Bioelectrical Impedance Vector Analysis, Nutritional Ultrasound®, and Handgrip Strength as Innovative Methods for Monitoring Critical Anorexia Nervosa Physical Recovery: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Psychiatric and Nutritional Intervention in EDHU
2.3. Anthropometric Measurements
2.4. Bioelectrical Impedance Vector Analysis
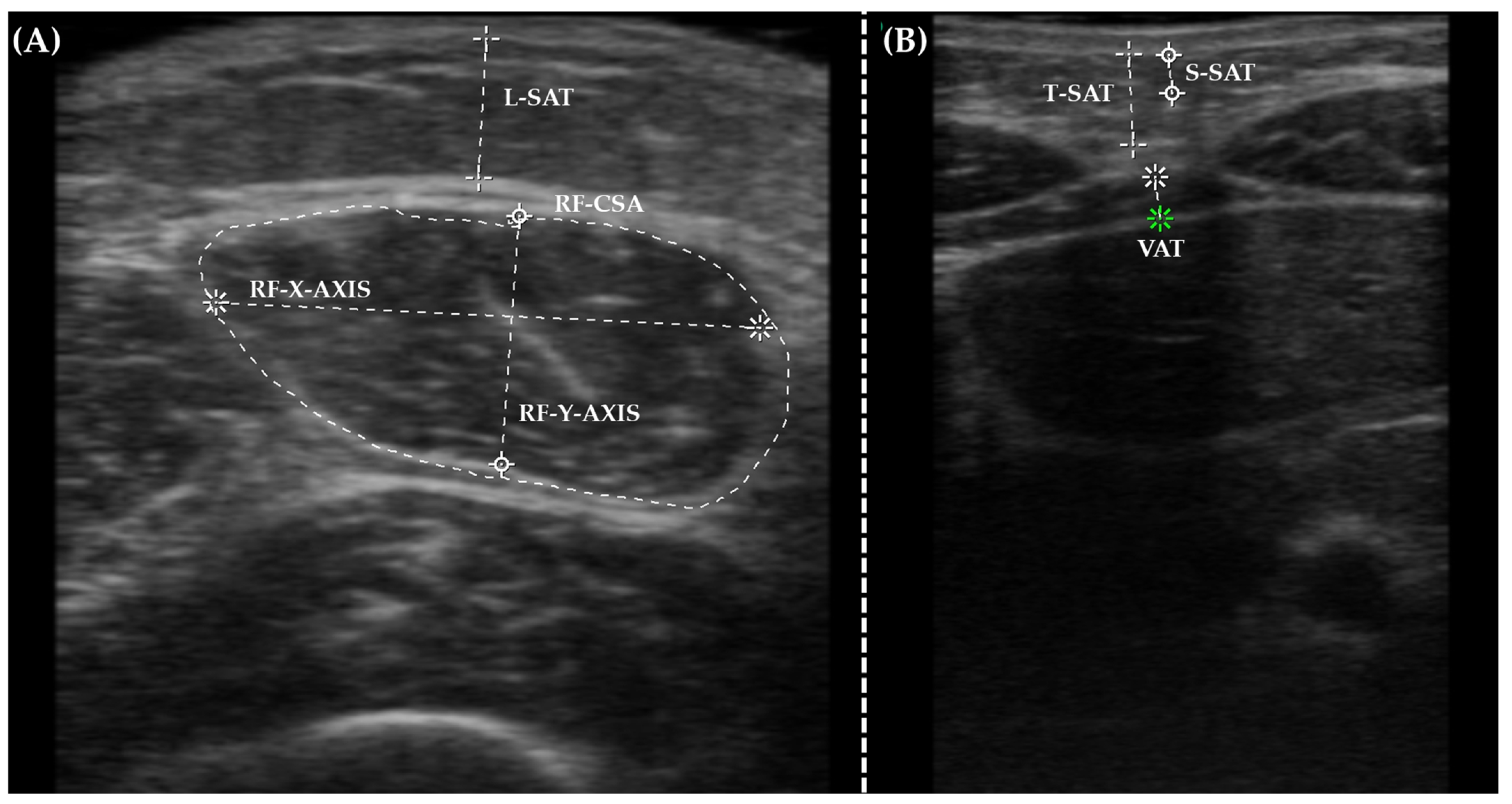
2.5. Nutritional Ultrasound®
2.6. Handgrip Strength Analysis
2.7. Biochemical Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Body Composition Analysis in Critical AN Patients
3.2. Pearson’s Correlation Matrix Analysis
3.3. Partial Least Squares-Discriminant Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteleone, A.M.; Pellegrino, F.; Croatto, G.; Carfagno, M.; Hilbert, A.; Treasure, J.; Wade, T.; Bulik, C.; Zipfel, S.; Hay, P.; et al. Treatment of Eating Disorders: A Systematic Meta-Review of Meta-Analyses and Network Meta-Analyses. Neurosci. Biobehav. Rev. 2022, 142, 104857. [Google Scholar] [CrossRef] [PubMed]
- Murray Hurtado, M.; Martín Rivada, Á.; Quintero Alemán, C.; Ruiz Alcántara, M.P.; Ramallo Fariña, Y. Body Composition and Nutritional Status Changes in Adolescents with Anorexia Nervosa. An. Pediatría (Engl. Ed.) 2023, 99, 162–169. [Google Scholar] [CrossRef] [PubMed]
- López-Gil, J.F.; Jiménez-López, E.; Fernández-Rodríguez, R.; Garrido-Miguel, M.; Victoria-Montesinos, D.; Gutiérrez-Espinoza, H.; Tárraga-López, P.J.; Mesas, A.E. Prevalence of Disordered Eating and Its Associated Factors From a Socioecological Approach Among a Sample of Spanish Adolescents: The EHDLA Study. Int. J. Public Health 2023, 68, 1605820. [Google Scholar] [CrossRef] [PubMed]
- Eddy, K.T.; Tabri, N.; Thomas, J.J.; Murray, H.B.; Keshaviah, A.; Hastings, E.; Edkins, K.; Krishna, M.; Herzog, D.B.; Keel, P.K.; et al. Recovery From Anorexia Nervosa and Bulimia Nervosa at 22-Year Follow-Up. J. Clin. Psychiatry 2017, 78, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bardone-Cone, A.M.; Hunt, R.A.; Watson, H.J. An Overview of Conceptualizations of Eating Disorder Recovery, Recent Findings, and Future Directions. Curr. Psychiatry Rep. 2018, 20, 79. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Alliance (UK). Eating Disorders: Recognition and Treatment; National Institute for Health and Care Excellence: Guidelines; National Institute for Health and Care Excellence (NICE): London, UK, 2017. [Google Scholar]
- Zeeck, A.; Herpertz-Dahlmann, B.; Friederich, H.-C.; Brockmeyer, T.; Resmark, G.; Hagenah, U.; Ehrlich, S.; Cuntz, U.; Zipfel, S.; Hartmann, A. Psychotherapeutic Treatment for Anorexia Nervosa: A Systematic Review and Network Meta-Analysis. Front. Psychiatry 2018, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Plank, L.D. Dual-Energy X-Ray Absorptiometry and Body Composition. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Albano, D.; Gitto, S.; Tofanelli, L.; Bazzocchi, A.; Ulivieri, F.M.; Guglielmi, G.; Sconfienza, L.M. Body Composition with Dual Energy X-Ray Absorptiometry: From Basics to New Tools. Quant. Imaging Med. Surg. 2020, 10, 1687–1698. [Google Scholar] [CrossRef]
- Carrera, O.; Adan, R.A.H.; Gutierrez, E.; Danner, U.N.; Hoek, H.W.; van Elburg, A.A.; Kas, M.J.H. Hyperactivity in Anorexia Nervosa: Warming Up Not Just Burning-Off Calories. PLoS ONE 2012, 7, e41851. [Google Scholar] [CrossRef]
- Silva, A.M.; Campa, F.; Stagi, S.; Gobbo, L.A.; Buffa, R.; Toselli, S.; Silva, D.A.S.; Gonçalves, E.M.; Langer, R.D.; Guerra-Júnior, G.; et al. The Bioelectrical Impedance Analysis (BIA) International Database: Aims, Scope, and Call for Data. Eur. J. Clin. Nutr. 2023, 77, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Bellido Guerrero, D.; Bretón Lesmes, I.; Tinahones Madueño, F.J. Nutritional Ultrasound®: Conceptualisation, Technical Considerations and Standardisation. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2023, 70 (Suppl. S1), 74–84. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torralvo, F.-J.; Porras, N.; Abuín-Fernández, J.; García-Torres, F.; Tapia, M.-J.; Lima, F.; Soriguer, F.; Gonzalo, M.; Rojo-Martínez, G.; Olveira, G.; et al. Valores de Normalidad de Dinamometría de Mano En España. Relación Con La Masa Magra. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Ellison, J.M.; Crosby, R.D.; Engel, S.G.; Mitchell, J.E.; Crow, S.J.; Peterson, C.B.; Le Grange, D.; Wonderlich, S.A. The Validity of DSM-5 Severity Specifiers for Anorexia Nervosa, Bulimia Nervosa, and Binge-Eating Disorder. Int. J. Eat. Disord. 2017, 50, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, E.; López-Plaza, B.; López-Sobaler, A.M.; Ortega, R.M. Prevalencia de Sobrepeso y Obesidad En Adultos Españoles. Nutr. Hosp. 2011, 26, 355–363. [Google Scholar] [PubMed]
- Prior-Sánchez, I.; Herrera-Martínez, A.D.; Zarco-Martín, M.T.; Fernández-Jiménez, R.; Gonzalo-Marín, M.; Muñoz-Garach, A.; Vilchez-López, F.J.; Cayón-Blanco, M.; Villarrubia-Pozo, A.; Muñoz-Jiménez, C.; et al. Prognostic Value of Bioelectrical Impedance Analysis in Head and Neck Cancer Patients Undergoing Radiotherapy: A VALOR® Study. Front. Nutr. 2024, 11, 1335052. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis-Part II: Utilization in Clinical Practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A New Method for Monitoring Body Fluid Variation by Bioimpedance Analysis: The RXc Graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, T.O.; de Magalhães Oliveira, F.; Kattah, F.M.; Pena, N.F.; Soares, M.M.S.; da Gama Torres, H.O. Body Composition and Energy Expenditure in Anorexia Nervosa: Preliminary Data of Outpatients with Recovering and Active Disease. J. Eat. Disord. 2022, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Reed, K.K.; Hübel, C.; Bulik-Sullivan, E.C.; Tang, Q.; Bulik, C.M.; Carroll, I.M. Comparison of Dual-Energy X-Ray Absorptiometry and Bioelectrical Impedance Analysis in the Assessment of Body Composition in Women with Anorexia Nervosa upon Admission and Discharge from an Inpatient Specialist Unit. Int. J. Environ. Res. Public Health 2021, 18, 11388. [Google Scholar] [CrossRef] [PubMed]
- Haas, V.; Riedl, A.; Hofmann, T.; Nischan, A.; Burghardt, R.; Boschmann, M.; Klapp, B. Bioimpedance and Bioimpedance Vector Analysis in Patients with Anorexia Nervosa. Eur. Eat. Disord. Rev. 2012, 20, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Himmerich, H.; Dalton, B.; Patsalos, O.; Schmidt, U.; Campbell, I.C. Cytokines and Water Distribution in Anorexia Nervosa. Mediat. Inflamm. 2021, 2021, 8811051. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, N.; Corey, M.; Rossi, M.F.; Goldberg, E.; Pencharz, P. Changes in Body Composition during Refeeding of Patients with Anorexia Nervosa. J. Pediatr. 1988, 113, 925–929. [Google Scholar] [CrossRef]
- Toutain, M.; Gauthier, A.; Leconte, P. Exercise Therapy in the Treatment of Anorexia Nervosa: Its Effects Depending on the Type of Physical Exercise-A Systematic Review. Front. Psychiatry 2022, 13, 939856. [Google Scholar] [CrossRef] [PubMed]
- Lackner, S.; Mörkl, S.; Müller, W.; Fürhapter-Rieger, A.; Oberascher, A.; Lehofer, M.; Bieberger, C.; Wonisch, W.; Amouzadeh-Ghadikolai, O.; Moser, M.; et al. Novel Approaches for the Assessment of Relative Body Weight and Body Fat in Diagnosis and Treatment of Anorexia Nervosa: A Cross-Sectional Study. Clin. Nutr. 2019, 38, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, E.; Ciccarese, F.; Di Pietro, E.; Facchini, G.; Moscano, F.; Iero, L.; Monaldi, A.; Battista, G.; Bazzocchi, A. Follow-up of Bone Mineral Density and Body Composition in Adolescents with Restrictive Anorexia Nervosa: Role of Dual-Energy X-ray Absorptiometry. Eur. J. Clin. Nutr. 2014, 68, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Soyka, L.A.; Miller, K.K.; Grinspoon, S.; Levitsky, L.L.; Klibanski, A. Regional Body Composition in Adolescents with Anorexia Nervosa and Changes with Weight Recovery. Am. J. Clin. Nutr. 2003, 77, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- de Alvaro, M.T.G.; Muñoz-Calvo, M.T.; Barrios, V.; Martínez, G.; Martos-Moreno, G.A.; Hawkins, F.; Argente, J. Regional Fat Distribution in Adolescents with Anorexia Nervosa: Effect of Duration of Malnutrition and Weight Recovery. Eur. J. Endocrinol. 2007, 157, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Caldwell, M.E.; Eddy, K.T.; Rutkove, S.B.; Breithaupt, L. Anorexia Nervosa and Muscle Health: A Systematic Review of Our Current Understanding and Future Recommendations for Study. Int. J. Eat. Disord. 2023, 56, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, S.; Sun, G.X.; Leung, S.-P.; Siddique, A.; Cooper, S.; Ezenwa, N.C.; Treasure, J.; Robinson, P. The Sit Up Squat Stand Test and Hand Grip Strength: What Is the Role of Tests of Muscle Power in Risk Assessment in Anorexia Nervosa? Eur. Eat. Disord. Rev. 2021, 29, 670–679. [Google Scholar] [CrossRef]
- Mueller, S.M.; Immoos, M.; Anliker, E.; Drobnjak, S.; Boutellier, U.; Toigo, M. Reduced Bone Strength and Muscle Force in Women 27 Years After Anorexia Nervosa. J. Clin. Endocrinol. Metab. 2015, 100, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Germain, N.; Genteuil, C.D.; Belleton, G.; Da Silva, T.L.; Exbrayat, C.; Degas, F.; Hammour, A.; Gay, A.; Ravey, B.; Massoubre, C.; et al. Continuous Glucose Monitoring Assessment in Patients Suffering from Anorexia Nervosa Reveals Chronic Prolonged Mild Hypoglycemia All over the Nycthemeron. Eur. Eat. Disord. Rev. 2023, 31, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Uotani, N.; Noma, S.; Akamine, M.; Miyawaki, T. Continuous Glucose Monitoring for Detection of Glycemic Variability, Hypoglycemia, and Hyperglycemia in Women with Eating Disorders. BioPsychoSocial Med. 2022, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Oh, E.S.; Lee, R.W.; Finucane, T.E. Serum Albumin and Prealbumin in Calorically Restricted, Nondiseased Individuals: A Systematic Review. Am. J. Med. 2015, 128, 1023.e1–1023.e22. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Hübel, C.; Hindborg, M.; Lindkvist, E.; Kastrup, A.M.; Yilmaz, Z.; Støving, R.K.; Bulik, C.M.; Sjögren, J.M. Increased Lipid and Lipoprotein Concentrations in Anorexia Nervosa: A Systematic Review and Meta-Analysis. Int. J. Eat. Disord. 2019, 52, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Dolezalova, R.; Lacinova, Z.; Dolinkova, M.; Kleiblova, P.; Haluzikova, D.; Housa, D.; Papezova, H.; Haluzik, M. Changes of Endocrine Function of Adipose Tissue in Anorexia Nervosa: Comparison of Circulating Levels versus Subcutaneous MRNA Expression. Clin. Endocrinol. 2007, 67, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Miller, K.K.; Mathur, V.A.; Misra, M.; Meenaghan, E.; Herzog, D.B.; Klibanski, A. Hormonal and Nutritional Effects on Cardiovascular Risk Markers in Young Women. J. Clin. Endocrinol. Metab. 2007, 92, 3089–3094. [Google Scholar] [CrossRef]
- Mariani, S.; di Giorgio, M.R.; Barbaro, G.; Contini, S.; Lubrano, C.; Donini, L.M.; Lenzi, A.; Gnessi, L. Inverse Association of Circulating SIRT1 and Adiposity: A Study on Underweight, Normal Weight, and Obese Patients. Front. Endocrinol. 2018, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Haluzíková, D.; Dostálová, I.; Kaválková, P.; Roubíček, T.; Mráz, M.; Papežová, H.; Haluzík, M. Serum Concentrations of Adipocyte Fatty Acid Binding Protein in Patients with Anorexia Nervosa. Physiol. Res. 2009, 58, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Miller, K.K.; Tsai, P.; Stewart, V.; End, A.; Freed, N.; Herzog, D.B.; Goldstein, M.; Riggs, S.; Klibanski, A. Uncoupling of Cardiovascular Risk Markers in Adolescent Girls with Anorexia Nervosa. J. Pediatr. 2006, 149, 763–769.e1. [Google Scholar] [CrossRef] [PubMed]
- Caso, J.R.; MacDowell, K.S.; Soto, M.; Ruiz-Guerrero, F.; Carrasco-Díaz, Á.; Leza, J.C.; Carrasco, J.L.; Díaz-Marsá, M. Dysfunction of Inflammatory Pathways and Their Relationship with Psychological Factors in Adult Female Patients with Eating Disorders. Front. Pharmacol. 2022, 13, 846172. [Google Scholar] [CrossRef] [PubMed]
- Kells, M.R.; Roske, C.; Watters, A.; Puckett, L.; Wildes, J.E.; Crow, S.J.; Mehler, P.S. Vitamin D and Hypophosphatemia in Patients with Anorexia Nervosa and Avoidant/Restrictive Food Intake Disorder: A Case Control Study. J. Eat. Disord. 2023, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Casper, R.C. Might Starvation-Induced Adaptations in Muscle Mass, Muscle Morphology and Muscle Function Contribute to the Increased Urge for Movement and to Spontaneous Physical Activity in Anorexia Nervosa? Nutrients 2020, 12, 2060. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Coëffier, M.; Jésus, P.; Charles, J.; Rimbert, A.; Déchelotte, P.; Grigioni, S. Bone Mineral Density after Weight Gain in 160 Patients with Anorexia Nervosa. Front. Nutr. 2017, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.P.; Robinson, L.; Stubbs, B.; dos Santos Alvarenga, M.; Araújo Martini, L.; Campbell, I.C.; Schmidt, U. Associations between Bone Mineral Density, Body Composition and Amenorrhoea in Females with Eating Disorders: A Systematic Review and Meta-Analysis. J. Eat. Disord. 2022, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial Least Squares-Discriminant Analysis (PLS-DA) for Classification of High-Dimensional (HD) Data: A Review of Contemporary Practice Strategies and Knowledge Gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef] [PubMed]

| EDHU Admission | 95% CI Admission | EDHU Discharge | 95% CI Discharge | p-Value | |
| Anthropometry | |||||
| Weight (kg) | 37.4 (4.5) | 28–47.1 | 42.8 (4.5) | 32.1–51.9 | <0.001 |
| BMI (kg/m2) | 14.3 (1.5) | 11.4–17.3 | 16.3 (1.1) | 13.1–18.6 | <0.001 |
| AC (cm) | 18.2 (6.1) | 14.18–21.5 | 19.9 (9.2) | 16–23 | <0.001 |
| CC (cm) | 27.8 (11.2) | 20–31 | 29.1 (14.3) | 23–33 | 0.052 |
| TST (mm) | 4.4 (2.7) | 1.2–8.5 | 5.7 (3.3) | 1.5–11 | <0.01 |
| BIVA | |||||
| PhA (°) | 4.8 (0.7) | 3.3–6 | 4.7 (0.5) | 3.5–5.9 | 0.972 |
| FM (kg) | 3.5 (2) | 1.7–10 | 5.3 (2.7) | 1.7–12.6 | <0.001 |
| FFM (kg) | 33.9 (3.8) | 26.2–42.6 | 37.5 (4.1) | 25.8–47.9 | <0.001 |
| TBW (L) | 26.1 (2.7) | 19.6–31.9 | 29.2 (7.3) | 21.4–71.6 | <0.05 |
| ECW (L) | 13.3 (2.0) | 9.6–18 | 15 (4.4) | 8.9–38.4 | <0.05 |
| BCM (kg) | 15.8 (2.5) | 10.2–20.9 | 17.4 (2.2) | 11.3–21.8 | <0.01 |
| TMM (kg) | 18.1 (2.5) | 13.3–23 | 19.2 (2.8) | 14.2–27.6 | 0.110 |
| ASMM (kg) | 12.5 (1.7) | 9–16.2 | 13.4 (1.9) | 10–18 | <0.05 |
| Functional measurement | |||||
| HGS max (kg) | 21.6 (9.1) | 8–35 | 25.9 (12.3) | 14–37 | <0.05 |
| Nutritional Ultrasound® | |||||
| RF-CSA (cm2) | 3.2 (1.5) | 1.4–4.0 | 3.7 (1.3) | 3–4.4 | 0.284 |
| RF-X-axis (cm) | 3.4 (1.6) | 2.9–4.0 | 3.2 (1.1) | 2.9–3.4 | 0.750 |
| RF-Y-axis (cm) | 1.2 (0.55) | 0.8–1.7 | 1.5 (0.5) | 1.3–1.7 | 0.413 |
| L-SAT (cm) | 0.4 (0.26) | 0.1–1.1 | 0.7 (0.27) | 0.4–1.3 | 0.270 |
| T-SAT (cm) | 0.5 (0.3) | 0.2–1.1 | 0.9 (0.3) | 0.7–1.3 | <0.05 |
| S-SAT (cm) | 0.3 (0.2) | 0.1–0.8 | 0.4 (0.2) | 0.3–0.6 | 0.074 |
| VAT (cm) | 0.3 (0.1) | 0.1–0.5 | 0.4 (0.1) | 0.2–0.6 | 0.154 |
| Biochemical analysis | |||||
| Glucose (mg/dL) | 69.4 (27.5) | 41–85 | 76.7 (14.5) | 56–93 | <0.01 |
| Creatinine (mg/dL) | 0.7 (0.3) | 0.5–1.1 | 0.6 (0.2) | 0.5–0.9 | 0.051 |
| Proteins (g/dL) | 6.6 (2.7) | 4.5–8.5 | 6.8 (2.2) | 4–8.4 | 0.541 |
| Albumin (mg/dL) | 4.3 (1.7) | 3.1–5.7 | 4.4 (1.2) | 3.3–5.7 | 0.731 |
| Prealbumin (mg/dL) | 29.8 (21.0) | 16–101 | 27.3 (7.2) | 20–37 | 0.488 |
| CPR (mg/L) | 4.3 (8.6) | 0.2–32 | 0.5 (0.5) | 0.2–3.2 | <0.05 |
| Total cholesterol (mg/dL) | 160.9 (98.4) | 0.2–403 | 174.1 (60.2) | 1.5–269 | 0.373 |
| Triglycerides (mg/dL) | 96.6 (59) | 30–280 | 70 (41.5) | 21–205 | <0.05 |
| Calcium (mg/dL) | 9 (3.4) | 7.8–10.4 | 9.1 (2.4) | 7.7–9.9 | 0.455 |
| Phosphorus (mg/dL) | 3.6 (1.6) | 1.9–5.3 | 4.3 (0.8) | 3.5–5.2 | <0.001 |
| Magnesium (mg/dL) | 2.0 (0.8) | 1.8–2.9 | 1.9 (0.5) | 1.6–2.3 | 0.099 |
| Potassium (mg/dL) | 4.2 (1.7) | 3.6–5 | 4.2 (0.8) | 1.4–5.3 | 0.790 |
| Anthropometry | BIVA | Functional | Ultrasound | ||||
| Weight | BMI | FM | BCM | HGS Max | RF-CSA | T-SAT | |
| Anthropometry | |||||||
| Weight (kg) | 1 ** | 0.510 ** | 0.424 ** | 0.789 ** | 0.386 * | - | - |
| BMI (kg/m2) | 0.510 ** | 1 ** | 0.593 ** | 0.400 ** | - | - | −0.842 * |
| AC (cm) | 0.414 * | 0.516 ** | 0.430 * | - | - | - | −0.937 ** |
| CC (cm) | - | - | - | - | - | - | - |
| TST (mm) | - | 0.430 * | 0.589 ** | - | −0.414 * | - | - |
| BIVA | |||||||
| PhA (°) | - | 0.491 ** | 0.424 ** | 0.554 ** | - | - | - |
| FM (kg) | 0.424 ** | 0.593 ** | 1 ** | - | −0.372 * | - | - |
| FFM (kg) | 0.805 ** | - | - | 0.818 ** | 0.658 ** | 0.883 * | - |
| TBW (L) | 0.351 * | - | - | 0.365 ** | 0.359 * | - | - |
| ECW (L) | 0.318 * | - | −0.365 * | - | 0.399 * | - | - |
| BCM (kg) | 0.789 * | 0.400 ** | - | 1 ** | 0.671 ** | - | - |
| TMM (kg) | - | - | −0.435 ** | 0.356 ** | 0.473 ** | 0.966 ** | - |
| ASMM (kg) | - | - | −0.406 ** | 0.387 ** | 0.482 ** | 0.965 ** | - |
| Functional parameters | |||||||
| HGS max (kg) | 0.386 * | - | −0.372 * | 0.671 ** | 1 ** | - | - |
| Time spent on the unit (days) | - | - | −0.347 * | - | - | - | - |
| Nutritional Ultrasound® | |||||||
| RF-CSA (cm2) | - | - | - | - | - | 1 ** | - |
| RF-X-axis (cm) | - | - | - | - | - | - | - |
| RF-Y-axis (cm) | - | −0.828 * | - | - | - | - | 0.933 ** |
| L-SAT (cm) | - | - | - | - | - | - | - |
| T-SAT (cm) | - | −0.842 * | - | - | - | - | 1 ** |
| S-SAT (cm) | - | - | - | - | - | - | - |
| VAT (cm) | - | - | - | - | - | - | - |
| Biochemical analysis | |||||||
| Glucose (mg/dL) | - | - | - | - | - | - | - |
| Creatinine (mg/dL) | - | - | - | - | - | - | 0.918 ** |
| Proteins (g/dL) | - | - | 0.364 * | - | - | - | - |
| Albumin (mg/dL) | −0.351 * | - | - | −0.324 * | - | - | - |
| Prealbumin (mg/dL) | - | - | - | - | - | - | - |
| CPR (mg/L) | −0.424 ** | −0.373 * | - | −0.460 ** | - | - | - |
| Total cholesterol (mg/dL) | - | - | - | - | - | - | - |
| Triglycerides (mg/dL) | −0.369 * | −0.352 * | - | −0.365 ** | - | - | - |
| Calcium (mg/dL) | - | - | 0.331 * | - | - | - | - |
| Phosphorus (mg/dL) | - | - | - | - | - | - | - |
| Magnesium (mg/dL) | 0.342 * | - | - | - | - | - | - |
| Potassium (mg/dL) | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Márquez, J.M.; Novo-Rodríguez, M.; Novo-Rodríguez, C.; Siles-Guerrero, V.; Herrera-Montes, I.; Navarro-Pelayo, F.G.; López-de-la-Torre-Casares, M.; Muñoz-Garach, A. Bioelectrical Impedance Vector Analysis, Nutritional Ultrasound®, and Handgrip Strength as Innovative Methods for Monitoring Critical Anorexia Nervosa Physical Recovery: A Pilot Study. Nutrients 2024, 16, 1539. https://doi.org/10.3390/nu16101539
Romero-Márquez JM, Novo-Rodríguez M, Novo-Rodríguez C, Siles-Guerrero V, Herrera-Montes I, Navarro-Pelayo FG, López-de-la-Torre-Casares M, Muñoz-Garach A. Bioelectrical Impedance Vector Analysis, Nutritional Ultrasound®, and Handgrip Strength as Innovative Methods for Monitoring Critical Anorexia Nervosa Physical Recovery: A Pilot Study. Nutrients. 2024; 16(10):1539. https://doi.org/10.3390/nu16101539
Chicago/Turabian StyleRomero-Márquez, Jose M., María Novo-Rodríguez, Cristina Novo-Rodríguez, Víctor Siles-Guerrero, Isabel Herrera-Montes, Francisco Garzón Navarro-Pelayo, Martín López-de-la-Torre-Casares, and Araceli Muñoz-Garach. 2024. "Bioelectrical Impedance Vector Analysis, Nutritional Ultrasound®, and Handgrip Strength as Innovative Methods for Monitoring Critical Anorexia Nervosa Physical Recovery: A Pilot Study" Nutrients 16, no. 10: 1539. https://doi.org/10.3390/nu16101539
APA StyleRomero-Márquez, J. M., Novo-Rodríguez, M., Novo-Rodríguez, C., Siles-Guerrero, V., Herrera-Montes, I., Navarro-Pelayo, F. G., López-de-la-Torre-Casares, M., & Muñoz-Garach, A. (2024). Bioelectrical Impedance Vector Analysis, Nutritional Ultrasound®, and Handgrip Strength as Innovative Methods for Monitoring Critical Anorexia Nervosa Physical Recovery: A Pilot Study. Nutrients, 16(10), 1539. https://doi.org/10.3390/nu16101539







