Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Assessment of Malnutrition and the Risk of Sarcopenia
2.3. Ultrasound Measurements
2.4. Study Variables
2.5. Categories of Sarcopenia
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. General Characteristics of Patients
3.2. Prevalence of Sarcopenia
3.3. Correlation between Ultrasound Variables, Handgrip Strength, BIA, and TUG
3.4. Ultrasound Cut-Off Points for Detecting Sarcopenia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127 (Suppl. S5), 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Torralvo, F.J.; Porras, N.; Abuín Fernández, J.; García Torres, F.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo, M.; Rojo Martínez, G.; Olveira, G. Normative reference values for hand grip dynamometry in Spain. Association with lean mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Mateo Lázaro, M.L.; Penacho Lázaro, M.A.; Berisa Losantos, F.; Plaza Bayo, A. New tables on hand strength in the adult population from Teruel. Nutr. Hosp. 2008, 23, 35–40. [Google Scholar] [PubMed]
- Mateos-Angulo, A.; Galán-Mercant, A.; Cuesta-Vargas, A.I. Ultrasound muscle assessment and nutritional status in institutionalized older adults: A pilot study. Nutrients 2019, 11, 1247. [Google Scholar] [CrossRef]
- García Almeida, J.M.; García García, C.; Bellido Castañeda, V.; Bellido Guerrero, B. A new nutritional approach. Assessment of the patient nutritional status: Function and body composition. Nutr. Hosp. 2018, 35, 1–4. [Google Scholar]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Bellido Guerrero, D.; Bretón Lesmes, I.; Tinahones Madueño, F.J. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70 (Suppl. 1), 74–84. [Google Scholar] [CrossRef]
- Perkisas, S.; Baudry, S.; Bauer, J.; Beckwee, D.; De Cock, A.-M.; Hobbelen, H.; Jager-Wittenaar, H.; Kasiukiewicz, A.; Landi, F.; Marco, E.; et al. Application of ultrasound for muscle assessment in sarcopenia: Towards standardized measurements. Eur. Geriatr. Med. 2018, 9, 739–757. [Google Scholar] [CrossRef]
- Perkisas, S.; Bastijns, S.; Baudry, S.; Bauer, J.; Beaudart, C.; Beckwée, D.; Cruz-Jentoft, A.; Gasowski, J.; Hobbelen, H.; Jager-Wittenaar, H.; et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur. Geriatr. Med. 2021, 12, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.; Esme, M.; Aycicek, G.S.; Armagan, B.; Kilic, L.; Ertenli, A.I.; Halil, M.G.; Akdogan, A. Evaluating skeletal muscle mass with ultrasound in patients with systemic sclerosis. Nutrition 2020, 84, 110999. [Google Scholar] [CrossRef] [PubMed]
- Barotsis, N.; Galata, A.; Hadjiconstanti, A.; Panayiotakis, G. The ultrasonographic measurement of muscle thickness in sarcopenia. A prediction study. Eur. J. Phys. Rehabil. Med. 2020, 56, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Ikezoe, T.; Taniguchi, M.; Yamada, Y.; Sawano, S.; Minani, S.; Asai, T.; Kimura, M.; Ichihashi, N. Cut-off values for lower limb muscle thickness to detect low muscle mass for sarcopenia in older adults. Clin. Interv. Aging 2021, 16, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Eşme, M.; Karcıoğlu, O.; Öncel, A.; Ayçiçek, G.; Deniz, O.; Ulaşlı, S.S.; Köksal, D.; Doğu, B.B.; Cankurtaran, M.; Halil, M. Ultrasound assessment of sarcopenia in patients with sarcoidosis. J. Ultrasound Med. 2021, 41, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, D.; Marco, E.; Davalos-Yerovi, V.; Lopez-Escobar, J.; Messaggi-Sartor, M.; Barrera, C.; Ronquillo-Moreno, N.; Vazquez-Ibar, O.; Calle, A.; Inzitari, M.; et al. Translation and validation of the spanish version of the SARC-F questionnaire to assess sarcopenia in older people. J. Nutr. Health Aging 2019, 23, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, R.; Wan, Q.; Wu, Y.; Zhou, Y.; Wang, Y.; Cui, Y.; Shen, X.; Wu, X. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies. Cancer Med. 2020, 9, 7964–7978. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Dasgupta, B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther. Adv. Musculoskelet. Dis. 2012, 4, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Meschi, T.; Narici, M.V.; Lauretani, F.; Maggio, M. Muscle Ultrasound and sarcopenia in older individuals: A clinical perspective. J. Am. Med. Dir. Assoc. 2017, 18, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.; Whatnall, M.C.; Hutchesson, M.J.; Haslam, R.L.; Bezzina, A.; Collins, C.E.; Ashton, L.M. Effectiveness of gender-targeted versus gender-neutral interventions aimed at improving dietary intake, physical activity and/or overweight/obesity in young adults (aged 17–35 years): A systematic review and meta-analysis. Nutr. J. 2020, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Primo, D.; Izaola, O.; Gómez, J.J.L.; de Luis, D. Correlation of the Phase Angle with Muscle Ultrasound and Quality of Life in Obese Females. Dis. Markers 2022, 2022, 7165126. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.J.; Izaola-Jauregui, O.; Almansa-Ruiz, L.; Jiménez-Sahagún, R.; Primo-Martín, D.; Pedraza-Hueso, M.; Ramos-Bachiller, B.; González-Gutiérrez, J.; De Luis-Román, D. Use of Muscle Ultrasonography in Morphofunctional Assessment of Amyotrophic Lateral Sclerosis (ALS). Nutrients 2024, 16, 1021. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Lopez Gomez, J.J. Morphofunctional Nutritional assessment in clinical Practice: A new approach to assessing Nutritional Status. Nutrients 2023, 5, 4300. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.J.; García-Beneitez, D.; Jiménez-Sahagún, R.; Izaola-Jauregui, O.; Primo-Martín, D.; Ramos-Bachiller, B.; Gómez-Hoyos, E.; Delgado-García, E.; Pérez-López, P.; De Luis-Román, D.A. Nutritional Ultrasonography, a Method to Evaluate Muscle Mass and Quality in Morphofunctional Assessment of Disease Related Malnutrition. Nutrients 2023, 15, 3923. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.J.; Gutierrez, C.; Izaola, O.; Primo, D.; Gomez, E.; Jimenez, R.; de Luis, D. Real World practice study of the effect of a specific oral nutritional supplement for diabetes mellitus on the morphofuncitional assessment and proteinenergy requirements. Nutrients 2022, 14, 4802. [Google Scholar] [CrossRef]
- Nies, I.; Ackermans, L.L.G.C.; Poeze, M.; Blokhuis, T.J.; Bosch, J.A.T. The Diagnostic Value of Ultrasound of the Rectus Femoris for the diagnosis of Sarcopenia in adults: A systematic review. Injury 2022, 53, S23–S29. [Google Scholar] [CrossRef]
- Lee, S.J.; Janssen, I.; Heymsfield, S.B.; Ross, R. Relation between whole-body and regional measures of human skeletal muscle. Am. J. Clin. Nutr. 2004, 80, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Meschi, T.; Maggio, M.; Narici, M.V. Application of ultrasound for muscle assessment in sarcopenia: The challenge of implementing protocols for clinical practice. Eur. Geriatr. Med. 2018, 10, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.M.G.; Bellido, D.; De Luis, D.; Rolo, G.G.; Olveira, G. Protocol for a prospective cohort study on the feasibility of application of nutritional ultrasound in the diagnosis and follow-up of patients with nutritional risk at hospital discharge: Study on body composition and function (DRECO). BMJ Open 2023, 13, e074945. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Patients | Men | Women | Difference | p |
|---|---|---|---|---|---|
| (n = 991) | (n = 585) | (n = 406) | (n = 991) | ||
| Age, years, mean ± SD | 58.5 ± 16.5 | 58.9 ± 16.5 | 57.8 ± 16.3 | −1.1 ± 1.1 | 0.33 |
| Weight, kg, mean ± SD | 63.7 ± 14.8 | 68.5 ± 14.2 | 56.7 ± 12.9 | −11.8 ± 0.9 | <0.0001 |
| BMI, kg/m2, mean ± SD | 22.9 ± 4.8 | 23.4 ± 4.7 | 22.3 ± 4.9 | −1.1 ± 0.3 | 0.0004 |
| Handgrip strength, kg, mean ± SD (n = 963) | 25.0 ± 10.8 | 30.0 ± 10.2 | 17.8 ± 6.8 | −12.2 ± 0.5 | <0.0001 |
| EWGSOP2 cut-off (men 27 kg, women 16 kg) (n = 963) | |||||
| Normal, n (%) | 321 (33.3) | 188 (33.1) | 133 (33.7) | −55 (0.60) | 0.889 |
| Abnormal, n (%) | 642 (66.7) | 380 (66.9) | 262 (66.3) | −118 (0.60) | |
| BIA, mean ± SD | |||||
| Phase angle, degrees, (n = 907) | 5.02 ± 1.11 | 5.20 ± 1.17 | 4.76 ± 0.96 | −0.44 ± 0.1 | <0.0001 |
| Total body water, % (n = 939) | 73.53 ± 6.14 | 74.05 ± 5.85 | 72.75 ± 6.48 | −1.3 ± 0.4 | 0.001 |
| Fat mass, kg (n = 958) | 15.13 ± 8.40 | 14.63 ± 8.17 | 15.85 ± 8.67 | 1.22 ± 0.5 | 0.027 |
| Lean mass, kg (n = 968) | 48.06 ± 10.09 | 53.36 ± 8.90 | 40.52 ± 6.10 | −12.84 ± 0.5 | <0.0001 |
| Body cell mass, kg (n = 934) | 23.46 ± 6.40 | 26.25 ± 6.14 | 19.46 ± 4.28 | −6.79 ± 0.3 | <0.0001 |
| Appendicular skeletal muscle mass, kg/m2 (n = 937) | 6.33 ± 1.63 | 6.75 ± 1.71 | 5.72 ± 1.27 | −1.03 ± 0.1 | <0.0001 |
| EWGSOP2 cut-off (men 7 kg/m2, women 5.5 kg/m2) (n = 937) | |||||
| Normal, n (%) | 474 (50.6) | 265 (47.4) | 209 (55.3) | −56 (7.9) | 0.019 |
| Abnormal, n (%) | 463 (49.4) | 294 (52.6) | 169 (44.7) | −125 (−7.9) | |
| Ultrasound rectus femoris, mean ± SD | |||||
| Cross-sectional area, cm2 (n = 869) | 3.80 ± 1.37 | 4.09 ± 1.42 | 3.33 ± 1.13 | −0.76 ± 0.1 | <0.0001 |
| X-axis, mm, (n = 979) | 37.16 ± 5.87 | 38.65 ± 5.73 | 34.99 ± 5.39 | −3.66 ± 0.4 | <0.0001 |
| Y-axis, mm (n = 981) | 10.45 ± 3.54 | 11.10 ± 3.80 | 9.51 ± 2.89 | −1.59 ± 0.2 | <0.0001 |
| X-axis/Y-axis ratio, mm (n = 979) | 3.93 ± 1.35 | 3.88 ± 1.42 | 4.0 ± 1.26 | 0.12 ± 0.1 | 0.18 |
| Total fat tissue, mm (n = 940) | 7.09 ± 4.73 | 5.44 ± 3.38 | 9.41 ± 5.35 | 3.97 ± 0.3 | <0.0001 |
| TUG, s, mean ± SD (n = 829) | 13.65 ± 7.70 | 12.53 ± 6.64 | 15.21 ± 8.73 | 2.68 ± 0.5 | <0.0001 |
| EWGSOP2 cut-off ≥ 20 s in men and women, (n = 829) | |||||
| Normal, n (%) | 696 (84.0) | 426 (88.4) | 270 (77.8) | −156 (−10.6) | 0.0005 |
| Abnormal, n (%) | 133 (16.0) | 56 (11.6) | 77 (22.2) | 21 (10.6) | |
| Biochemical data, mean ± SD | |||||
| Albumin, g/dL (n = 925) | 3.45 ± 0.76 | 3.45 ± 0.73 | 3.45 ± 0.81 | 0 ± 0.1 | 0.977 |
| Prealbumin, mg/dL (n = 677) | 17.89 ± 8.22 | 17.77 ± 8.47 | 18.07 ± 7.86 | 0.30 ± 0.5 | 0.638 |
| C-reactive protein (CRP), mg/L (n = 905) | 45.56 ± 65.97 | 48.0 ± 63.8 | 42.1 ± 68.9 | −5.9 ± 4.3 | 0.185 |
| CPR/prealbumin ratio (n = 659) | 5.15 ± 12.62 | 5.90 ± 13.14 | 4.09 ± 11.78 | −1.81 ± 0.8 | 0.07 |
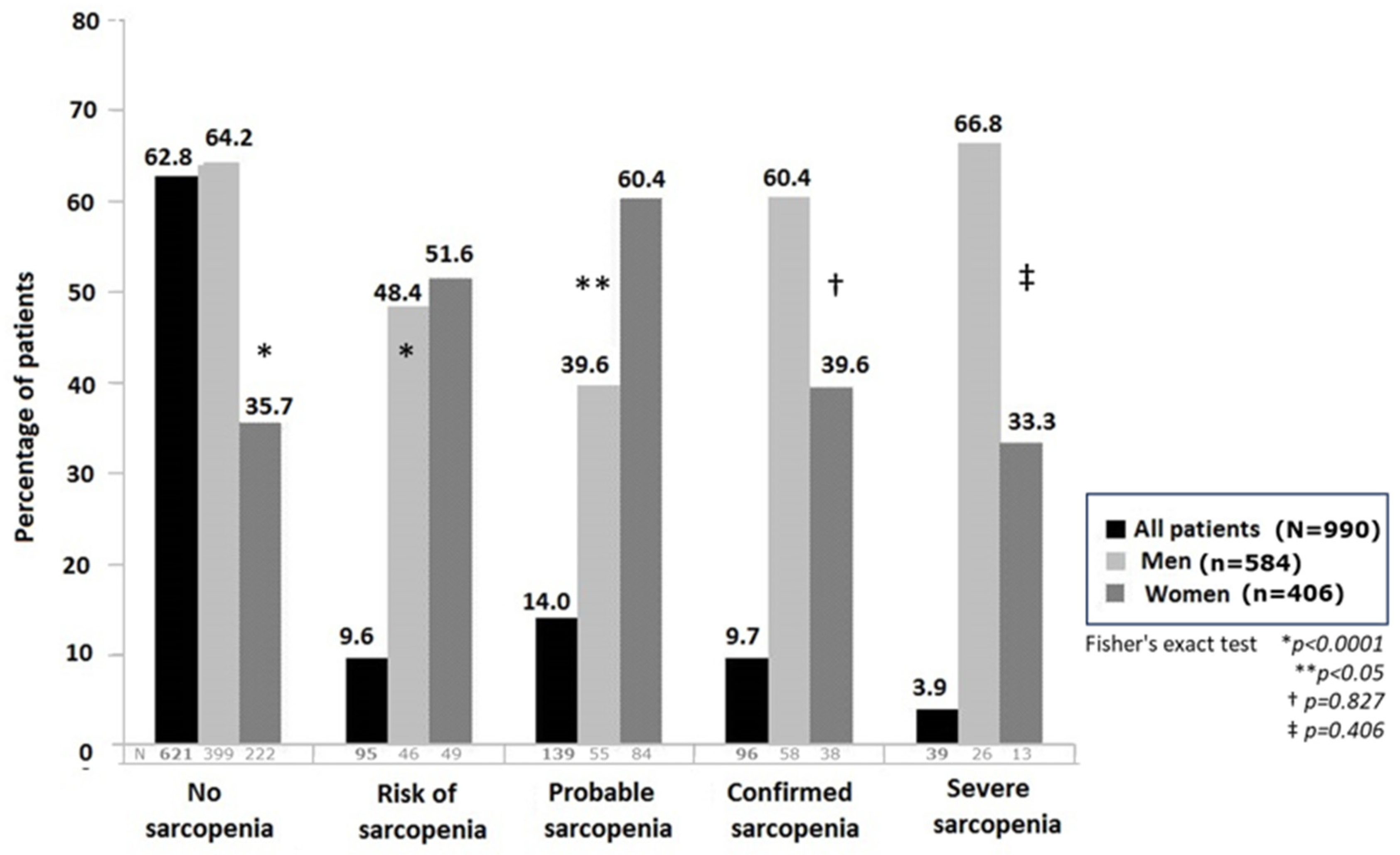
| Categories | All Patients (n = 990) | Men (n = 584) | Women (n = 406) | Difference (n = 990) |
|---|---|---|---|---|
| Sarcopenia, n (%) | ||||
| Absence | 621 (62.8) | 399 (68.3) | 222 (54.7) | −177 (−13.6) |
| At risk | 95 (9.6) | 46 (7.9) | 49 (12.1) | 3 (4.2) |
| Probable | 139 (14.0) | 55 (9.4) | 84 (20.7) | 29 (11.3) |
| Confirmed | 96 (9.7) | 58 (9.9) | 38 (9.4) | −20 (−0.5) |
| Severe | 39 (3.9) | 26 (4.5) | 13 (3.2) | −13 (−1.3) |
| Variables | CSA cm2 | X-Axis mm | Y-Axis mm | Handgrip Strength, kg | BIA, Body Cell Mass, kg | TUG, s |
|---|---|---|---|---|---|---|
| CSA, cm2 | - | n = 867 rho = 0.624 p < 0.001 | n = 869 rho = 0.788 p < 0.001 | n = 850 rho = 0.426 p < 0.001 | n = 822 rho = 0.519 p < 0.001 | n = 738 rho = −0.290 p < 0.001 |
| X-axis, mm | n = 867 rho = 0.624 p < 0.001 | - | n = 979 rho = 0.393 p < 0.001 | n = 955 rho = 0.411 p < 0.001 | n = 924 rho = 0.368 p < 0.001 | n = 822 rho = −0.246 p < 0.001 |
| Y-axis, mm | n = 869 rho = 0.788 p < 0.001 | n = 979 rho = 0.393 p < 0.001 | - | n = 957 rho = 0.391 p < 0.001 | n = 926 rho = 0.548 p < 0.001 | n = 823 rho = −0.340 p < 0.001 |
| Handgrip strength, kg | n = 850 rho = 0.425 p < 0.001 | n = 955 rho = 0.411 p < 0.001 | n = 957 rho = 0.391 p < 0.001 | - | n = 912 rho = 0.633 p < 0.001 | n = 815 rho = −0.466 p < 0.001 |
| BIA, body cell mass, kg | n = 822 rho = 0.519 p < 0.001 | n = 924 rho = 0.368 p < 0.001 | n = 926 rho = 0.548 p < 0.001 | n = 912 rho = 0.633 p < 0.001 | - | n = 786 rho = −0.300 p < 0.001 |
| TUG, s | n = 738 rho = −0.290 p < 0.001 | n = 822 rho = −0.242 p < 0.001 | n = 823 rho = −0.340 p < 0.001 | n = 815 rho = −0.466 p < 0.001 | n = 786 rho = −0.300 p < 0.001 | - |
| Variables | Sarcopenia Category | Study Patients | Cut-Off Value | AUC | Sensitivity % | Specificity % | Predictive Values | |
|---|---|---|---|---|---|---|---|---|
| Positive % | Negative % | |||||||
| Cross-sectional area (CSA), cm2 | Risk of sarcopenia | All patients | 3.37 | 0.629 | 58.5 | 61.5 | 45.8 | 72.7 |
| Men | 3.48 | 0.647 | 56.4 | 66.6 | 42.4 | 77.8 | ||
| Women | 2.97 | 0.556 | 50.0 | 62.5 | 51.7 | 60.8 | ||
| Probable sarcopenia | All patients | 3.37 | 0.634 | 64.4 | 59.1 | 28.7 | 86.6 | |
| Men | 3.48 | 0.700 | 66.7 | 66.9 | 35.9 | 87.8 | ||
| Women | 3.37 | 0.548 | 70.0 | 41.5 | 20.9 | 86.2 | ||
| Confirmed sarcopenia | All patients | 3.66 | 0.680 | 81.0 | 49.5 | 20.0 | 94.3 | |
| Men | 3.48 | 0.777 | 81.4 | 66.4 | 26.9 | 85.9 | ||
| Women | 2.4 | 0.483 | 89.1 | 16.3 | 14.8 | 90.2 | ||
| Severe sarcopenia | All patients | 3.41 | 0.669 | 78.1 | 55.3 | 6.4 | 98.5 | |
| Men | 3.41 | 0.818 | 95.2 | 66.6 | 10.8 | 99.7 | ||
| Women | 3.12 | 0.597 | 72.7 | 49.7 | 4.8 | 98.1 | ||
| X-axis, mm | Risk of sarcopenia | All patients | 37.37 | 0.583 | 58.3 | 56.0 | 44.3 | 69.1 |
| Men | 40.1 | 0.579 | 68.6 | 45.8 | 37.2 | 75.7 | ||
| Women | 37.41 | 0.534 | 72.5 | 35.0 | 48.3 | 60.3 | ||
| Probable sarcopenia | All patients | 33.55 | 0.610 | 37.6 | 79.3 | 34.5 | 80.4 | |
| Men | 40.21 | 0.634 | 77.4 | 46.0 | 30.7 | 86.8 | ||
| Women | 32.57 | 0.620 | 51.2 | 73.7 | 34.1 | 84.9 | ||
| Confirmed sarcopenia | All patients | 38.3 | 0.579 | 73.3 | 46.7 | 18.3 | 91.5 | |
| Men | 38.3 | 0.687 | 76.2 | 59.8 | 24.6 | 93.6 | ||
| Women | 34.41 | 0.584 | 74.5 | 43.4 | 16.4 | 91.9 | ||
| Severe sarcopenia | All patients | 38.3 | 0.613 | 76.9 | 45.4 | 5.7 | 97.8 | |
| Men | 37.82 | 0.725 | 76.9 | 62.4 | 9.2 | 98.2 | ||
| Women | 37.69 | 0.579 | 53.8 | 67.9 | 5.8 | 97.8 | ||
| Y-axis, mm | Risk of sarcopenia | All patients | 9.59 | 0.628 | 56.9 | 63.5 | 48.3 | 71.2 |
| Men | 9.66 | 0.652 | 55.7 | 70.2 | 46.6 | 77.2 | ||
| Women | 8.57 | 0.563 | 48.9 | 65.1 | 53.9 | 60.4 | ||
| Probable sarcopenia | All patients | 9.59 | 0.645 | 62.4 | 61.2 | 31.8 | 84.7 | |
| Men | 9.66 | 0.691 | 64.2 | 70.0 | 39.8 | 86.4 | ||
| Women | 7.85 | 0.583 | 44.0 | 73.7 | 30.8 | 83.2 | ||
| Confirmed sarcopenia | All patients | 9.66 | 0.686 | 71.9 | 59.4 | 22.3 | 92.8 | |
| Men | 9.66 | 0.775 | 78.6 | 69.7 | 30.8 | 94.9 | ||
| Women | 10.4 | 0.534 | 74.5 | 35.4 | 14.7 | 90.3 | ||
| Severe sarcopenia | All patients | 8.77 | 0.716 | 74.4 | 67.6 | 9.0 | 98.4 | |
| Men | 8.65 | 0.801 | 80.8 | 77.3 | 14.9 | 98.8 | ||
| Women | 8.77 | 0.558 | 61.5 | 56.0 | 4.6 | 97.7 | ||
| X/Y axis ratio | Risk of sarcopenia | All patients | 5.19 | 0.598 | 89.9 | 25.3 | 60.0 | 66.7 |
| Men | 4.63 | 0.624 | 35.7 | 83.8 | 50.7 | 73.5 | ||
| Women | 4.95 | 0.552 | 24.7 | 86.6 | 60.81 | 57.8 | ||
| Probable sarcopenia | All patients | 4.63 | 0.598 | 37.1 | 79.9 | 35.0 | 81.3 | |
| Men | 4.64 | 0.638 | 40.1 | 83.1 | 42.3 | 81.7 | ||
| Women | 4.95 | 0.533 | 27.4 | 83.8 | 31.1 | 81.2 | ||
| Confirmed sarcopenia | All patients | 4.19 | 0.661 | 60.0 | 68.3 | 23.5 | 91.2 | |
| Men | 4.66 | 0.708 | 52.4 | 84.0 | 36.1 | 91.1 | ||
| Women | 4.16 | 0.582 | 62.7 | 61.0 | 19.4 | 91.6 | ||
| Severe sarcopenia | All patients | 4.19 | 0.666 | 66.7 | 66.7 | 79.5 | 97.9 | |
| Men | 4.67 | 0.577 | 57.7 | 82.2 | 13.8 | 97.5 | ||
| Women | 4.26 | 0.602 | 69.2 | 63.0 | 6.08 | 98.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Luis Roman, D.; García Almeida, J.M.; Bellido Guerrero, D.; Guzmán Rolo, G.; Martín, A.; Primo Martín, D.; García-Delgado, Y.; Guirado-Peláez, P.; Palmas, F.; Tejera Pérez, C.; et al. Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk. Nutrients 2024, 16, 1552. https://doi.org/10.3390/nu16111552
de Luis Roman D, García Almeida JM, Bellido Guerrero D, Guzmán Rolo G, Martín A, Primo Martín D, García-Delgado Y, Guirado-Peláez P, Palmas F, Tejera Pérez C, et al. Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk. Nutrients. 2024; 16(11):1552. https://doi.org/10.3390/nu16111552
Chicago/Turabian Stylede Luis Roman, Daniel, José Manuel García Almeida, Diego Bellido Guerrero, Germán Guzmán Rolo, Andrea Martín, David Primo Martín, Yaiza García-Delgado, Patricia Guirado-Peláez, Fiorella Palmas, Cristina Tejera Pérez, and et al. 2024. "Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk" Nutrients 16, no. 11: 1552. https://doi.org/10.3390/nu16111552
APA Stylede Luis Roman, D., García Almeida, J. M., Bellido Guerrero, D., Guzmán Rolo, G., Martín, A., Primo Martín, D., García-Delgado, Y., Guirado-Peláez, P., Palmas, F., Tejera Pérez, C., García Olivares, M., Maíz Jiménez, M., Bretón Lesmes, I., Alzás Teomiro, C. M., Guardia Baena, J. M., Calles Romero, L. A., Prior-Sánchez, I., García-Luna, P. P., González Pacheco, M., ... Olveira, G. (2024). Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk. Nutrients, 16(11), 1552. https://doi.org/10.3390/nu16111552








