Dietary (Poly)phenols and the Gut–Brain Axis in Ageing
Abstract
1. Introduction
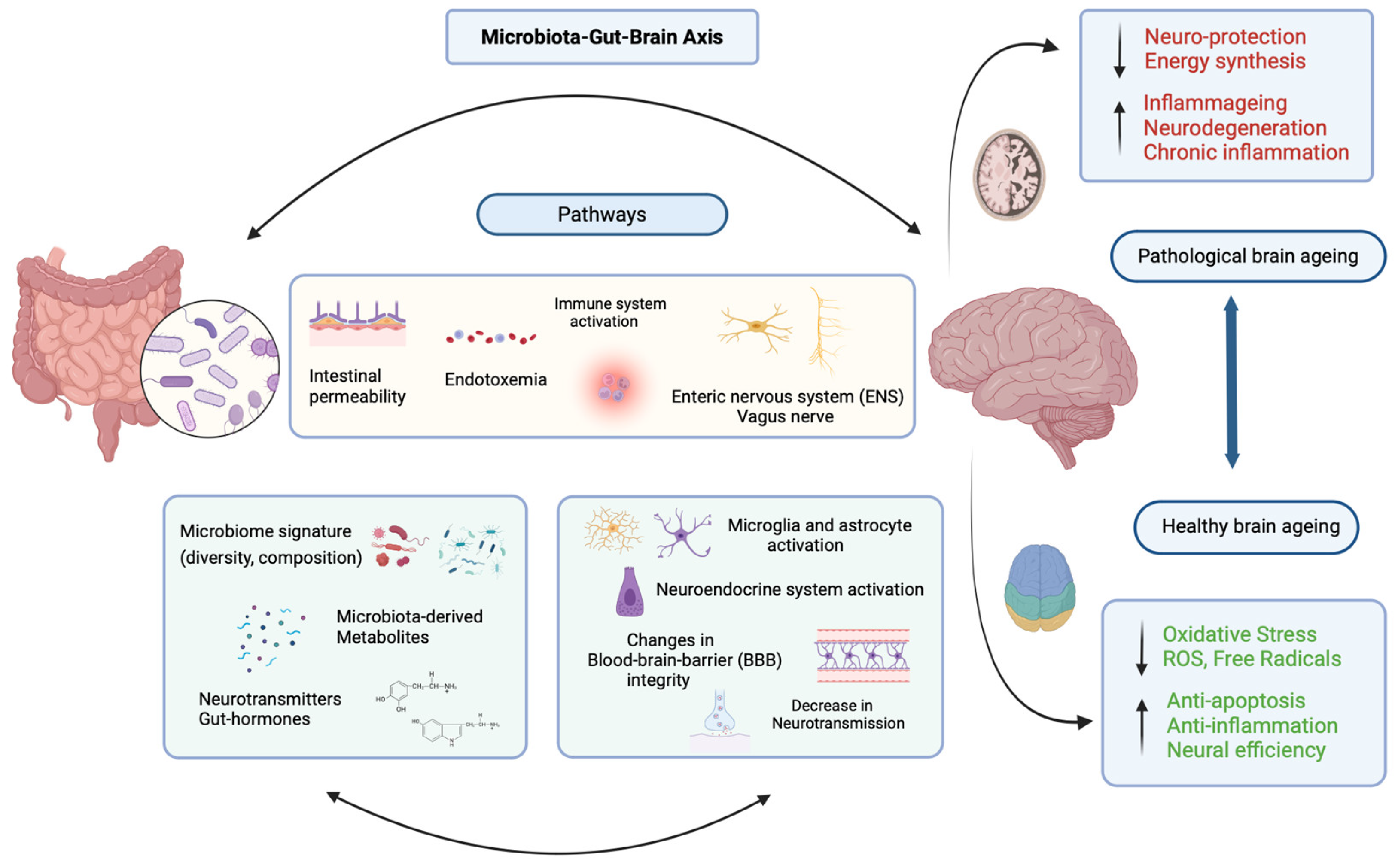
2. The Microbiota–Gut–Brain Axis and Ageing
2.1. The Gut Microbiome and Ageing
2.2. Gut Microbiota, Inflammation, and the Blood-Brain Barrier
2.3. The Gut–Brain Axis and Neurological Disorders
2.4. Diet, Gut Microbiota, and Gut–Brain Communication
3. (Poly)phenols, Gut–Microbiota–Brain Axis and Ageing
3.1. Preclinical Evidence
3.1.1. (Poly)phenols, Gut Microbiome Modulation and Related Brain Health
3.1.2. Insight into Associated Molecular Mechanisms
| Polyphenol Type | Study Model, Sample Size, Dose, and Duration | Changes in Microbiome Structure | Changes in Microbial Metabolites | Functions | Refs. |
|---|---|---|---|---|---|
| Isolated compounds | |||||
| Flavanols | |||||
| Epigallocatechin gallate (EGCG) | Female Sprague-Dawley (SD) rats (n = 60), 75 mg/kg, 150 mg/kg, and 300 mg/kg, 12 weeks | ↑ bacterial and species richness, ↓ Proteobacteria. ↑ abundance of Akkermansia and Bifidobacterium. ↓ Enterococcus and Escherichia-Shigella | NC | ↓ activation of microglia (↓ Iba-1 positive cells in hippocampus), ↓ TLR4/NF-κB signalling pathway (TLR4 and IRAK proteins) and ↓ IL-1β, IL-6 and TNF-α. ↓ LPS levels | [68] |
| Epigallocatechin gallate (EGCG) | Male C57BL/6 mice, GTP 0.1% (w/w), 4 weeks | ↑ Bacteroidetes and Actinobacteria. ↑ abundance of Lactobacillus. ↑ intestinal flora species | ↑ metabolic levels of daidzein and O-DMA, O-acetyl -L-carnitine, trans-caffeic acid and daidzein | ↑ regulation of astrocytes and oligodendrocytes, adjusted expression of core clock genes (Csnk1d, Clock, Per3, Cry2, and BhIhe41), ↑ lipid and amino acid metabolism | [107] |
| Eigallocatechin-3-gallate (EGCG) | Drosophila melanogaster male, n = 60, 0.1 mM or 0.5 mM EGCG, 3 days and 20 days | Normalized the microbial diversity, ↓ Proteobacteria, Acetobacter and Lactobacillus | NC | Improved locomotive functions, rescued ↓ life span | [112] |
| Epicatechin (EC) | C57BL/6J mice (n = 50), 2 or 20 mg/kg body weight, 24 weeks | ↑ Firmicutes, Acidobacteria, Bacteroidetes and Nitrospirae and ↓ Actinobacteria. ↑ alpha diversity (higher dose group) | Changed metabolomic profile (↑ organic acids, nucleotides, and fatty acid isobutyrate) ↓ Alanine, valerate, cytosine and citrate | No effects on recognition, spatial memory and learning, mitigated anxiety-related behaviour. ↑ BDNF mRNA levels (32% higher EC group), ↓ glucocorticoids in the brain. Correlation between metabolome, microbiome, and behaviour test (OF) | [113] |
| Flavonols | |||||
| Quercetin-3-O-glucuronide (Q3G) | Male C57BL/6J mice, n = 30, 50 mg per kg/day, 4 weeks | ↑ Barnesiella and Lactobacillus; ↓ Alistipes and Rikenella | ↑ Short-chain fatty acids (SCFAs) | Alleviated spatial memory impairment, ↓ Aβ accumulation, ↓ tau phosphorylation | [114] |
| Quercetin | Aged male ICR mice, n = 32, 0.08% quercetin, 21 days | ↑ gut microbiota α-diversity, ↓ Verrucomicrobia, Blautia and Anaerotruncus, ↑ Tenericutes | NC | Partial reversed of dAGEs-induced cognitive impairment, ↓ protein expression related to A-Beta generation and tau phosphorylation (Cathesin B and p-Tauser396 & 404) ↓ reactive astrocytes | [100] |
| Quercetin | Sprague Dawley rats, n = 30 10 mL/kg or of 50 mg/kg, 12 weeks | ↑ Actinobacteria, ↓ Porphyromonadaceae, Oxalobacteraceae, Oxalobacter and Klebsiella | NC | Prevented myelin and axonal damage, ↓ RO | [69] |
| Quercetin | Male C57BL/6 mice, n = 24 50 mg/kg, 7 days | ↑ intestinal permeability, normalized microbiome abundance | ↑ Short-chain fatty acids (SCFAs) acetate and propionate levels | ↓ neuropsychiatric problems, ↑ expression of occludin and doublecortin in frontal cortex and hippocampus, ↑ level of this tight junction protein, ↓ anxiety-like behaviour | [83] |
| Fisetin | Male C57BL/6 mice, n = 24, 100 ng/kg/bw/d, 30 days | ↑ Lachnospiraceae, ↓ Escherichia- Shigella and Bacillus | NC | Improved behavioural impairments, ↑ tyrosine hydroxylase | [73] |
| Icariin (Herba Epimedii) | Male C57BL/6J mice, n = 20 100 mg/kg, (15 days) | ↑ epithelial inflammation, offer a distinct taxonomic profile related to young mice in the old mice, ↑ SCFA-producing bacteria. ↑ beneficial bacterial profile | NC | ↑ motor learning and coordination in aged mice, ↑ SOD, ↓ H2O2 and ROS levels (Malonaldehyde (MDA) levels, ↑ Nrf2 activity ↓ expression of FOXO1 and ANXA3 in old mice | [74] |
| Flavones | |||||
| Baicalein, Scutellariae baicalensis Georgi | Male SAMP8 and SAMR1 mice, n = 42, 200 mg/kg/d, 6 weeks | ↓ Mucispirillum, Parabacteroides, Prevotella, Bacteroides, and Sutterella, ↑ Christensenellaceae | NC | ↓ grading score of senescence, ↑ cognitive functions, inhibited release of proinflammatory cytokines in the brain cortex. Christensenellaceae correlates positively with the recognition index | [75] |
| Baicalein, Scutellariae baicalensis Georgi | Male C57BL/6J mice, n = 135, 25,50 and 100 mg/kg, 7 days | Restored Firmicutes and Bacteroidetes ratio, ↑ Halomonas_smyrnensis ↓ Parabacteroides_johnsonii and Bacteroides_uniformis | ↓ TMA and TMAO plasma levels | ↑ recognition memory, spatial learning, and memory, ↑ brain functional connectivity, restored the hippocampal neuronal plasticity, ↓ pro-inflammatory cytokines | [76] |
| Isootientin (ISO) | Male PP/PS1 mice, n = 45, 25 mg/kg or 50 mg/kg, 2 months | Regulated abnormal gut microbiome, ↑ α-diversity in caecum. ↑ Mollicutes, Prevotellaceae, and Prevotellaceae UCG 001 bacteria | NC | ↓ β plaque deposition in cortex and hippocampus of AD Mice, TNF-α ↓, IL-6 ↓, IL-4 ↑, IL-10 ↑; iNOS ↓, COX-2 ↓, ROS ↓ | [102] |
| Apigenin | Male Sprague Dawley rats, n = 36, 20 mg/kg, 10 days | Mitigated stress-induced dysbiosis of gut microbiota, regulated composition, and abundance of gut microbiota | NC | ↑ intestinal barrier function (expression levels of occludin and ZO-1), reversed mast cell and microglial activation, inhibited the activation of mast cells, prevented microglial activation | [77] |
| Vitexin, millet-derived flavonoid | Male C57BL/6 mice, n = 24, Low dose 10 mg/kg, high dose 30 mg/kg + HF diet, 4 weeks | ↑ α-diversity, reversed HF diet microbiome alterations, ↑ Akkermansia, ↓ Lachnospiraceae | NC | ↓ expression level of inflammatory cytokines in brain and intestine (TNF-a and IL-1B), improve oxidative stress and blood lipids parameters. ↓ MDA levels, ↑ GHS | [101] |
| Phenolic compounds | |||||
| Curcumin | APP/PS1 double transgenic mice, n = 15, High group 200 mg/kg body weight, low group 50 mg/kg, 3 months | ↓ Bacteroidaceae, Prevotellaceae, and Lactobacillaceae and ↑ Rikenellaceae | ↑ demethylcurcumin (M1) and bisdemethoxycurcumin metabolites | ↑ spatial learning and memory, ↓ Aβ pathology in the hippocampus | [70] |
| Curcumin | C57BL/6 mice, n = 30, 100 mg/g/day, 7 days | Restored levels of Bacteroidetes and Deinococcus-Thermus, ↑ Muribaculaceae | Changed metabolites related to glycerophospholipid metabolism, specific regulation of 1-butylimidazole and tryptophan by curcumin | Alleviated anxiety-like behaviours restored lipid metabolism, ↑ phosphatidylcholine in the prefrontal cortex | [71] |
| Sesamol, (Sesamum indicum) | Male C57BL/6 ApoE knockout mice (ApoE−/−), n = 60, 100 mg/kg/bw, 8 weeks | ↑ gut bacteria producing short-chain fatty acids (SCFAs), ↑ Bacillales, Fusobacterium and Lactococus | ↑ SCFA acetate | ↑ synapse ultrastructure and inhibited Aβ accumulation, prevented gut barrier damages and systemic inflammation, ↑ cognition | [115] |
| Sesamol, (Sesamum indicum) | APPswe/PS1dE9 AD mice, n = 24, 100 mg/kg/bw, 8 weeks | Reshaped gut microbiota composition, ↑ Rikenellaceae and Bifidobacterium (slight change), ↓ H. hepaticus, Clostridium, and Bacillaceae | ↑ SCFAs acetate, propionate, isobutyrate, butyrate, and valerate | Inhibited plaque deposition in cortex and hippocampal CA1, repressed expression of APP and β-secretase (Bace1), ↓ neuroinflammation (TNF-a and IL-B) | [72] |
| p-coumaric acid (PCA) | Male C57BL/6 J Low-dose (20 mg·kg−1), high-dose (40 mg·kg−1), 28 days | Corrected gut microbiota abnormalities | Regulated arachidonic acid, tyrosine metabolism, and unsaturated fatty acid biosynthesis, glycolysis/glycogenesis and glycerophospholipid (neuroinflammation and energy metabolism) | ↓ Aβ1–42, p-Tau proteins in the brain, ↓ ROS and MDA, ameliorated neuroinflammation | [103] |
| Chlorogenic acid (CGA) | Male C57 BL/6J mice, n = 40, 30 mg/kg/day, 11 days | ↑ Lactobacillus, Firmicutes, ↓ Bacteroides microbiome-related neurotransmitters | ↓ kynurenic and quinolinic acid (KYN and Quin) | Alleviated TMT-induced epilepsy-like seizure and cognitive impairment, ↓ hippocampal neuronal degeneration and neuroinflammation. ↑ levels of SCFAs (propionic and isobutyric acid) in hippocampus. ↑ DL-kynurenine and acetylcholine chloride | [111] |
| Chicoric acid (CA) (Echinacea purpurea) | C57BL/6J mice, n = 60, 60 mg kg−1, 12 days | ↓ microbial dysbiosis, ↓ Bacteroidetes and Parabacteroide, ↑ Firmicutes, Lactobacillus and Ruminiclostridium. ↑ Lachnospiraceae, Lactobacillus, Riminiclostridium and Lachnoclostridium | Restored normal SCFA production | Better motor performance, ↓ TNF-α and IL-1β in the serum, striatum and colon, ↓ neuroinflammation and gut integrity. ↑ expression of BDNF and GDNF, prevented neurotrophic suppression, Promoted colonic epithelial integrity | [116] |
| Corylin, Psoraleae Fructus | Female C57BL/6J mice, n = 72, low dose 10 mg/kg/day, medium 30 mg/kg/day, and high 90 mg/kg/day, 4 days | ↓ Bacteroides and Escherichia-Shigella, ↑ Enterorhabdus and Candidatic_Stoqueficus and ↓ Turicibacter. Key bacterial type maintained by Corylin were Muribacylaceae, Dubosiella and Lactobacillus | NC | Maintained BBB structural and functional integrity, ↓ neuroinflammation and ↑ expression of TJ proteins. ↑ 5-HT, 5-HTP, and Trp levels. Dose dependent effects. Ameliorate colon damage and inflammatory response (↓ IL-6 and TNF-α), reversed bacterial composition and diversity | [108] |
| Stilbenes | |||||
| Resveratrol (Res) | C57BL/6 mice, n = 55, 30/mg/day, 8 weeks | ↑ Ruminoclostridium, Odoriabacter, Prevotellaceae, Rikenellaceae, Alistipes and Blautia, ↓ Fimicutes/Bacteroidetes, ↓ Lachnospiraceae, Ruminococcaceae, Lactobacillus, Lachnospiraceae and Akkermansia. | NC | Alleviated mice phenotype from PD progression. ↑ motor functions, ↑ intestinal transit rate, alleviated dopaminergic neurodegeneration, ↓ relative abundance of inflammatory cytokines (TNF-α, IL-6 and IL-1β) | [117] |
| Polyphenols-rich extracts and Botanicals | |||||
| Blackberry anthocyanin-rich extract (BE) | Male Wistar rats, n = 24, 25 mg/kg/body weight/day, 17 weeks | Restored changes in gut dysbiosis induced by HF diet, ↑ Rumminococcus, Pseudoflavonifractor, Sporobacter and ↓ Oscillobacter | ↓ tryptophan | ↓ LPS, ↓ Tryptophan positively correlated to TCK-1 expression in the hippocampus, ↑ | [78] |
| Blueberry anthocyanin-rich extracts (BAE) | Male C57BL/6 mice, n = 24, 100 mg/kg body weight/day or 200 mg/kg/body weight/day, 8 weeks | ↑ Bifidobacterium, Lactobacillus, Roseburia, Faecalibaculum, Parabacteroides and Ruminiclostridium, and ↓ Staphylococcus | ↑ Short chain fatty acid (SCFA) butyrate | ↑ SOD and GSH-Px in the liver, ↑ 5-HT, ↑ dopamine, normalized neuron morphology | [79] |
| Polyphenol-rich blueberry-mulberry extract (BME), Vaccinium uliginosum L. and Muros nigra L. | Male C57BL/6J mice, n = 32, 300 mg/kg/d, 6 weeks | ↑ Lactobacillus, Streptococcus, Lactococcus, Corynebacteriaceae, Aerococcus, Enterococcus, Leuconostoc and Weisella, ↓ Blautia, Lachnoclostridium, Roseburia and Anaerotruncus. Restored beta diversity | ↑ 21 metabolites: fatty acids, amino acids, benzoid acids and indoles. Blautia positive correlation with methylcysteine, and negative with vanillic acid, Lactobacillus negative correlation with methylcysteine and positive with vanillic acid, Streptoccocus negatively correlated with methylcysteine and positively with EPA, linoleic acid and other fatty acids | Improved cognitive performance, ↓ neuronal loss, ↓ IL-6 and TNF-α levels in brain and intestine, ↑ levels of intestinal tight junction proteins (ZO-1 and occludin) | [86] |
| Anthocyanin rich extract Rubus idaeus (raspberries) | Male C57Bl/6J mice and APP/PS-1, n = 100, 100 mg/day (yellow or red raspberries), 24 weeks | ↓ Bacteroidetes, ↑ Proteobacteria, No changes in bacterial richness | NC | No difference in cognitive functions, no improvement in microvascular architecture, modulated endogenous metabolites in brain/plasma | [118] |
| Grape-derived bioactive dietary polyphenol preparation (BDPP) | C57BL6/J male mice, n = 122, Grape seed extract 200 mg/kg/bw, resveratrol 400 mg/kg BW, grape juice polyphenol content 183 mg/kg BW, 13 days | ↑ microbial α-diversity | NC | BDPP restored the SD-induced memory impairment. microbiota dysbiosis ↓ efficacy of dietary polyphenols, ↓ bioavailability of BDPP-derived phenolic acids | [119] |
| Flavanol-rich preparation (FRP) (cocoa) | Humanized gnotobiotic mice (FMT), n = 13, 40 mg FRP flavanol/kg BW/day, 15 days | Unique bacterial composition from human donors (HuA and HuB) with Bacteroides ovatus, Bacteroides thetaiotanomicron, Bacteroides uniformis and Eggerthella lenta | ↑ DHCA and 3-HPPA plasma levels, unique phenolic acid metabolites in the caecum | ↑ bioavailability of plasma-circulating DHCA and brain-accumulating 3-HPPA and 3,4-diHBA, linked to ↓ of Aβ misfolding, ↓ inflammation and ↑ in brain resilience | [120] |
| Citrus limon polyphenols (LPP) | Male SAMP1 mice, n = 36, 0.1% (w/v) LPP, throughout life | ↓ Bacteroidetes/Firmicutes ratio | NC | ↑ lifespan (3 weeks), ↓ ageing-related scores (e.g., peri-ophthalmic lesions) and locomotor atrophy | [121] |
| Coffee cherry husks (CCHP) | Female C57BL/6 J mice, n = 18, low dose 10 mg/kg, high dose, 30 mg/kg 0.5, 1, 7 days | ↑ Bacteroides and Bacteroidota, ↓ Allobaculum, Helicobacter, and Enterococcus | NC | Restored inflamed gut microbiome, ↓ TNF-α, IL-1β, IL-6 and Cox-2, inhibition of TLR4/Myd88/NF-κB signalling pathway | [122] |
| Hawthorn fruit | Female KM female mice, n = 72, 100, 200, 400 mg/kg/d, 35 days | ↑ Dubosiella, Alloprevotella, and Bifidobacterium ↓ Acinetobacter, Akkermansia, Lachnospiraceae_NK4A136_group, and Staphylococcus, ↑ richness and diversity | ↑ Docosapentaenoic acid (DPA), sphingolipid (SM), phosphatidylcholine (PC), phosphatidylethanolamine (LPE) and lysophospholipid (LPC), ↓ succinic acid, hexadecanedioic acid, tetradecanedioic acid, 2-butoxyacetic acid, l-ascorbic acid 2-sulfate, and (R)-3-hydroxy myristic acid | ↑ cognitive function, ↓ Aβ1–42 level in the hippocampus, inhibited abnormal activation of microglia, changed serum metabolites related to microbiota. ↓ MDA and ↑ enzymatic activity (SOD and GSH-Px) | [80] |
| Dendrobium nobile Lindl. (D. nobile) | Female Kunming mice, 200 mg/kg/bw, 8 weeks | Improved gut microbiota dysbiosis and reversed age-related changes in microbiome (reversed Firmicutes) | NC | ↑ SOD, CAT and GSH-Px activities in the blood, and SOD and GSH-Px activities in the brain (↑ antioxidant activities), regulates endocrine system pathway genes, improved pathological tissue changes, positively affects gene expression levels related to ageing | [81] |
| Peanut shell (PS), Arachis hypogaea L. fruit | Male ICR mice, n= 120, low 100 mg/kg/day, medium 300 mg/kg/day and high dose 900 mg/kg/day, 2 weeks | ↓ inflammatory response in the small intestine, ↑ alpha-diversity of gut microbiota, ↑ Lachnospiraceae | NC | ↓ depression-like symptoms, ↓ inflammatory responses in the brain, in serum, and in small intestine, regulation of gut barrier tight-junction proteins, ↓ IL-1β, IL-6, TNF-α, in cortex and hippocampus | [123] |
| Astragalus membranaceus | C57BL/6J mice, n = 26, high dose 50 mg/kg/day, medium dose 25 mg/kg/day, low dose 5 mg/kg/day, 16 weeks | ↑ species richness, ↑ butyrate-producing bacteria | NC | ↓ fasting blood glucose, ↓ Aβ aggregation in the brain, ↑ expression of PSD95 and synapsin, positively modified mitochondrial biogenesis in the hippocampus, protected BBB and gut barrier. ↓ inflammatory cytokines and LPS | [124] |
| Ficus pandurata Hance var. angustifolia, Ficus of Moraceae | Male C57BL/6J mice, n = 30, 0.1% (w/w), 4 weeks | ↓ Firmicutes. ↑ Aerococcus, Bifidobacterium, Faecalibacterium, Bacteroides, Akkermansia, Allobaculum, and Prevotella. ↓ Pediococcus | ↑ actinonin, 4-methylumbelliferone, genioin, decosahexaenoic acid ethyl ester (DHA-ee) and enoxacin and ↓ neuropathic metabolites (stearoylcarnitine, 2-monolinolein, 4-hydroxybutyric acid and benzenoids). ↓ secondary bile acids, cholesterol metabolism and isoflavonoid biosynthesis. ↑catecholamine transferase inhibitor, caffeine metabolism and isoflavone biosynthesis | Improved exploration and memory behaviours. ↑ intestinal barrier functions, ↑ expression of occludin, ↓ expression of Aβ in the hippocampus, ↓ IL-6 level | [84] |
| Seabuckthorn (Hippophae rhamnoides L.) | Male adult ICR mice, n = 24, 20 mg/kg/day or 100 mg/kg/day, 14 days | Normalized Firmicutes levels, ↓ Lactobacillaceae, ↑ Lachnospiraceae, regulated gut microbiome. ↑ α-diversity | NC | Restored CUMS-induced damage to the hippocampus, ↑ levels of neurotransmitters, ↑ levels of neurotrophins, ↓ expression of IL-1β, IL-6 and TNF-α in cortex and hippocampus. Positive correlation between Lachnospiraceae and neurotransmitters and negative with inflammation and stress-hormones | [125] |
| Acanthopanax senticosus (AS) | Male KM mice, n = 35, 250 mg/kg/d, 1, 3,7, 14 and 28 days | Changed microbiota composition, ↓ Helicobacter, ↑ Lactobacillus, Ruminococcaceae, Peptosreptococcaceae, Clostridiales_vadinBB60_group and Porphyromonodaceae | NC | Prevented learning and memory loss, ↑ tight junction protein, ↑ expression of BDNF and NF-κB, maintained hippocampal neurons, restored GABA levels, ↑ 5-HT. Positive correlation between 5-HT and Lactobacillus and Ruminococcaceae | [126] |
| Nopal (Opuntia ficus indica) | Wistar rats, n = 25, 5% nopal water content, 7 months | ↑ alpha diversity, ↑ Ruminococcus bromii, Rumminococcus flavefaciens, Lactobacillus reuteri, Bacteroides fragilis and Akkermansia muciniphila. ↓ Bacteroides acidifaciens, Blautia producta, Faecalibacterium prausnitzii, Butyricicoccus pullicaecorum and Clostridium citroniae | NC | Restored the mucus layer, improved cognitive functions, ↑ abundance of occludin, ↑ intestinal permeability, ↓ LPS serum levels, ↓ expression of proinflammatory genes Tnf-α, and NADPH oxidase, ↓ brain malondialdehyde (MDA) concentration, ↓expression of inflammation and oxidative stress in adipose tissues | [85] |
| Polyphenol blends/mixtures | |||||
| Xanthohumol, quercetin and phlorotannin extracts | Sprague Dawley rats, n = 54, X 10/mg/kg/day, Q 20 mg/kg/day and P 20/kg/day respectively, 8 weeks | Change in ß diversity, ↑ Enterorhabdus, Asteroplasma, Lachnospiraceae and Coprococcus | ↑ BCFAs, isobutyrate and valerate | Antidepressant and anxiolytic effects, ↓ corticosterone (xanthohumol), ↑ BDNF, 5-HT | [127] |
| Grape Seed Polyphenolic Extract (GSPE) and resveratrol | Male C57BL/6J mice, n = 36 GSPE, 0.4 g and resveratrol 0.4 g in water, 2 weeks | ↓ Firmicutes, ↑ Bacteroides, ↓ Clostridium, ↑ Parasutteralla and Akkermensia | NC | ↑ locomotor response, mitigated behavioural response to opioids | [128] |
| Chlorogenic acid (CGA) and epigallocatechin-3-gallate | Female ICR mice, n = 35, 20 mg kg−1 d−1 chlorogenic acid, 20 mg kg−1 d−1 EGCG, or 20 mg kg−1 d−1 chlorogenic acid plus 20 mg kg−1 d−1 EGCG, 8 weeks | ↓ Firmicutes/Bacteroides, ↓ Lactobacillaceae, Erysipelotrichaceae, Deferribacteraceae, ↑ Lachnospiraceae, Muribaculaceae, and Rikenellaceae | NC | ↓ gut permeability, ↓ endotoxemia and colon inflammation markers. Combination CGA plus EGCG recovered moving ability, ↓ gut inflammation, ↓ reactive oxygen species accumulation | [104] |
| Triphala polyherbal formulation (Emblica officinalis, Terminalia chebula, and Terminalia bellerica mixture) | APP/PS1 mice, n = 30, 250 mg/kg (extract) and 500 mg/kg (Powder)/day, 60 days | ↓ gut dysbiosis, ↑ Verrucomicrobia, Bacteroidetes, Proteobacteria, Actinobacteria | ↑ SCFAs acetic acid, propionic acid and butyric acid | ↑ cognitive functions, ↓ LPS level, anti-inflammatory parameters (TNF-α, IFN-γ and IL) | [129] |
| Triphala polyherbal formulation (Emblica officinalis, Terminalia chebula, and Terminalia bellerica mixture) | 5XFAD mice, n = 45, 500 mg/kg twice a day, 60 days | ↓ Cyanobacteria, ↑ gut transition time | ↑ SCFAs butyrate levels | ↑ learning and memory function, mitigates dysbiosis in prolonged antibiotics treatment | [130] |
| Quercetin and 2-hydroxypropyl-B-cyclodextrin | C57BL/6 J mice, n = 18, 40 mg/kg/d of Quercetin complex, 6 days | ↑ Firmicutes, ↓ Bacteroidota, reversed the changes in the relative abundance | NC | ↑ spontaneous activity behaviour and short-term memory ability as well as anxiety level, ↓ TNF-α and IL-6 levels, and ↓ intestinal and hippocampal inflammation | [131] |
| Hizikia fusiforme, Brown algae—Polyphenol Polyssacharide Complex (PPC) | Kunming mice, n = 108, 10 mg/mL PPC, 37 days | Changed intestinal flora diversity, ↑ Firmicute/Bacteroidetes (F/B) ratio | NC | Activate the Nrf2-ARE signal pathway, and related antioxidant pathways (Nqo1 and SOD1) in brain mice, ↓ MDA and improved LPO clearance rate | [82] |
| Polyphenol blend (citrus pulp, carrot, and spinach) and fish oil | Dogs, n = 40, Blend of test food with 106 mg/g polyphenols and lycopene 0.054 ppm, fish oil (0.5%), 30 days | ↑ Coxiellaceae, Blautia, Parabacteroides, Eubacterium, biforme, Acholeplasma, and Odoribacter. ↓ Flexispira and Gammaproteobacteria | ↓ 4-EPS and sphingomyelin levels in serum, ↑ azelate and choline in faeces | 4-Ethylphenyl sulphate negatively correlates with metabolites related to anxiety-like disorders | [132] |
| Symbiotic | |||||
| Grape-derived Bioactive dietary polyphenol preparation (BDPP) with Lactobacillus plantarum and Bifidobacterium longum | C57BL/6J male mice, n = 43, 1% w/v resveratrol, 1% w/v grape seed polyphenol extract, and 5% w/v concord grape extract | ↑ SCFAs producing bacteria | ↑ plasma and brain bioavailability of microbial-derived phenolic metabolites, ↑ polyphenolic and tryptophan metabolites | ↓ chronic-stress inflammatory responses (ileum and prefrontal cortex), ↑ brain resilience, ↓ IL-6, TLR, IL-1 (symbiotic) | [133] |
| Nanoparticles | |||||
| Chlorogenic acid (CGA), nano system with selenium | APP/PS1 transgenic mice, n = 40, 80 mg/kg body weight/day, 16 weeks | ↑ diversity and richness of gut microbiota. ↑ Turicibacter, Colidextribacter, Ruminococcus, Alloprevotella, and Alistipes. ↑ Bacteroidetes | NC | ↓ Aβ aggregate-related neuroinflammation and glucose homeostasis disorder in the brain, ↑cognitive impairment, ↓ oxidative stress | [134] |
| Polyphenol-armoured chitosan and tannic acid (CHI/TA) | Female C57BL/6J mice, n = 102, 1 mg mL−1, 8 days | Retains relative abundance of Lactobacillaceae, Muribaculaceae and Bifidobacteriaceae. ↓ Enterococcaceae. Prebiotic activities modulated gut microbiota diversity and homeostasis | NC | ↑ learning and cognitive abilities, ↓ anxiety-and depression-like behaviours and cognitive impairment. Inhibited expression of GABA receptors | [135] |
| Resveratrol (Res), selenium/chitosan | ICR mice, n = 40, 50 mg/kg body weight or 60 mg/kg body weight, 24 weeks | Regulation of Entercoccus, Colidextribacter, Rikenella, Ruminococcus, Candidatus_Saccharimonas, Alloprevotella and Lachnospiraceae_UCG-006. ↑ alpha-diversity. Regulated F/B ratio to normal levels, ↑ gut bacteria linked to ↓ antioxidation, lipid deposition and anti-inflammation | NC | Inhibited lipid deposition, ↓ oxidative stress and neuroinflammation, ameliorated glucose tolerance, ↓ MDA | [105] |
| Dietary pattern | |||||
| Pre-Hispanic Mexican diet (PMD), Diet rich in fibre, polyphenols, a healthy ratio of omega 6/omega 3 fatty acids, vegetable protein rich in β-carotenes, polyphenols, lycopene, | Male Sprague–Dawley rats, n = 24, Food combination (nopal, polyphenols, omega 3), 3 months | ↓ Fimicutes, ↑ Bacteroidetes, Bifidobacteria and Lactobacillus | NC | ↑ cognitive functions, ↓ glucose intolerance, serum and liver triglycerides and leptin, ↓ hepatic levels of ROS, oxidized proteins and GSSG/GSH ratio, ↓ MDA levels, ↓ adiponectin | [106] |
3.2. Clinical Evidence
| Polyphenol Type | Study Model, Sample Size, Dose, and Duration | Changes in Microbiome Structure | Changes in Microbial Metabolites | Functions | Refs. |
|---|---|---|---|---|---|
| Ellagitannins | |||||
| Polyphenols from walnuts | Parkinson’s disease patients, n = 52 30 g of walnuts, 3 days | ↑ Enterobacteriaceae, Desulfovibrionaceae, Lactobacillaceae, Enterococcaceae, Actinomycetaceae, and Olsenella in PD patients. ↓ Ruminococcaceae, Lachnospiraceae, Faecalibacterium, ↓ urolithin-producing bacteria | ↓ Urolithin production in PD patients, ↓ anti-inflammatory metabolites, ↓SCFA butyrate, ↑ p.cresol production | ↑ LPS, ↑ intestinal mucus breakdown, ↑ tyrosine degradation, correlation with health-related microbial biomarkers | [136] |
| Anthocyanins | |||||
| Wild blueberry (WB) | Dietary intervention, n = 61, 302 mg anthocyanins, 12 weeks | No change in gut microbiota composition | NC | ↑ vascular and cognitive function, ↓ 24 h ambulatory systolic BP | [143] |
| Flavanones | |||||
| Flavonoid-Rich Orange Juice | Depressive adults, n = 40, Daily 380 mL, 600 ± 5.4 mg flavonoids, 8 weeks | ↑ Lachnospiraceae, Eubacterium, Roseburia, Coprococcus, Agathobacter, Bifidobacterium and Bacteroides | NC | Serum BDNF level significantly positively correlated with abundance of the Lachnospiraceae, and Gemella with homocysteine levels and depression | [144] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murman, D.L. The Impact of Age on Cognition. Semin Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Álvarez-Mon, M.A.; García-Montero, C.; Fraile-Martínez, O.S.; Monserrat, J.; Martinez-Rozas, L.; Rodríguez-Jiménez, R.; Álvarez-Mon, M.; Lahera, G. Microbiota–gut–brain axis mechanisms in the complex network of bipolar disorders: Potential clinical implications and translational opportunities. Mol. Psychiatry 2023, 28, 2645–2673. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Sun, L.-J.; Li, J.-N.; Nie, Y.-Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Ahlman, H.; Nilsson, O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001, 12, S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2017, 57, 1–24. [Google Scholar] [CrossRef]
- Daniel, H. Diet and Gut Microbiome and the “Chicken or Egg” Problem. Front. Nutr. 2022, 8, 828630. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Sozio, C.; La Rosa, G.; Guida, B.; Faraonio, R.; Santillo, M.; Mondola, P. Metabolism Regulation and Redox State: Insight into the Role of Superoxide Dismutase 1. Int. J. Mol. Sci. 2020, 21, 6606. [Google Scholar] [CrossRef] [PubMed]
- Narduzzi, L.; Agulló, V.; Favari, C.; Tosi, N.; Mignogna, C.; Crozier, A.; Del Rio, D.; Mena, P. (Poly)phenolic compounds and gut microbiome: New opportunities for personalized nutrition. Microbiome Res. Rep. 2022, 1, 16. [Google Scholar] [CrossRef]
- Tomasova, L.; Grman, M.; Ondrias, K.; Ufnal, M. The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health. Nutr. Metab. 2021, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut–brain communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Hoyles, L. Human microbiome myths and misconceptions. Nat. Microbiol. 2023, 8, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Yang, Q.; Wang, X.; Bai, Y.; Liu, Z. Isolation and Cultivation of Human Gut Microorganisms: A Review. Microorganisms 2023, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.D.P.; Ayres, L.F.A.; Barreto, D.S.; Henriques, B.D.; Prado, M.R.M.C.; Passos, C.M.D. Acquisition of microbiota according to the type of birth: An integrative review. Rev. Lat.-Am. Enferm. 2021, 29, e3446. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, M.-X.; Abuduaini, R.; Yu, H.-Y.; Li, D.-H.; Wang, Y.-J.; Zhou, N.; Jiang, M.-Z.; Niu, P.-X.; Han, S.-S.; et al. Enlightening the taxonomy darkness of human gut microbiomes with a cultured biobank. Microbiome 2021, 9, 119. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Lawley, T.D.; Walker, A.W. Intestinal colonization resistance. Immunology 2012, 138, 1–11. [Google Scholar] [CrossRef]
- Rolhion, N.; Chassaing, B. When pathogenic bacteria meet the intestinal microbiota. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150504. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Broderick, N.A.; Buchon, N.; Lemaitre, B.; McFall-Ngai, M.J. Microbiota-Induced Changes in Drosophila melanogaster Host Gene Expression and Gut Morphology. mBio 2014, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Fiske, A.; Wetherell, J.L.; Gatz, M. Depression in Older Adults. Annu. Rev. Clin. Psychol. 2009, 5, 363–389. [Google Scholar] [CrossRef]
- Salazar, N.; Valdés-Varela, L.; González, S.; Gueimonde, M.; de los Reyes-Gavilán, C.G. Nutrition and the gut microbiome in the elderly. Gut Microbes 2016, 8, 82–97. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Meehan, C.J.; Koenig, J.E.; Dhanani, A.S.; Rose, R.A.; Howlett, S.E.; Beiko, R.G. Microbial shifts in the aging mouse gut. Microbiome 2014, 2, 50. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. Toward an improved definition of a healthy microbiome for healthy aging. Nat. Aging 2022, 2, 1054–1069. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood–brain Barrier: Structural Components and Function Under Physiologic and Pathologic Conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef]
- Al-Obaidi, M.M.J.; Desa, M.N.M. Mechanisms of Blood Brain Barrier Disruption by Different Types of Bacteria, and Bacterial–Host Interactions Facilitate the Bacterial Pathogen Invading the Brain. Cell. Mol. Neurobiol. 2018, 38, 1349–1368. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Hoyles, L.; Pontifex, M.G.; Rodriguez-Ramiro, I.; Anis-Alavi, M.A.; Jelane, K.S.; Snelling, T.; Solito, E.; Fonseca, S.; Carvalho, A.L.; Carding, S.R.; et al. Regulation of blood–brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome 2021, 9, 235. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Stachulski, A.V.; Knausenberger, T.B.A.; Shah, S.N.; Hoyles, L.; McArthur, S. A host–gut microbial amino acid co-metabolite, p-cresol glucuronide, promotes blood–brain barrier integrity in vivo. Tissue Barriers 2022, 11, 2073175. [Google Scholar] [CrossRef]
- Cirillo, G.; Negrete-Diaz, F.; Yucuma, D.; Virtuoso, A.; Korai, S.A.; De Luca, C.; Kaniusas, E.; Papa, M.; Panetsos, F. Vagus Nerve Stimulation: A Personalized Therapeutic Approach for Crohn’s and Other Inflammatory Bowel Diseases. Cells 2022, 11, 4103. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Ippolito, C.; Segnani, C.; Dolfi, A.; Errede, M.; Virgintino, D.; Fornai, M.; Antonioli, L.; Garelli, F.; Nericcio, A.; et al. Pathological remodelling of colonic wall following dopaminergic nigrostriatal neurodegeneration. Neurobiol. Dis. 2020, 139, 104821. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.; Antonini, A.; Colosimo, C.; Marconi, R.; Morgante, L.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, G.; Ceravolo, R.; et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 2009, 24, 1641–1649. [Google Scholar] [CrossRef]
- Bernal-Pacheco, O.; Limotai, N.; Go, C.L.; Fernandez, H.H. Nonmotor manifestations in Parkinson disease. Neurologist 2012, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Pahwa, R. The impact and management of nonmotor symptoms of Parkinson’s disease. Am. J. Manag. Care 2011, 17 (Suppl. S12), S308–S314. [Google Scholar]
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota–gut–brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 2022, 79, 80. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Micek, A.; Mena, P.; Del Rio, D.; Galvano, F.; Castellano, S.; Grosso, G. Dietary (Poly)phenols and Cognitive Decline: A Systematic Review and Meta-Analysis of Observational Studies. Mol. Nutr. Food Res. 2023, 68, 2300472. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Tomás-Barberán, F.A. Contribution of plant food bioactives in promoting health effects of plant foods: Why look at interindividual variability? Eur. J. Nutr. 2019, 58, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373. [Google Scholar] [CrossRef]
- Mulder, D.; Aarts, E.; Arias Vasquez, A.; Bloemendaal, M. A systematic review exploring the association between the human gut microbiota and brain connectivity in health and disease. Mol. Psychiatry 2023, 28, 5037–5061. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, Y.; Cheng, L.; Shi, M.; Li, X.; Zhang, L.; Zhao, H. Tea polyphenols ameliorates memory decline in aging model rats by inhibiting brain TLR4/NF-kappaB inflammatory signaling pathway caused by intestinal flora dysbiosis. Exp. Gerontol. 2021, 153, 111476. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Li, X.Y.; Wang, S.; Shen, L.; Ji, H.F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Y.; Chen, H.; Jiang, H.; Zhou, F.; Lv, B.; Xu, M. Curcumin Alleviates DSS-Induced Anxiety-Like Behaviors via the Microbial-Brain-Gut Axis. Oxidative Med. Cell. Longev. 2022, 2022, 6244757. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, T.; Xi, Y.; Li, L.; Mo, F.; Liu, X.; Liu, Z.; Gao, J.M.; Yuan, T. Sesamol Attenuates Amyloid Peptide Accumulation and Cognitive Deficits in APP/PS1 Mice: The Mediating Role of the Gut-Brain Axis. J. Agric. Food Chem. 2021, 69, 12717–12729. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Feng, Y.; Liu, T.; Wu, T.T.; Chen, Y.J.; Li, X.; Li, Q.; Wu, Y.C. Fisetin Regulates Gut Microbiota and Exerts Neuroprotective Effect on Mouse Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 549037. [Google Scholar] [CrossRef]
- Li, X.; Khan, I.; Xia, W.; Huang, G.; Liu, L.; Law, B.Y.K.; Yin, L.; Liao, W.; Leong, W.; Han, R.; et al. Icariin enhances youth-like features by attenuating the declined gut microbiota in the aged mice. Pharmacol. Res. 2021, 168, 105587. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, J.; Zhou, Y.; Huang, X.; Qin, X.; Du, G. Effects of Baicalein on Cortical Proinflammatory Cytokines and the Intestinal Microbiome in Senescence Accelerated Mouse Prone 8. ACS Chem. Neurosci. 2018, 9, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Wang, Y.; Si, C.; Wang, X.; Wang, R.T.; Lv, Z. Baicalin ameliorates neuropathology in repeated cerebral ischemia-reperfusion injury model mice by remodeling the gut microbiota. Aging 2020, 12, 3791–3806. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Peng, S.; Lin, M.; Duan, H.; Yuan, F.; Shao, M.; Tan, W.; Luo, H. Apigenin attenuates visceral hypersensitivity in water avoidance stress rats by modulating the microbiota-gut-brain axis and inhibiting mast cell activation. Biomed. Pharmacother. 2023, 167, 115562. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Bi, J.; Chen, Q.; Cui, H.; Bao, Y.; Tian, J.; Shu, C.; Wang, Y.; Tan, H.; Zhang, W.; et al. Effect of Blueberry Anthocyanin-Rich Extracts on Peripheral and Hippocampal Antioxidant Defensiveness: The Analysis of the Serum Fatty Acid Species and Gut Microbiota Profile. J. Agric. Food Chem. 2021, 69, 3658–3666. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, J.; Liu, R.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. Hawthorn flavonoid ameliorates cognitive deficit in mice with Alzheimer’s disease by increasing the levels of Bifidobacteriales in gut microbiota and docosapentaenoic acid in serum metabolites. Food Funct. 2022, 13, 12371–12382. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Luo, Y.; Lei, Y.; Long, W.; Wang, K.; Zhou, J.; Lei, M.; Yang, N.; Zou, H.; et al. Various Fractions of Alcoholic Extracts from Dendrobium nobile Functionalized Antioxidation and Antiaging in D-Galactose-Induced Aging Mice. Front. Biosci. (Landmark Ed.) 2022, 27, 315. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, Y.; Zhong, S.; Li, Y.; Di, Y.; Wang, Q.; Ren, D.; Liu, S.; Li, D.; Cao, F. Antioxidant and Anti-Aging Properties of Polyphenol-Polysaccharide Complex Extract from Hizikia fusiforme. Foods 2023, 12, 3725. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Bazaz, M.R.; Pasam, T.; Sharief, N.; Velip, L.; Samanthula, G.; Dandekar, M.P. Involvement of Microbiome Gut-Brain Axis in Neuroprotective Effect of Quercetin in Mouse Model of Repeated Mild Traumatic Brain Injury. Neuromol. Med. 2023, 25, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, J.; Liu, Y.; Zhang, X.; Cheng, K. Effects of Ficus pandurata Hance var. angustifolia Cheng Flavonoids on Intestinal Barrier and Cognitive Function by Regulating Intestinal Microbiota. Foods 2023, 12, 1682. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tapia, M.; Aguilar-Lopez, M.; Perez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, A.R.; Torres, N. Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci. Rep. 2017, 7, 4716. [Google Scholar] [CrossRef]
- Li, H.; Xiao, C.; Wang, F.; Guo, X.; Zhou, Z.; Jiang, Y. Blueberry-Mulberry Extract Alleviates Cognitive Impairment, Regulates Gut Metabolites, and Inhibits Inflammation in Aged Mice. Foods 2023, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4/NF-kappaB pathway activation. Food Nutr. Res. 2019, 63, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.T.; Ryu, S.; Shin, J.S.; Choi, J.H.; Park, H.J.; Lee, K.T. Euscaphic acid isolated from roots of Rosa rugosa inhibits LPS-induced inflammatory responses via TLR4-mediated NF-kappaB inactivation in RAW 264.7 macrophages. J. Cell. Biochem. 2012, 113, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Wong, X.; Madrid, A.M.; Tralma, K.; Castillo, R.; Carrasco-Pozo, C.; Navarrete, P.; Beltrán, C.; Pastene, E.; Gotteland, M. Polyphenol extracts interfere with bacterial lipopolysaccharide in vitro and decrease postprandial endotoxemia in human volunteers. J. Funct. Foods 2016, 26, 406–417. [Google Scholar] [CrossRef]
- Lundin, J.I.; Checkoway, H. Endotoxin and cancer. Environ. Health Perspect. 2009, 117, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.; Barr, L.C.; Wiscombe, S.; McAuley, D.F.; Simpson, A.J.; Rostron, A.J. Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation. Eur. Respir. J. 2020, 56, 1901298. [Google Scholar] [CrossRef] [PubMed]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Plotnikov, E.Y.; Silachev, D.N.; Zorova, L.D.; Pevzner, I.B.; Zorov, S.D.; Babenko, V.A.; Jankauskas, S.S.; Popkov, V.A.; Savina, P.S. Microbiota and mitobiota. Putting an equal sign between mitochondria and bacteria. Biochemistry 2014, 79, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biology 2021, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8, 10–1128. [Google Scholar] [CrossRef]
- Zhou, Y.; Blanco, L.P.; Smith, D.R.; Chapman, M.R. Bacterial amyloids. Methods Mol. Biol. 2012, 849, 303–320. [Google Scholar] [CrossRef]
- Luo, Y.P.; Tang, X.F.; Zhang, Y.C.; Chen, S.M.; Wu, Q.; Li, W.J. Epigallocatechin-3-gallate alleviates galactose-induced aging impairment via gut-brain communication. Food Funct. 2022, 13, 11200–11209. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, H.; Wang, G.; Zhong, X.H.; Shen, Q.L.; Zhang, X.J.; Li, R.Y.; Chen, L.H.; Zhang, Y.H.; Wan, Z. Quercetin is protective against short-term dietary advanced glycation end products intake induced cognitive dysfunction in aged ICR mice. J. Food Biochem. 2020, 44, e13164. [Google Scholar] [CrossRef]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.; Lv, H.; Chen, Y.; Zhang, C.; Guan, X. Vitexin alleviates high-fat diet induced brain oxidative stress and inflammation via anti-oxidant, anti-inflammatory and gut microbiota modulating properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tan, X.; Sun, X.; Wei, J.; Li, Q.X.; Wu, Z. Isoorientin Affects Markers of Alzheimer’s Disease via Effects on the Oral and Gut Microbiota in APP/PS1 Mice. J. Nutr. 2022, 152, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zeng, M.N.; Hao, F.X.; Hao, Z.Y.; Zhang, Z.K.; Liang, X.W.; Wu, Y.Y.; Zhang, Y.H.; Feng, W.S.; Zheng, X.K. P-coumaric acid ameliorates Abeta(25-35)-induced brain damage in mice by modulating gut microbiota and serum metabolites. Biomed. Pharmacother. 2023, 168, 115825. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Su, Z.; Mackenzie, G.G. Chlorogenic acid combined with epigallocatechin-3-gallate mitigates D-galactose-induced gut aging in mice. Food Funct. 2023, 14, 2684–2697. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Zheng, G.; Li, Z.; Mei, J. Resveratrol-loaded selenium/chitosan nano-flowers alleviate glucolipid metabolism disorder-associated cognitive impairment in Alzheimer’s disease. Int. J. Biol. Macromol. 2023, 239, 124316. [Google Scholar] [CrossRef] [PubMed]
- Avila-Nava, A.; Noriega, L.G.; Tovar, A.R.; Granados, O.; Perez-Cruz, C.; Pedraza-Chaverri, J.; Torres, N. Food combination based on a pre-hispanic Mexican diet decreases metabolic and cognitive abnormalities and gut microbiota dysbiosis caused by a sucrose-enriched high-fat diet in rats. Mol. Nutr. Food Res. 2017, 61, 1501023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, L.; Liu, Y.; Zhang, R.; Wu, Z.; Cheng, K.; Zhang, X. Omics Analyses of Intestinal Microbiota and Hypothalamus Clock Genes in Circadian Disturbance Model Mice Fed with Green Tea Polyphenols. J. Agric. Food Chem. 2022, 70, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Chen, L.H.; Xu, J.; Xu, Q.X.; Xu, W.; Yang, X.W. Corylin ameliorates chronic ulcerative colitis via regulating the gut-brain axis and promoting 5-hydroxytryptophan production in the colon. Phytomedicine 2023, 110, 154651. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, M.A.; Azcoitia, I.; Gonzalez-Burgos, I.; Garcia-Segura, L.M. Signaling mechanisms mediating the regulation of synaptic plasticity and memory by estradiol. Horm. Behav. 2015, 74, 19–27. [Google Scholar] [CrossRef]
- Brann, D.W.; Dhandapani, K.; Wakade, C.; Mahesh, V.B.; Khan, M.M. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids 2007, 72, 381–405. [Google Scholar] [CrossRef]
- Xi, Y.; Li, H.; Yu, M.; Li, X.; Li, Y.; Hui, B.; Zeng, X.; Wang, J.; Li, J. Protective effects of chlorogenic acid on trimethyltin chloride-induced neurobehavioral dysfunctions in mice relying on the gut microbiota. Food Funct. 2022, 13, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, M.; Xue, J.; Xiang, L.; Li, Y.; Xiao, J.; Xiao, G.; Wang, H.L. EGCG ameliorates neuronal and behavioral defects by remodeling gut microbiota and TotM expression in Drosophila models of Parkinson’s disease. FASEB J. 2020, 34, 5931–5950. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, Z.; Cremonini, E.; Le Gall, G.; Pontifex, M.G.; Muller, M.; Vauzour, D.; Oteiza, P.I. (-)-Epicatechin mitigates anxiety-related behavior in a mouse model of high fat diet-induced obesity. J. Nutr. Biochem. 2022, 110, 109158. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Huang, H.; Mo, X.; Zhu, Y.; Chen, X.; Li, X.; Peng, X.; Xu, Z.; Chen, L.; Rong, S.; et al. Quercetin-3-O-Glucuronide Alleviates Cognitive Deficit and Toxicity in Abeta(1-42) -Induced AD-Like Mice and SH-SY5Y Cells. Mol. Nutr. Food Res. 2021, 65, e2000660. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Chu, C.; Shi, R.; Cui, T.; Zhang, X.; Zhao, Y.; Shi, X.; Hui, Y.; Pan, J.; Qian, R.; et al. ApoE-Dependent Protective Effects of Sesamol on High-Fat Diet-Induced Behavioral Disorders: Regulation of the Microbiome-Gut-Brain Axis. J. Agric. Food Chem. 2019, 67, 6190–6201. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, B.N.; Hu, B.; Cheng, Y.L.; Guo, Y.H.; Qian, H. Neuroprotection of chicoric acid in a mouse model of Parkinson’s disease involves gut microbiota and TLR4 signaling pathway. Food Funct. 2022, 13, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; An, Y.; Xu, L.; Wang, Y.; Wang, C.; Li, P.; Li, M.; Yan, D.; Wang, M.; Zhong, G.; et al. The protective role of microbiota in the prevention of MPTP/P-induced Parkinson’s disease by resveratrol. Food Funct. 2023, 14, 4647–4661. [Google Scholar] [CrossRef] [PubMed]
- Alonso Torrens, A.; Mitchell, C.A.; Pourshahidi, L.K.; Murphy, B.O.; Allwood, W.; Rizzetto, L.; Scholz, M.; Tuohy, K.; Pereira-Caro, G.; Moreno-Rojas, J.M.; et al. Long-term supplementation with anthocyanin-rich or -poor Rubus idaeus berries does not influence microvascular architecture nor cognitive outcome in the APP/PS-1 mouse model of Alzheimer’s disease. Int. J. Food Sci. Nutr. 2023, 74, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Frolinger, T.; Sims, S.; Smith, C.; Wang, J.; Cheng, H.; Faith, J.; Ho, L.; Hao, K.; Pasinetti, G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019, 9, 3546. [Google Scholar] [CrossRef]
- Ho, L.; Zhao, D.; Ono, K.; Ruan, K.; Mogno, I.; Tsuji, M.; Carry, E.; Brathwaite, J.; Sims, S.; Frolinger, T.; et al. Heterogeneity in gut microbiota drive polyphenol metabolism that influences alpha-synuclein misfolding and toxicity. J. Nutr. Biochem. 2019, 64, 170–181. [Google Scholar] [CrossRef]
- Shimizu, C.; Wakita, Y.; Inoue, T.; Hiramitsu, M.; Okada, M.; Mitani, Y.; Segawa, S.; Tsuchiya, Y.; Nabeshima, T. Effects of lifelong intake of lemon polyphenols on aging and intestinal microbiome in the senescence-accelerated mouse prone 1 (SAMP1). Sci. Rep. 2019, 9, 3671. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Zhang, Y.; Lv, H.; Luo, L.; Wang, S.; Guan, X. Polyphenolic Extracts of Coffee Cherry Husks Alleviated Colitis-Induced Neural Inflammation via NF-kappaB Signaling Regulation and Gut Microbiota Modification. J. Agric. Food Chem. 2022, 70, 6467–6477. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.X.; Xia, T.C.; Peng, Z.T.; Wu, Q.Y.; Zhu, Y.; Dong, T.T.; Tsim, K.W. The ethanolic extract of peanut shell attenuates the depressive-like behaviors of mice through modulation of inflammation and gut microbiota. Food Res. Int. 2023, 168, 112765. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, T.; Gu, J.; Wang, Z.; Lin, J.; Wang, R.; Duan, T.; Li, Z.; Dong, R.; Wang, W.; et al. Intake of flavonoids from Astragalus membranaceus ameliorated brain impairment in diabetic mice via modulating brain-gut axis. Chin. Med. 2022, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.X.; Gao, A.X.; Zhu, Y.; Dong, T.T.; Tsim, K.W. Flavonoids from Seabuckthorn (Hippophae rhamnoides L.) restore CUMS-induced depressive disorder and regulate the gut microbiota in mice. Food Funct. 2023, 14, 7426–7438. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yin, Y.; Qin, Y.; Li, T.; Zeng, D.; Ju, T.; Duan, F.; Zhang, Y.; Lu, W. Acanthopanax senticosus extract alleviates radiation-induced learning and memory impairment based on neurotransmitter-gut microbiota communication. CNS Neurosci. Ther. 2023, 29 (Suppl. S1), 129–145. [Google Scholar] [CrossRef] [PubMed]
- Donoso, F.; Egerton, S.; Bastiaanssen, T.F.S.; Fitzgerald, P.; Gite, S.; Fouhy, F.; Ross, R.P.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology 2020, 116, 104673. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Hofford, R.S.; Meckel, K.R.; Dave, Y.A.; Zeldin, S.M.; Shipman, A.L.; Lucerne, K.E.; Trageser, K.J.; Oguchi, T.; Kiraly, D.D. Dietary polyphenols drive dose-dependent behavioral and molecular alterations to repeated morphine. Sci. Rep. 2023, 13, 12223. [Google Scholar] [CrossRef]
- Upadhyay, P.; Tyagi, A.; Agrawal, S.; Kumar, A.; Gupta, S. Bidirectional Effect of Triphala on Modulating Gut-Brain Axis to Improve Cognition in the Murine Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2023, e2300104. [Google Scholar] [CrossRef]
- Upadhyay, P.; Gupta, S. Dual mode of Triphala in the reversal of cognition through gut restoration in antibiotic mediated prolonged dysbiosis condition in 5XFAD mice. Exp. Neurol. 2023, 367, 114473. [Google Scholar] [CrossRef]
- Hu, J.; Jiao, W.; Tang, Z.; Wang, C.; Li, Q.; Wei, M.; Song, S.; Du, L.; Jin, Y. Quercetin inclusion complex gels ameliorate radiation-induced brain injury by regulating gut microbiota. Biomed. Pharmacother. 2023, 158, 114142. [Google Scholar] [CrossRef]
- Ephraim, E.; Brockman, J.A.; Jewell, D.E. A Diet Supplemented with Polyphenols, Prebiotics and Omega-3 Fatty Acids Modulates the Intestinal Microbiota and Improves the Profile of Metabolites Linked with Anxiety in Dogs. Biology 2022, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Caracci, F.; Zhao, D.; Wu, Q.L.; Frolinger, T.; Simon, J.; Pasinetti, G.M. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav. Immun. 2021, 91, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, G.; Wang, N.; Liang, H.; Li, C.; Wang, Y.; Cui, Y.; Yang, L. A Flower-like Brain Targeted Selenium Nanocluster Lowers the Chlorogenic Acid Dose for Ameliorating Cognitive Impairment in APP/PS1 Mice. J. Agric. Food Chem. 2023, 71, 2883–2897. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Qin, Q.; Xu, F.; Chen, Y.; Rao, S.; Wang, C.; Jiang, X.; Lu, X.; Xie, C. Oral polyphenol-armored nanomedicine for targeted modulation of gut microbiota-brain interactions in colitis. Sci. Adv. 2023, 9, eadf3887. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Fernandez-Villalba, E.; Gil-Martinez, A.L.; Cuenca-Bermejo, L.; Espin, J.C.; Herrero, M.T.; Selma, M.V. Urolithins: Potential biomarkers of gut dysbiosis and disease stage in Parkinson’s patients. Food Funct. 2022, 13, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Knausenberger, T.B.A.; Connell, E.; Gall, G.L.; Hardy, T.A.J.; Randall, D.W.; McCafferty, K.; Yaqoob, M.M.; Solito, E.; Müller, M.; et al. Cerebrovascular damage caused by the gut microbe-derived uraemic toxin p-cresol sulfate is prevented by blockade of the epidermal growth factor receptor. bioRxiv 2022. bioRxiv:11.12.516113. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Casamenti, F.; Stefani, M. Olive polyphenols: New promising agents to combat aging-associated neurodegeneration. Expert. Rev. Neurother. 2017, 17, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Luciano, M.; Corley, J.; Cox, S.R.; Valdés Hernández, M.C.; Craig, L.C.A.; Dickie, D.A.; Karama, S.; McNeill, G.M.; Bastin, M.E.; Wardlaw, J.M.; et al. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology 2017, 88, 449–455. [Google Scholar] [CrossRef]
- Tor-Roca, A.; Sánchez-Pla, A.; Korosi, A.; Pallàs, M.; Lucassen, P.J.; Castellano-Escuder, P.; Aigner, L.; González-Domínguez, R.; Manach, C.; Carmona, F.; et al. A Mediterranean Diet-Based Metabolomic Score and Cognitive Decline in Older Adults: A Case–Control Analysis Nested within the Three-City Cohort Study. Mol. Nutr. Food Res. 2023, 2300271. [Google Scholar] [CrossRef]
- Huhn, S.; Kharabian Masouleh, S.; Stumvoll, M.; Villringer, A.; Witte, A.V. Components of a Mediterranean diet and their impact on cognitive functions in aging. Front. Aging Neurosci. 2015, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.; Hein, S.; Mesnage, R.; Fernandes, F.; Abhayaratne, N.; Xu, Y.; Zhang, Z.; Bell, L.; Williams, C.; Rodriguez-Mateos, A. Wild blueberry (poly)phenols can improve vascular function and cognitive performance in healthy older individuals: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2023, 117, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, J.; Lee, H.J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Láng, L.; McArthur, S.; Lazar, A.S.; Pourtau, L.; Gaudout, D.; Pontifex, M.G.; Müller, M.; Vauzour, D. Dietary (Poly)phenols and the Gut–Brain Axis in Ageing. Nutrients 2024, 16, 1500. https://doi.org/10.3390/nu16101500
Láng L, McArthur S, Lazar AS, Pourtau L, Gaudout D, Pontifex MG, Müller M, Vauzour D. Dietary (Poly)phenols and the Gut–Brain Axis in Ageing. Nutrients. 2024; 16(10):1500. https://doi.org/10.3390/nu16101500
Chicago/Turabian StyleLáng, Léonie, Simon McArthur, Alpar S. Lazar, Line Pourtau, David Gaudout, Matthew G. Pontifex, Michael Müller, and David Vauzour. 2024. "Dietary (Poly)phenols and the Gut–Brain Axis in Ageing" Nutrients 16, no. 10: 1500. https://doi.org/10.3390/nu16101500
APA StyleLáng, L., McArthur, S., Lazar, A. S., Pourtau, L., Gaudout, D., Pontifex, M. G., Müller, M., & Vauzour, D. (2024). Dietary (Poly)phenols and the Gut–Brain Axis in Ageing. Nutrients, 16(10), 1500. https://doi.org/10.3390/nu16101500











