.; do Rosario, V.; Netzel, M.E.; Hong, H.T.; Netzel, G.; Phan, A.D.T.; Charlton, K.E. Low anthocyanin plum nectar does not impact cognition, blood pressure and gut microbiota in healthy older adults: A randomized crossover trial. Nutr. Res. 2020, 82, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ikram, M.; Park, J.S.; Park, T.J.; Kim, M.O. Gut Microbiota, Its Role in Induction of Alzheimer’s Disease Pathology, and Possible Therapeutic Interventions: Special Focus on Anthocyanins. Cells 2020, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Jamar, G.; Estadella, D.; Pisani, L.P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. BioFactors 2017, 43, 507–516. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef]
- Jayarathne, S.; Stull, A.J.; Park, O.-H.; Kim, J.H.; Thompson, L.; Moustaid-Moussa, N. Protective Effects of Anthocyanins in Obesity-Associated Inflammation and Changes in Gut Microbiome. Mol. Nutr. Food Res. 2019, 63, 1900149. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, C. The role of short-chain fatty acids in central nervous system diseases. Mol. Cell. Biochem. 2022, 477, 2595–2607. [Google Scholar] [CrossRef] [PubMed]
- Bayazid, A.B.; Jeong, Y.H.; Jeong, S.A.; Lim, B.O. Sodium butyrate alleviates potential Alzheimer’s disease in vitro by suppressing Aβ and tau activation and ameliorates Aβ-induced toxicity. Food Agric. Immunol. 2023, 34, 2234100. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 424615. [Google Scholar]
- He, X.; Zhang, T.; Zeng, Y.; Pei, P.; Liu, Y.; Jia, W.; Zhao, H.; Bi, M.; Wang, S. Sodium butyrate mediates histone crotonylation and alleviated neonatal rats hypoxic–ischemic brain injury through gut–brain axis. Front. Microbiol. 2022, 13, 993146. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium ruthenicum Anthocyanins Attenuate High-Fat Diet-Induced Colonic Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, 2000745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shen, G.X. Impact of anthocyanin component and metabolite of Saskatoon berry on gut microbiome and relationship with fecal short chain fatty acids in diet-induced insulin resistant mice. J. Nutr. Biochem. 2023, 111, 109201. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lin, Z.; Zhao, S.; Zhang, B.; Luo, L.; Zeng, L. Pu-erh tea alleviated colitis-mediated brain dysfunction by promoting butyric acid production. Food Chem. Toxicol. 2023, 172, 113594. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Tiwari, A.; Sharma, S.; Tiwari, V.; Sheoran, B.; Ali, U.; Garg, M. Effect of anthocyanins on gut health markers, Firmicutes-Bacteroidetes ratio and short-chain fatty acids: A systematic review via meta-analysis. Sci. Rep. 2023, 13, 1729. [Google Scholar] [CrossRef]
- Sun, Y.; O’Riordan, M.X.D. Chapter Three—Regulation of Bacterial Pathogenesis by Intestinal Short-Chain Fatty Acids. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 85, pp. 93–118. [Google Scholar]
- do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Paterson, A.; Roodenrys, S.; Thomas, S.; Bliokas, V.; Potter, J.; Walton, K.; Weston–Green, K.; et al. Food anthocyanins decrease concentrations of TNF-α in older adults with mild cognitive impairment: A randomized, controlled, double blind clinical trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 950–960. [Google Scholar] [CrossRef]
- Carbonel, A.A.F.; Cecyn, M.N.; Girão, J.H.R.C.; da Silva Sasso, G.R.; de Mello Ponteciano, B.; Vellozo, E.P.; Simões, R.S.; de Jesus Simões, M.; Girão, M.J.B.C.; de Oliveira, D.R. Flavonoids as Modulators of Synaptic Plasticity: Implications for the Development of Novel Therapeutic Strategies for Healthy Lifestyle. In Flavonoids—A Coloring Model for Cheering up Life; IntechOpen: London, UK, 2019. [Google Scholar]
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins Reversed D-Galactose-Induced Oxidative Stress and Neuroinflammation Mediated Cognitive Impairment in Adult Rats. Mol. Neurobiol. 2017, 54, 255–271. [Google Scholar] [CrossRef]
- Milenkovic, D.; Krga, I.; Dinel, A.-L.; Morand, C.; Laye, S.; Castanon, N. Nutrigenomic modification induced by anthocyanin-rich bilberry extract in the hippocampus of ApoE-/- mice. J. Funct. Foods 2021, 85, 104609. [Google Scholar] [CrossRef]
- Xu, J.; Gao, H.; Zhang, L.; Rong, S.; Yang, W.; Ma, C.; Chen, M.; Huang, Q.; Deng, Q.; Huang, F. Melatonin alleviates cognition impairment by antagonizing brain insulin resistance in aged rats fed a high-fat diet. J. Pineal Res. 2019, 67, e12584. [Google Scholar] [CrossRef] [PubMed]
- Mangalmurti, A.; Lukens, J.R. How neurons die in Alzheimer’s disease: Implications for neuroinflammation. Curr. Opin. Neurobiol. 2022, 75, 102575. [Google Scholar] [CrossRef]
- Dong, G.; Xu, N.; Wang, M.; Zhao, Y.; Jiang, F.; Bu, H.; Liu, J.; Yuan, B.; Li, R. Anthocyanin Extract from Purple Sweet Potato Exacerbate Mitophagy to Ameliorate Pyroptosis in Klebsiella pneumoniae Infection. Int. J. Mol. Sci. 2021, 22, 11422. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Maes, M.; Corbi, G.; Zarrelli, A.; Willcox, D.C.; Scapagnini, G. Dietary phytochemicals and neuro-inflammaging: From mechanistic insights to translational challenges. Immun. Ageing 2016, 13, 16. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Hwang, U.K.; Jang, Y.A.; Jeong, Y.H.; Jo, Y.C.; Lim, B.O. Andrographis paniculata Leaves Extract Alleviates UVB-Induced HaCaT Cells Through Suppressing Mitogen-Activated Protein Kinases Activation. Nat. Prod. Commun. 2024, 19, 1934578X241238137. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Cox, D.; Ormsby, A.R.; Reid, G.E.; Hatters, D.M. Protein painting reveals pervasive remodeling of conserved proteostasis machinery in response to pharmacological stimuli. NPJ Syst. Biol. Appl. 2022, 8, 46. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Therriault, J.; Zimmer, E.R.; Benedet, A.L.; Pascoal, T.A.; Gauthier, S.; Rosa-Neto, P. Staging of Alzheimer’s disease: Past, present, and future perspectives. Trends Mol. Med. 2022, 28, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Koya, J.; Reznik, S.E. Insulin Resistance Exacerbates Alzheimer Disease via Multiple Mechanisms. Front. Neurosci. 2021, 15, 687157. [Google Scholar] [CrossRef] [PubMed]
- Weilinger, N.L.; Lohman, A.W.; Rakai, B.D.; Ma, E.M.M.; Bialecki, J.; Maslieieva, V.; Rilea, T.; Bandet, M.V.; Ikuta, N.T.; Scott, L.; et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 2016, 19, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Malosio, M.L.; Tecchio, F.; Squitti, R. Molecular mechanisms underlying copper function and toxicity in neurons and their possible therapeutic exploitation for Alzheimer’s disease. Aging Clin. Exp. Res. 2021, 33, 2027–2030. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Lee, J.; Sin, J.S.; Kim, S.-k.; Kim, C.J.; Park, M.H.; Cho, W.-S.; Moon, M.; Kim, D.H.; Jung, J.W. Effects of Perilla frutescens var. acuta in amyloid β toxicity and Alzheimer’s disease-like pathology in 5XFAD mice. Food Chem. Toxicol. 2022, 161, 112847. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-F.; Shen, X.-Y.; Lio, C.K.; Dai, Y.; Cheng, C.-S.; Liu, J.-X.; Yao, Y.-D.; Yu, Y.; Xie, Y.; Luo, P.; et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γ gene expression in metabolic syndrome subjects. Free. Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Fattorelli, N.; Martinez-Muriana, A.; Davis, E.; Wolfs, L.; Van Den Daele, J.; Geric, I.; Premereur, J.; Polanco, P.; Bijnens, B.; et al. Xenografted human microglia display diverse transcriptomic states in response to Alzheimer’s disease-related amyloid-β pathology. Nat. Neurosci. 2024, 27, 886–900. [Google Scholar] [CrossRef]
- Jaber, M.; Shawahna, R.; Abu-Issa, M.; Radwan, F.; Dweik, M. Anesthesia considerations for patients with epilepsy: Findings of a qualitative study in the Palestinian practice. Epilepsy Behav. 2021, 123, 108278. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.-S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Shih, P.-H.; Wu, C.-H.; Yeh, C.-T.; Yen, G.-C. Protective Effects of Anthocyanins against Amyloid β-Peptide-Induced Damage in Neuro-2A Cells. J. Agric. Food Chem. 2011, 59, 1683–1689. [Google Scholar] [CrossRef]
- Kshirsagar, V.; Thingore, C.; Juvekar, A. Insulin resistance: A connecting link between Alzheimer’s disease and metabolic disorder. Metab. Brain Dis. 2021, 36, 67–83. [Google Scholar] [CrossRef]
- Kellar, D.; Craft, S. Brain insulin resistance in Alzheimer’s disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Ye, X.; Chen, W.; Huang, X.-F.; Yan, F.-J.; Deng, S.-G.; Zheng, X.-D.; Shan, P.-F. Anti-diabetic effect of anthocyanin cyanidin-3-O-glucoside: Data from insulin resistant hepatocyte and diabetic mouse. Nutr. Diabetes 2024, 14, 7. [Google Scholar] [CrossRef]
- de Mello, J.E.; Teixeira, F.C.; dos Santos, A.; Luduvico, K.; Soares de Aguiar, M.S.; Domingues, W.B.; Campos, V.F.; Tavares, R.G.; Schneider, A.; Stefanello, F.M.; et al. Treatment with Blackberry Extract and Metformin in Sporadic Alzheimer’s Disease Model: Impact on Memory, Inflammation, Redox Status, Phosphorylated Tau Protein and Insulin Signaling. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ridzwan, N.; Jumli, M.N.; Baig, A.A.; Rohin, M.A.K. Pomegranate-derived anthocyanin regulates MORs-cAMP/CREB-BDNF pathways in opioid-dependent models and improves cognitive impairments. J. Ayurveda Integr. Med. 2020, 11, 478–488. [Google Scholar] [CrossRef]
- Williams, C.M.; El Mohsen, M.A.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P.E. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free. Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Cozachenco, D.; Ribeiro, F.C.; Ferreira, S.T. Defective proteostasis in Alzheimer’s disease. Ageing Res. Rev. 2023, 85, 101862. [Google Scholar] [CrossRef]
- Polling, S.; Ormsby, A.R.; Wood, R.J.; Lee, K.; Shoubridge, C.; Hughes, J.N.; Thomas, P.Q.; Griffin, M.D.W.; Hill, A.F.; Bowden, Q.; et al. Polyalanine expansions drive a shift into α-helical clusters without amyloid-fibril formation. Nat. Struct. Mol. Biol. 2015, 22, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Bourdenx, M.; Fujimaki, M.; Karabiyik, C.; Krause, G.J.; Lopez, A.; Martín-Segura, A.; Puri, C.; Scrivo, A.; Skidmore, J.; et al. The different autophagy degradation pathways and neurodegeneration. Neuron 2022, 110, 935–966. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.; Jardim, C.; Figueira, I.; Almeida, A.F.; McDougall, G.J.; Stewart, D.; Yuste, J.E.; Tomás-Barberán, F.A.; Tenreiro, S.; Outeiro, T.F.; et al. (Poly)phenol-digested metabolites modulate alpha-synuclein toxicity by regulating proteostasis. Sci. Rep. 2018, 8, 6965. [Google Scholar] [CrossRef]
- Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Cui, S.; Chen, W. Anti-aging effects and mechanisms of anthocyanins and their intestinal microflora metabolites. Crit. Rev. Food Sci. Nutr. 2024, 64, 2358–2374. [Google Scholar] [CrossRef]
- Li, H.; Zheng, T.; Lian, F.; Xu, T.; Yin, W.; Jiang, Y. Anthocyanin-rich blueberry extracts and anthocyanin metabolite protocatechuic acid promote autophagy-lysosomal pathway and alleviate neurons damage in in vivo and in vitro models of Alzheimer’s disease. Nutrition 2022, 93, 111473. [Google Scholar] [CrossRef] [PubMed]
- Francis, Y.I.; Fà, M.; Ashraf, H.; Zhang, H.; Staniszewski, A.; Latchman, D.S.; Arancio, O. Dysregulation of Histone Acetylation in the APP/PS1 Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 18, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Z.; Sun, T. The role of microRNAs in neurodegenerative diseases: A review. Cell Biol. Toxicol. 2023, 39, 53–83. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, B.; Shen, L. The Epigenetics of Alzheimer’s Disease: Factors and Therapeutic Implications. Front. Genet. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Day, J.J.; Sweatt, J.D. Epigenetic Mechanisms in Cognition. Neuron 2011, 70, 813–829. [Google Scholar] [CrossRef]
- Qazi, T.J.; Quan, Z.; Mir, A.; Qing, H. Epigenetics in Alzheimer’s Disease: Perspective of DNA Methylation. Mol. Neurobiol. 2018, 55, 1026–1044. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Wang, D.; Huo, Y.; Ji, B. Anthocyanin-rich extracts from blackberry, wild blueberry, strawberry, and chokeberry: Antioxidant activity and inhibitory effect on oleic acid-induced hepatic steatosis in vitro. J. Sci. Food Agric. 2016, 96, 2494–2503. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Sanlier, N. Effects of nutrient and bioactive food components on Alzheimer’s disease and epigenetic. Crit. Rev. Food Sci. Nutr. 2019, 59, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, A.; Scialò, E.; Puglisi, I.; Lo Piero, A.R. Anthocyanin Biosynthesis and DNA Methylation Dynamics in Sweet Orange Fruit [Citrus sinensis L. (Osbeck)] under Cold Stress. J. Agric. Food Chem. 2020, 68, 7024–7031. [Google Scholar] [CrossRef] [PubMed]


| Source | Content | Refs. |
|---|---|---|
| Red Grape | Cyanidin-3-glucoside, Delphinidin-3-glucoside, Malvidin-3-glucoside, Peonidin-3-glucoside | [20] |
| Blackcurrant | Delphinidin-3-rutinoside, Delphinidin-3-glucoside, Cyanidin-3-rutinoside, Cyanidin-3-glucoside | [21,22] |
| Purple Potato | Petunidin glucoside, Peonidin glucoside, Malvidin glucoside | [14] |
| Eggplant | Delphinidin 3-O-rutinoside-5-glucoside, Delphinidin 3-O-glucoside, Cyanidin 3-O-rutinoside | [23] |
| Red Cabbage | Cyanidin-3-diglucoside-5-glucoside. | [24] |
| Blueberry | Delphinidin 3-galactoside, Cyanidin 3-galactoside, Cyanidin 3-arabinoside, Peonidin 3-galactoside, Peonidin 3-arabinoside | [25] |
| Blackberry | Naringenin-7-O-glucoside, Quercetin-3-O-glucoside, Kaempferol-3-O-rutinoside | [26] |
| Raspberry | Cyanidins, Pelargonidins | [27] |
| Strawberry | Peonidin-3- glucoside, Peonidin-3-rutinoside, Cyanidin-3-glucoside | [7] |
| Cherry | Cyanidin-3-O-glucoside, Cyanidin-3-O-rutinoside | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayazid, A.B.; Lim, B.O. Therapeutic Effects of Plant Anthocyanin against Alzheimer’s Disease and Modulate Gut Health, Short-Chain Fatty Acids. Nutrients 2024, 16, 1554. https://doi.org/10.3390/nu16111554
Bayazid AB, Lim BO. Therapeutic Effects of Plant Anthocyanin against Alzheimer’s Disease and Modulate Gut Health, Short-Chain Fatty Acids. Nutrients. 2024; 16(11):1554. https://doi.org/10.3390/nu16111554
Chicago/Turabian StyleBayazid, Al Borhan, and Beong Ou Lim. 2024. "Therapeutic Effects of Plant Anthocyanin against Alzheimer’s Disease and Modulate Gut Health, Short-Chain Fatty Acids" Nutrients 16, no. 11: 1554. https://doi.org/10.3390/nu16111554
APA StyleBayazid, A. B., & Lim, B. O. (2024). Therapeutic Effects of Plant Anthocyanin against Alzheimer’s Disease and Modulate Gut Health, Short-Chain Fatty Acids. Nutrients, 16(11), 1554. https://doi.org/10.3390/nu16111554






