Abstract
Neurodevelopmental disorders appear to be rising in prevalence, according to the recent Global Burden of Disease Study. This rise is likely to be multi-factorial, but the role of certain nutrients known to facilitate neurodevelopment should be considered. One possible contributing factor could be attributed to deficits in choline intake, particularly during key stages of neurodevelopment, which includes the first 1000 days of life and childhood. Choline, a key micronutrient, is crucial for optimal neurodevelopment and brain functioning of offspring. The present narrative review discusses the main research, describing the effect of choline in neurodevelopmental disorders, to better understand its role in the etiology and management of these disorders. In terms of findings, low choline intakes and reduced or altered choline status have been reported in relevant population subgroups: pregnancy (in utero), children with autism spectrum disorders, people with attention deficit hyperactivity disorder and those with dyslexia. In conclusion, an optimal choline provision may offer some neuronal protection in early life and help to mitigate some cognitive effects in later life attributed to neurodevelopmental conditions. Research indicates that choline may act as a modifiable risk factor for certain neurodevelopmental conditions. Ongoing research is needed to unravel the mechanisms and explanations.
1. Introduction
Neurodevelopmental disorders (NDDs) are a class of disorders impacting brain development and function [1]. In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) NDDs are defined as a group of conditions with onset in the developmental period, inducing deficits that produce impairments of functioning [2]. Within this definition, NDDs consist of: autism spectrum disorder (ASD; a communication disorder); attention-deficit/hyperactivity disorder (ADHD); intellectual disabilities; neurodevelopmental motor disorders (including tic disorders) and specific learning disorders (including dyslexia) [2,3]. A high level of comorbidity also exists between conditions. For example, ASD and ADHD are known to have shared genetic heritability, with both being associated with social and executive functioning impairments [4]. Most individuals with ASD exhibit ADHD symptoms, and around 15–25% of ADHD individuals have ASD symptoms [5]. Similarly, research from twin studies found that there was more than an eightfold increase in the prevalence of NDDs (termed ‘neurodevelopmental disorders and problems’ in this study) in individuals with dyslexia, compared with typical readers [6].
The global burden of NDDs appears to be rising, as demonstrated in Figure 1. Both ASD and ADHD are reported to have risen in prevalence over the past 10 years [5]. An analysis using data from 204 countries and territories forming part of the Global Burden of Disease Study 2019 showed that for ASD, age-standardized rates had risen by around 0.06% annually over the last three decades [7]. The total global prevalence of ASD in 1990 was 20.3 million, increasing to 28.3 million in 2019 [8]. For males, ASD prevalence was 15.6 million in 1990 and 21.6 million in 2019 [8]. For females, the prevalence of ASD in 1990 was 4.7 million, rising by an additional 2 million to 6.7 million by 2019 [8]. For ADHD, in 1990 the reported prevalence was 72.4 million, rising to 84.7 million in 2019 [8]. In males, the global prevalence of ADHD was higher—52.6 million in 1990, increasing to 61.5 million in 2019 [8]. Amongst females, the 1990 global prevalence of ADHD was 19.8 million, rising to 23.2 million in 2019 [8]. Subsequently, the burdens of ADHD and ASD appear to have been greater in males than females [8]. ADHD and ASD remain under-recognized and underdiagnosed in many countries, especially amongst girls and women [9,10]; thus, prevalence rates could be even higher. Prevalence is also reported to be higher in certain population groups, such as looked-after children [11,12]. ADHD prevalence transitions into adulthood in around 30–50% of cases [13].
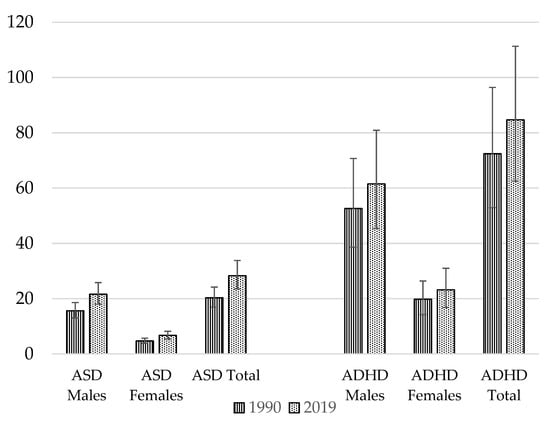
Figure 1.
Global prevalence (in millions) of NDDs. Source: Data extracted from the Global Burden of Disease Study [8].
Dyslexia is highly prevalent, affecting around 20% (1 in 5) of the global population, and males/females equally [14]. It occurs across a range of cognitive and language abilities, a range which includes both higher-than- and lower-than-average levels of functioning [15]. There are different definitions of dyslexia, but Reid (2016) defines it succinctly as a “processing difference, often characterized by difficulties in literacy acquisition affecting reading, writing, and spelling. It can also have an impact on cognitive processes such as memory, speed of processing, time management, coordination, and automaticity. There may be visual and/or phonological discrepancies and there are usually some discrepancies in educational performances” [16].
NDDs are now the most frequently diagnosed conditions in child neurology/pediatric clinical practices [17,18]. There are many potential explanations as to why the prevalence of NDDs could be rising. Improved diagnostic screening might be one explanation [18]. Other factors such as maternal metabolic conditions [19] and misalignment with dietary and lifestyle recommendations have also been proposed [20]. Increasingly, the role of nutrition during gestation (pregnancy) and neurodevelopment is increasingly being recognized, with inadequate intakes of certain nutrients being linked to ADHD, ASD, altered cognition and visual and motor deficits [21]. Past research has focused heavily on nutrients such as the omega-3 fatty acid docosahexaenoic acid (DHA) [22,23,24], but now research has accrued for other nutrients, including choline, considering their roles in neurodevelopment and promoting optimal cognition [21,25,26,27,28].
In this narrative review, we focus on and examine the role(s) of choline as a potential modifiable risk factor for certain NDDs. We will focus on ADHD, ASD (now also referred to as ASC, autistic spectrum condition), and dyslexia, as this is where most research appears to sit.
2. Neurodevelopment
The brain is a central organ that orchestrates the whole body [29]. The human brain begins to develop as early as the third week into gestation when neural progenitor cells differentiate; this process extends into later adolescence and potentially across the lifespan [30]. Processes underpinning brain development include gene expression and environmental inputs, which are both crucial for normal brain development, with disruption of either significantly impacting upon neural outcomes [30]. The development of the brain’s circuitry begins as early as 2–3 weeks into gestation and requires the coordination of complex neurodevelopmental processes [31]. Stiles et al. (2010) explains that “brain development is aptly characterized as a complex series of dynamic and adaptive processes that operate throughout the course of development to promote the emergence and differentiation of new neural structures and functions” [30].
From approximately 6 weeks post-conception to mid-gestation, a number of cellular events occur, including neurogenesis followed by apoptosis, differentiation, migration and synapse formation [31]. Extended periods of cortical development occur across the lifespan. This ‘developmental’ phase occurs across childhood and adolescence when cortical development (outer layers of the cerebrum) transitions from lower-order, unimodal cortices with motor and sensory functions to higher-order, trans-modal cortices underpinning executive, socioemotional and mental brain functions [32].
The role of in utero programming, as described in the theory developed by Professor David Barker, and thus coined the ‘Barker Hypothesis’, is well-recognized [33]. If fetuses have a limited nutrient supply in utero they need to adapt; this is a process that can permanently modify their structure/metabolism, inducing ‘programme changes’ that can be the origins of other conditions later in life (the ‘fetal origins of adult disease’) [33]. It is now well recognized that changes in brain function can lead to a spectrum of NDDs [29].
Intrauterine exposures (including nutrients) have been linked to NDDs, although elucidating the timing and exact mechanisms can be challenging [34,35]. Such exposures during these sensitive windows of life are another factor considered to influence brain development [36]. Increasingly, it is well appreciated that normal neurodevelopment is central for brain functions across the lifespan, with any modulations potentially contributing to brain dysfunction [29]. Heland et al. (2022) recently proposed that nutritional deficits, to some extent, could potentially prevent neurodiversity, as certain nutrients have the ability to improve neurodevelopmental outcomes by mitigating pathological processes such as inflammation, hypoxia and oxidative stress [37]. This brings us on to the potential role of choline.
3. In Utero Origins
Zeisel et al. (2006) described how choline deficiency during sensitive periods of brain development could induce permanent changes in brain function and result in persistent cognitive and memory deficits [38]. As choline is important for brain development, Bernhard et al. (2013) raised concerns about choline levels in very low birth-weight infants, with nutritional intakes of preterm infants frequently being less than the estimated adequate intake, and shortages being apparent until day 10 postnatally [39].
In the Seychelles Child Development Nutrition Study, choline was listed as one of the key nutrients expected to have direct effects on neurodevelopment, both prenatally and postnatally, and was believed to have some correlation with fish consumption [40]. Another study looking at maternal egg consumption found that choline deficiency predicted fetal autonomic and brain maturation indices at 32- and 36-weeks’ gestation, respectively [41]. Poor availability of choline in utero has been further linked to impaired differentiation of retinal neuronal cells, indicating a role in the development of the visual system [42].
Derbyshire and Obeid (2020) [27] provided an updated systematic review using data from 38 animal and 16 human studies. In particular, it was concluded that choline over the first 1000 days of life could potentially: (1) support normal brain development; (2) protect against neural and metabolic insults, including alcohol; and (3) improve neural and cognitive functioning [27]. A further systematic review and meta-analysis collating evidence from 30 publications found that higher maternal choline intake was likely to be associated with improved child neurocognition/neurodevelopment [26].
Gestation
Choline deficiency is common in pregnancy (in utero exposure) [43,44]. Average choline intakes amongst women of childbearing age have been explored in a review of 23 studies, and were reported to range from 233 mg/day to 383 mg/day, even with the inclusion of choline from supplements, and thus are consistently lower than the estimated adequate intake (AI) of 480 mg/day for pregnant women [45,46]. In a recent study conducted in Germany, only 7% of pregnant women achieved adequate choline intakes [47]. Similarly, amongst an Australian sample of pregnant women, median choline intake was 362 mg/day in early pregnancy, with eggs providing around 17% of the choline [48]. The authors concluded that few pregnant women met the AI for choline, and that this may need to be improved [48].
4. Mechanistic Studies
Choline is an essential micronutrient, as recognized by the United States (U.S.) Institute of Medicine in 1998 [49]. Mechanistically, it is a precursor of the brain neurotransmitter acetylcholine and membrane phospholipids, including phosphatidylcholine [50,51]. It is also a methyl donor known to play a central role in brain growth and development, maintaining the functional and structural integrity of the cell membrane [36]. Through the actions of its metabolites, it partakes in pathways involved in the methylation of genes related to memory and cognitive functions [36].
As shown in Table 1, several mechanistic studies have investigated the effects and mechanisms of choline on brain development. The fetus and newborn are known to have high choline demands, with the micronutrient’s role in DHA and histone methylation believed to be as a modifier of genes involved in aspects of learning and memory [52]. Bekdash et al. (2016) describe how choline is an important epigenetic modifier of the genome (altering gene methylation, expression and cell function), with abnormal levels during fetal development and/or early postnatal life being linked to altered memory functions later in adult life [53]. Choline is also needed for normal memory development, possibly due to changes in the development of the memory center (hippocampus) in the brain [54]. Choline is thought to influence stem cell proliferation and apoptosis, therefore potentially modifying brain structure and function [55].
Murine models show that postnatal choline treatment can modulate neuronal plasticity, preventing deficits in motor coordination whilst enhancing density of dendritic spines and neuronal morphology [56]. Choline has further been found to partially restore dendritic structural complexity in murine hippocampal neurons that are iron deficient [57]. Other work shows that reduced choline supplies during gestation can impede and diminish the number of cortical neural progenitor brain cells (NPCs), with two types of NPCs—the radial glial and intermediate progenitor cells—being affected, indicating that choline supplies regulate cerebral cortex development [58]. A murine model focusing on autism showed that choline supplementation administered to offspring of methylenetetrahydrofolate reductase (MTHFR)-deficient mothers had the potential to attenuate the autistic-like phenotype [59]. Further research found choline supplementation to improve impairments in social interaction in a murine model of autism, helping to reduce deficits in social behavior and reduce anxiety [60].

Table 1.
Role(s) of choline in neurodevelopment and brain function.
Table 1.
Role(s) of choline in neurodevelopment and brain function.
| Author(s), Year | Publication | Choline Mechanisms |
|---|---|---|
| Blusztajn et al. (2017; 2012) [50,52] | Discussion paper | Precursor of PC, acetylcholine, and via betaine, the methyl group donor S-adenosylmethionine. Cho as a methyl donor influences DNA and histone methylation (regulates gene expression). |
| Bekdash et al. (2019; 2018; 2016) [36,53,61] | Discussion paper | Cho maintains structural and functional integrity of membranes and regulates cholinergic neurotransmission via acetylcholine synthesis. |
| Zeisel et al. (2020; 2017; 2011; 2006; 2000; 1997) [38,54,55,62,63,64] | Discussion paper | Cho can induce changes in the development of the memory center (hippocampus), modulate methylation via BHMT (and its metabolite, betaine) and regulate S-adenosylhomocysteine and S-adenosylmethionine levels. Plausible mechanisms: changes in transmembrane signal transduction, stem cell proliferation/differentiation, regulation of apoptosis, gene expression in neuronal and endothelial progenitor cells. |
| Bastian et al. (2022) [57] | Murine model | Cho restored dendritic function in developing hippocampal neurons that were iron-deficient. |
| Agam et al. (2020) [59] | MTHFR-deficient mice | Cho supplementation, even at adulthood, to offspring of MTHFR-deficient mothers attenuated the autistic-like phenotype. |
| Chin et al. (2019) [56] | Knockout murine model | Cho enhanced neuronal morphology, rescued deficits in motor coordination and increased density of dendritic spines. |
| Wang et al. (2016) [58] | Murine model | When Cho supply was reduced during gestation, the number of two types of cortical NPCs (radial glial cells and intermediate progenitor cells) were significantly reduced in fetal brains. |
| Langley et al. (2015) [60] | Mouse model of autism | Cho intake during early development can prevent/dramatically reduce deficits in social behavior and anxiety. |
Key: BHMT, betaine homocysteine methyltransferase; Cho, choline; DNA, deoxyribonucleic acid; MTHFR, Methylenetetrahydrofolate reductase; NPCs, neural progenitor cells; PC, phosphatidylcholine.
5. Brain Imaging (Animal Models)
Several imaging studies have investigated choline’s role in brain structure and function using animal models. Mudd et al. (2016) fed sows either a choline-deficient (CD) or choline-sufficient (CS) diet for the last half of gestation, finding that CD sows had smaller total brain volumes when measured using magnetic resonance imaging 30 days postnatally [65]. Concentrations of glycerophosphocholine-phosphocholine were also lower, indicating that choline deficiency appeared to delay neurodevelopment and induce structural and metabolic changes [65]. Other work by Mudd et al. (2018), using a similar approach, further showed that CD pigs had significantly reduced left- and right-cortex gray matter compared with prenatally CS pigs [66].
6. Human Studies
6.1. ASD
Nutritional status (including low choline) is regarded as playing a key role in the severity and incidence of core ASD symptoms [67]. Research from human studies has focused on the potential role(s) of choline in relation to ASD (Table 2).
Firstly, work using spectroscopic imaging on children with ASD (aged 3–4 years) found that gray matter and white matter levels of choline were reduced, compared with typically developed children [68,69]. Focusing on the white matter composition, other work also found that ASD and its severity was associated with lower levels of choline in brain white matter, along with the perisylvian cortex [70]. Similarly, Margari et al. (2018) found brain metabolite levels (choline/Cr ratios) to be significantly altered in the frontal lobe white matter in ASD subjects versus controls [71]. Hardan et al. (2008) observed lower levels of choline in the left side of the thalamus in children with autism [72]. Some research has found lower choline levels in the thalamus to be correlated with behavioral scores in ASD children (7–18 years), i.e., increased severity of stereotyped behaviors and communication impairments [73].
Regarding metabolic characteristics, Wang et al. (2022) studied 29 ASD and 30 typically developing boys (mean age ≈ 3 years) [74]. Boys with ASD had lower levels of plasma choline, which was adversely correlated with ABC-language scores–findings that aligned with Gabis et al. (2019) [74,75]. Indeed, the work by Wang et al. (2022) also found that choline metabolism intermediates such as phosphatidylcholine and lysophosphatidylcholine (involved in glycerophospholipid metabolism) were reduced, implying that this could impact on processes of choline metabolism and subsequent impairments in language ability in ASD children [74]. Additional work has investigated the effects of supplementation. Gabis et al. (2019) undertook a double-blind randomized trial examining the combined effects of donepezil and choline (350 mg/day for 8 weeks in the open label study phase) compared to a placebo [75]. The treatment group had a sustained effect on receptive language skills in ASD children for 6 months post-treatment, with more significant effects observed in those under 10 years of age [75].
Other research has described habitual choline intake and status. Scientists using data from the U.S. Autism Intervention Research Network for Physical Health (AIR-P) study found that 60–93% of ASD children were consuming less than the recommended adequate intake for choline [76]. Children with autism also had lower plasma choline levels than did healthy controls [76]. The authors concluded that choline intakes were inadequate in a significant number of ASD children, which could contribute to abnormalities in folate-dependent one-carbon metabolism observed in many children with autism [76]. Very similar findings were reported by Hyman et al. (2012) [77]. An analysis of 3-day food records from children with ASD aged 2–11 years showed that few ASD or matched controls met recommended chorine intake levels [77].

Table 2.
Key studies investigating ASD and choline.
Table 2.
Key studies investigating ASD and choline.
| Author, Year | Study Design/Approach | Age | No. of Participants | Key Findings |
|---|---|---|---|---|
| Wang et al. (2022) [74] | Metabolomic analysis | ASD 3.02 ± 0.67 y TD 3.13 ± 0.46 y | n = 28 boys ASD n = 30 boys TD | The level of Cho was inversely correlated with ABC-language score in ASD group. |
| O’Neill et al. (2020) [70] | Magnetic resonance spectroscopy | 5–60 y | n = 78 ASD n = 96 TD | Cho metabolites were lower in ASD than in TD. |
| Gabis et al. (2019) [75] | Randomized, DB, PC trial (12-wk int., 6-months washout) | NR | n = 60 ASD | Donepezil hydrochloride + Cho contributed to a sustainable effect on receptive language skills in ASD children for 6 months after treatment, with a more significant effect in those <10 y. |
| Margari et al. (2018) [71] | Case-control | 21 months to 14 y, 1 month | n = 75 ASD n = 50 controls | Cho/Cr ratios were significantly altered in ASD vs. controls. |
| Doyle-Thomas et al. (2014) [73] | Case-control | 7–18 y | n = ASD n = 16 TD controls | Boys had increased Cho in the thalamus. Cho in the three brain regions studies correlated with behavioral scores in the ASD group |
| Corrigan et al. (2013) [69] | Cross-sectional analysis | 3–4 y 7–9 y 9–10 y | n = 45 ASD n = 14 DD n = 14 TD | At 3–4 y, the ASD group exhibited lower Cho concentrations than did the TD group |
| Hamlin et al. (2013) [76] | Observational analysis | 2–11 y | n = 288 ASD | Cho intake was inadequate in a significant subgroup of ASD children and reflected in lower plasma levels. This may contribute to metabolic abnormalities. |
| Hyman et al. (2012) [77] | Prospective analysis | 2–11 y | n = 252 ASD | Few children met the recommended intakes for Cho. |
| Hardan et al. (2008) [72] | Case-control | 8–15 y | n = 18 M ASD n = 16 controls | Lower levels of Cho-containing metabolites were found on the left side of the thalamus in the ASD group vs. controls. |
| Friedman et al. (2006) [68] | Cross-sectional spectroscopic imaging | 3–4 y | n = 45 ASD n = 12 DD n = 10 TD | Children with ASD had significantly decreased gray matter concentrations of Cho (p < 0.001) |
Key: ASD, autism spectrum disorder; Cho, choline; DB, double-blind; DD, delayed development; int., intervention; M, male; PC, placebo-controlled; TD, typical development; y, years.
6.2. ADHD
Some human trials have included an analysis of choline status or circulating levels of brain metabolites and studied these factors in relation to ADHD (Table 3).
Focusing on imaging studies, Alger et al. (2021) used whole-brain diffusion tensor imaging [78]. The results showed that ADHD children (aged 8–13 years) exposed to ‘prenatal alcohol exposure’ (PAE) had more severe white-matter pathology compared with those without PAE [78]. Amongst those with ADHD + PAE, Tower Test Achievement scores (higher for better performance) correlated negatively with choline, with its role somewhat unclear and warranting continued investigation [78]. Additional neuroimaging work measuring prefrontal white matter discovered that anterior corona radiata levels of choline were 27% lower in children and adolescents with ADHD + PAE, compared with those with idiopathic ADHD [79]. Other magnetic resonance research showed that 20–25% of neurons may have died or been severely dysfunctional in pediatric ADHD patients, and there seemed to be mild hyperactivity of the cholinergic system (the system that encompasses the synthesis and secretion of acetylcholine) [80].
Additional research has looked at supplementation. One randomized controlled trial provided 2–5-year-olds with fetal alcohol spectrum disorder (FASD) with choline (1.25 g. choline bitartrate powder delivering 513 mg/day choline) or a placebo over 9-months, with follow up 4 years later (mean age 8.6 years) [81]. At follow-up the children who had received choline had fewer behavioral symptoms of ADHD than did the placebo group [81]. The children administered choline also had better working memory ability, verbal memory, visual-spatial skills, and non-verbal intelligence than the placebo group [81]. Borlase et al. (2020) studied the effects of micronutrient treatment on brain neurometabolites, since these appeared to be altered in children with ADHD, particularly in the prefrontal cortex and striatum [82]. Children with ADHD (mean age 10.8 years, non-medicated, n = 27) received daily micronutrients or a placebo over 10 weeks [82]. It was not specified which micronutrients or dosages were involved, but choline was identified as a metabolite of interest. In the treatment group, there was a trend for decreased (improved) choline in the left and right striatum, though changes were not regarded to be of significance [82].
Some research has studied choline levels in relation to methylphenidate administration. Earlier work proposed that choline does not appear to be sensitive to methylphenidate treatment in children [83]. However, an analysis of regional brain spectra in 2008 found a significantly reduced signal of choline-containing compounds following methylphenidate treatment [84]. This is believed to fit with a recent energetics hypothesis of ADHD—that insufficient lactate supply to oligodendrocytes leads to impairments in fatty acid synthesis and myelin sheath formation, which may account for the reduced choline levels [84,85]. According to Wiguna et al. (2012), stimulant (long-acting methylphenidate) treatment over 12 weeks appears to induce neurochemical changes (thought to reflect improved neuroplasticity) in the prefrontal cortices of children [86]. In particular, the choline/creatine ratio decreased significantly in children (mean age 8.5 years) by 12.4% in the right prefrontal cortex and 16% in the left prefrontal cortex [86]. This was a pilot study, so repeated investigation is needed.

Table 3.
Key studies investigating ADHD and choline.
Table 3.
Key studies investigating ADHD and choline.
| Author, Year | Study Design/Approach | Age | No. of Participants | Key Findings |
|---|---|---|---|---|
| Alger et al. (2021) [78] | Case-control | 8–13 y | n = 23 ADHD + PAE n = 19 ADHD − PAE n = 28 TD | Cho findings were less prominent in this study. |
| Borlase et al. (2020) [82] | Randomized PC trial | 11 y (mean) | n = 27 ADHD (non-medicated) | In the treatment group (12 capsules/day; dose NS) there was a trend for decreased choline in the striatum. |
| Wozniak et al. (2020) [81] | Randomized, DB, PC trial (9-months) | 2–5 y | n = 15 FASD Cho n = 16 FASD placebo | Children receiving Cho (1.25 g. Cho bitartrate powder delivering 513 mg/day Cho) had higher non-verbal intelligence, higher visual-spatial skill, higher working memory ability, better verbal memory and fewer behavioral symptoms of ADHD than those receiving the placebo. |
| O’Neill et al. (2019) [79] | Case-control | 7–17 y | n = 44, by subgroup | Low Cho may stem from abnormal Cho metabolism. |
| Wiguna et al. (2012) [86] | Prospective analysis | 9 y (mean) | n = 21 ADHD | The Cho/creatine ratio decreased 12% in the right prefrontal cortex after stimulant treatment. |
| Kronenberg et al. (2008) [84] | Spectroscopic test-retest study | Adults | n = 7 ADHD | Analysis of regional brain showed a significantly decreased signal of Cho-containing compounds following treatment with MPH. |
| Carrey et al. (2003) [83] | Magnetic resonance spectroscopy | NR | n = 14 ADHD | Cho metabolites did not demonstrate any change in response to medication. |
| Jin et al. (2001) [80] | Magnetic resonance spectroscopy | NR | n = 12 B ADHD n = 10 controls | In ADHD children the bilateral striatum Cho/Cr ratio showed a mild unilateral increase. There appears to be mild hyperactivity of the cholinergic system. |
Key: B, boys; Cho, choline; DB, double-blind; FASD, fetal alcohol spectrum disorder; MPH, methylphenidate; NR, not reported; NS, Not clearly specified; PC, placebo-controlled; TD, typical development; y, years.
6.3. Dyslexia
An emerging body of evidence has looked at choline levels/metabolites in relation to markers of dyslexia (Table 4). In one study, reading ability and executive function were measured in 24 children (8 to 12 years) with dyslexia and 30 typical readers [87]. For females with dyslexia there was a strong, statistically significant inverse correlation between processing speed and choline [87]. It is well appreciated that individuals with dyslexia have prolonged response times, and metabolite changes appear to be present which could hold promise as possible markers for dyslexia [87]. This is one of the first studies to identify metabolite changes in regions not regarded as being part of the ‘classic’ reading circuitry.
Earlier work has shown that choline levels were negatively correlated with reading and related linguistic measures in phonology and vocabulary (i.e., higher concentrations were associated with reduced performance) in the occipital lobe [88,89], left temporoparietal region [90] and the cerebellum [91]. It seems tenable that higher choline levels in reading-related regions for those with reading difficulties could be indicative of high membrane turnover, white matter, and cellular density [87]. This aligns with evidence that myelination is impaired in this population, particularly in the white-matter tracts that pass the temporoparietal regions and the occipital lobe [92,93].
Other research focusing on the visual and temporo-parietal cortex showed that children with dyslexia, compared to controls, had choline levels that were 7.6% lower in the left temporo-parietal region and 5.5% lower in the visual cortex [94]. Amongst children, the higher the choline concentration, the faster the Rapid Automatized Naming (RAN), though this did not reach a level of significance [94].

Table 4.
Key studies investigating markers of dyslexia.
Table 4.
Key studies investigating markers of dyslexia.
| Author, Year | Study Design/Approach | Age | No. of Participants | Key Findings |
|---|---|---|---|---|
| Kossowski et al. (2019) [94] | MEGA-PRESS single-voxel spectroscopy | 30.28 ± 4.09 D 28.02 ± 3.40 C 10.90 ± 0.98 D 11.21 ± 0.95 C | n = 36 adults (50% D) n = 52 children (50% D) | Adults vs. children were characterized by higher Cho in the left temporo-parietal and occipital cortices. |
| Horowitz-Kraus et al. (2018) [87] | Magnetic resonance spectroscopy | 8–12 y | n = 24 dyslexia n = 30 TR | After adjustment for multiple comparisons, F with dyslexia showed strong significant inverse correlations between processing speed and Cho (r = −0.54, p = 0.005) levels. |
| Del Tufo et al. (2018) [89] | Magnetic resonance spectroscopy | 8.1 y (mean) | n = 231, Metabolites measured in n = 70 | There was an inverse association between Cho and reading ability. |
| Pugh et al. (2014) [88] | Longitudinal analysis | 6.1–10.1 y | n = 75 reading abilities across a continuum (including those with RD) | Cho levels were inversely correlated with reading, phonology and vocabulary (possible links to white matter anomalies and hyperexcitability). |
| Bruno et al. (2013) [90] | Magnetic resonance spectroscopy | 18–30 y | n = 31 young adults | Lower scores on phonological decoding and sight word reading measures were associated with higher Cho concentrations. |
| Laycock et al. (2008) [91] | Whole-brain volumetric MRI | NR | n = 10 M dyslexic n = 11 M controls | The dyslexic group had a lower ratio of NAA/Cho in the right cerebellar hemisphere and a higher ratio of Cho/Cr in the left cerebellar hemisphere, possibly indicative of excessive connectivity or abnormal myelination. |
Key: C, Control; Cho, choline; D, Individuals with dyslexia; M, male; MRI, Magnetic resonance imaging; NAA, N-acetyl aspartate; NR, not reported; RD, reading difficulties (termed disability in cited publication); TR, typical readers; y, years.
6.4. Processing Speed and Attention
It is well recognized that processing speed and working memory can be reduced in both individuals with ADHD and those with dyslexia, with individuals having both conditions concurrently being most affected [95]. Caudill et al. (2018) examined the effects of maternal choline supplementation during pregnancy [96]. Women moving into their third trimester were randomly assigned to groups consuming 480 mg choline/d (n = 13) or 930 mg choline/d (n = 13) until delivery [96]. Infant information processing speed and visuospatial memory were studied at 4, 7, 10 and 13 months of age [96]. Intriguingly, mean reaction time (averaged across the four ages) was significantly quicker for infants born to mothers who took the higher levels of choline—930 (vs. 480) mg choline/d [96]. These findings suggest that maternal consumption of around twice the recommended amount of choline during the last trimester of pregnancy improves infant information processing speed.
When these children were followed-up at 7 years of age, children who had been exposed to choline at a level of intake of 930 mg/d from the third trimester of pregnancy had improved levels of sustained attention (i.e., a significantly better ability to maintain correct signal detections) [97]. This may be attributed to them being able to sustain cholinergic activity in the prefrontal cortex of the brain, a region which regulates attentional control [97]. These findings are interesting, and have wider potential implications, including potential studies focusing on attention/inattention and choline status in individuals with ADHD and/or dyslexia.
7. Discussion
In the past, there has been a tendency to focus on nutrients such as long-chain omega-3 fatty acids in relation to their effects on neurodevelopment and NDDs [22,23,98,99,100]. However, in recent years there has been an accumulation of evidence highlighting the potential role(s) of choline [27,38,54,63].
Choline is recognized as having three distinct physiological roles: (1) the synthesis of neurotransmitter acetylcholine, (2) acting as a major methyl donor and (3) preserving the structural integrity and lipid-mediated signaling for cell membranes [44,101]. Choline has an important role in neurodevelopment, with normal concentrations enlarging cholinergic neurons in size and number in the medial septum [28]. There is now an accruing body of science demonstrating that choline is important for neurological development and brain function [21,27]. Regarding mechanisms, it is possible that the role(s) of choline are different depending on the life stage and form of the NDD. Mechanisms are complex and have potential to be multi-faceted. Modulations in white-matter pathology, impaired myelination, altered levels of brain metabolites and potential compensatory mechanisms have all been described in relation to choline markers [66,68,70,78,85]. Further research is now warranted to build on present insights and disentangle these.
For ASD, we have seen how lower plasma choline levels have been correlated with diminished language scores, indicating that there could be plausible links between products of choline metabolism and language ability [74,75]. Choline is a major brain metabolite and essential component of different membrane phospholipids [102]. Interestingly, other work conducted with typically developed children has discovered links between speeded (rapid) naming (a measure of long-term memory) and choline levels which could potentially influence language ability [103]. We have seen in several studies included in this narrative review how choline intakes and/or status appear to be reduced in children with NDDs, particularly ASD [74,76,77]. These are intriguing findings worthy of future development and research.
Moving on to ADHD, the effect of stimulant medication (methylphenidate) appears to be inconclusive, with some authors reporting that choline is not sensitive to this [83] and others documenting that levels of choline-containing compounds are reduced [84,86]. Some studies have looked at the effects of medication on brain choline metabolite levels [84,104]. One spectroscopic study observed reduced choline levels in the anterior cingulum following chronic methylphenidate treatment [84]. Additional research found that children with ADHD on stimulant medication had significantly higher choline ratios in the left prefrontal region, indicating that the medication could normalize brain metabolite levels [104]. Further clinical trials are needed to investigate this further. Given the advances of new medications, these should also be studied in relation to any effects on choline levels/metabolites. Another important point to consider is that sensory issues and food selectivity in these population groups could further impact dietary intakes [105,106], including that of choline.
Turning to dyslexia, more research is needed to unravel the science. It appears that higher choline levels in those with reading difficulties for reading-related regions could be reflective of higher membrane turnover, white matter, and cellular density, indicating compromised myelination in this population [87,92,93]. For dyslexia, the visual magnocellular theory posits that omega-3 long chain polyunsaturated fatty acids (particularly docosahexaenoic acid; DHA) provide membranes with properties to enable rapid electrical activity of M-cells and the rapid opening and closure of these cells [107]. Thus, a lack of DHA can affect the integrity of these neurons and result in impaired visual magnocellular function [107]. Interestingly, in the in utero environment, there is now evidence that choline is needed for the appropriate development of the visual system, especially the regulation of temporal progression of retinogenesis [42]. This is a field that would be worthy of extended study in relation to dyslexia and visual processing.
Some organizations are now beginning to specifically mention choline’s role(s) in neurodevelopment. For example, both the American Academy of Pediatrics (AAP) and the American Medical Association (AMA) have communicated the importance of maternal choline intake during pregnancy and lactation and identified that failure to provide choline during the first 1000 days post-conception could result in lifelong shortfalls in brain function, despite subsequent nutrient repletion [108,109,110,111]. The AAP calls for pediatricians to move beyond simply recommending a ‘good diet’ and ensure that pregnant women and young children have access to food that provides adequate amounts of “brain-building” nutrients, with choline being listed as one of these [111]. The main food groups reported as contributing to choline intake are milk, egg, and their derived products, as well as meat, grains and fish [112]. The AMA explains that during pregnancy, cognitive, neural tube and hippocampus development are dependent on adequate choline intake, and have called for prenatal vitamin supplements to contain ‘evidenced-based’ amounts of choline [110].
A recent analysis of over 180 commercial prenatal supplements identified that these varied in content, frequently only providing a subset of essential vitamins, and containing amounts that tended to be below recommendations [113]. The authors concluded that choline was only included in 40% of prenatal supplements, at a median level of 25 mg [113]. They also reported the incidence of certain physical and mental health conditions in the U.S.—9.4% for ADHD, and 2% for autism—and recognized that choline is needed for fetal brain development, potentially lowering the risk of neural tube defects and autism [113]. It was concluded that women who do not eat several eggs per week may benefit from prenatal supplements containing choline. Dosages provisionally advised in this publication—at least 350 mg of choline during the first two trimesters, and approximately 600 mg in the third trimester—were rather high [113]. EFSA advises an AI of 480 mg/day choline for pregnant and 520 mg/day choline for lactating women [46]. Furthermore, it is important to consider that tolerable upper intake levels have not been formally established for choline. Some side-effects, such as gastrointestinal disturbances and fishy body odor, have been reported in earlier studies administering higher amounts of choline (8–20 g/day) [114,115,116], though these studies are dated and not representative of the population of interest.
Overall, there is an emergent evidence base accruing in this important field. It is evident that more clinical trials are needed before firm conclusions can be drawn. This paper aimed to provide a first insight into the field. One prudent point to consider is the nature of the terminologies used in scientific papers published within this field. We are now gradually moving away from phrases such as ‘reading disorders’ or ‘reading disabilities’ and ‘autism spectrum disorder’ to revised terms such as ‘reading difficulties’ and ASC (autism spectrum condition). Some older terms may have been used in this paper, but only when referring to older studies that used such terms. In the future, greater consistency is needed in aligning with revised, modernized terminologies.
On a final note, now is the time to pay greater attention to choline from the perspective of neurodevelopment and NDD. Many countries, including the United Kingdom, do not yet have formal choline intake recommendations [117]. Clearly this is a central starting point. A generic lack of awareness about the nutrient choline and its potential role(s) in neurodevelopment and NDDs is evident [108,109,117]. Firming up choline recommendations and guidance to women of childbearing age potentially has tremendous implications for supporting the neurodevelopment of the next generation.
8. Conclusions
All taken together, choline appears to play a central role in brain development, growth, and function. An accruing body of evidence indicates that choline could have underpinning roles in the etiology of ASD, ADHD and possibly other NDDs. The origins of these conditions are multi-faceted, but can be genetic and attributed to environmental factors, including dietary exposures such as choline (in utero and beyond). Mechanisms of choline in relation to brain function and neurochemistry may be different at different life stages, e.g., in utero versus later in life, and for the variations of NDDs that exist and co-exist. Future research is needed in this important field. Choline certainly appears to be a nutrient worthy of consideration when studying neurodevelopment and NDDs.
Author Contributions
E.D. conceptualized, researched, wrote, and edited the article. M.M. reviewed and edited the article. All authors have read and agreed to the published version of the manuscript.
Funding
Procter & Gamble funded E.D.’s writing of the paper. M.M. did not receive any funding for reviewing and contributing to the publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thank you to J. Cooper, Medical Statistician, for statistical expertise in plotting data from the Global Burden of Disease Study [8].
Conflicts of Interest
Procter & Gamble commissioned E.D. to write the manuscript.
References
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef]
- APA (American Psychiatric Association). Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Morris-Rosendahl, D.J.; Crocq, M.A. Neurodevelopmental disorders-the history and future of a diagnostic concept Dialogues Clin. Neurosci. 2020, 22, 65–72. [Google Scholar] [CrossRef]
- Antshel, K.M.; Russo, N. Autism Spectrum Disorders and ADHD: Overlapping Phenomenology, Diagnostic Issues, and Treatment Considerations. Curr. Psychiatry Rep. 2019, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Antshel, K.M.; Zhang-James, Y.; Wagner, K.E.; Ledesma, A.; Faraone, S.V. An update on the comorbidity of ADHD and ASD: A focus on clinical management. Expert. Rev. Neurother. 2016, 16, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Brimo, K.; Dinkler, L.; Gillberg, C.; Lichtenstein, P.; Lundstrom, S.; Asberg Johnels, J. The co-occurrence of neurodevelopmental problems in dyslexia. Dyslexia 2021, 27, 277–293. [Google Scholar] [CrossRef]
- Li, Z.; Yang, L.; Chen, H.; Fang, Y.; Zhang, T.; Yin, X.; Man, J.; Yang, X.; Lu, M. Global, regional and national burden of autism spectrum disorder from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Epidemiol. Psychiatr. Sci. 2022, 31, e33. [Google Scholar] [CrossRef]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Sayal, K.; Prasad, V.; Daley, D.; Ford, T.; Coghill, D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry 2018, 5, 175–186. [Google Scholar] [CrossRef]
- Lockwood Estrin, G.; Milner, V.; Spain, D.; Happe, F.; Colvert, E. Barriers to Autism Spectrum Disorder Diagnosis for Young Women and Girls: A Systematic Review. Rev. J. Autism Dev. Disord. 2021, 8, 454–470. [Google Scholar] [CrossRef]
- Heady, N.; Watkins, A.; John, A.; Hutchings, H. Prevalence of neurodevelopmental disorders and their impact on the health and social well-being among looked after children (LAC): A systematic review protocol. Syst. Rev. 2022, 11, 49. [Google Scholar] [CrossRef]
- Willis, R.; Dhakras, S.; Cortese, S. Attention-Deficit/Hyperactivity Disorder in Looked-After Children: A Systematic Review of the Literature. Curr. Dev. Disord. Rep. 2017, 4, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Altabella, L.; Zoratto, F.; Adriani, W.; Canese, R. MR imaging-detectable metabolic alterations in attention deficit/hyperactivity disorder: From preclinical to clinical studies. AJNR Am. J. Neuroradiol. 2014, 35, S55–S63. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, S.E.; Shaywitz, J.E.; Shaywitz, B.A. Dyslexia in the 21st century. Curr. Opin. Psychiatry 2021, 34, 80–86. [Google Scholar] [CrossRef]
- Wagner, R.K.; Zirps, F.A.; Edwards, A.A.; Wood, S.G.; Joyner, R.E.; Becker, B.J.; Liu, G.; Beal, B. The Prevalence of Dyslexia: A New Approach to Its Estimation. J. Learn. Disabil. 2020, 53, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Dyslexia: A Practitioner’s Handbook, 5th ed.; Wiley-Blackwell: Chichester, UK, 2016; p. 5. [Google Scholar]
- Acosta, M.T. Neurodevelopmental disorders: From the laboratory to the classroom. Med. B Aires 2022, 82 (Suppl. S1), 6–10. [Google Scholar]
- Savatt, J.M.; Myers, S.M. Genetic Testing in Neurodevelopmental Disorders. Front. Pediatr. 2021, 9, 526779. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, P.; Walker, C.K.; Bremer, A.A.; Baker, A.S.; Ozonoff, S.; Hansen, R.L.; Hertz-Picciotto, I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012, 129, e1121–e1128. [Google Scholar] [CrossRef]
- Loewen, O.K.; Maximova, K.; Ekwaru, J.P.; Asbridge, M.; Ohinmaa, A.; Veugelers, P.J. Adherence to Life-Style Recommendations and Attention-Deficit/Hyperactivity Disorder: A Population-Based Study of Children Aged 10 to 11 Years. Psychosom. Med. 2020, 82, 305–315. [Google Scholar] [CrossRef]
- Cortes-Albornoz, M.C.; Garcia-Guaqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutierrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Basak, S.; Mallick, R.; Duttaroy, A.K. Maternal Docosahexaenoic Acid Status during Pregnancy and Its Impact on Infant Neurodevelopment. Nutrients 2020, 12, 3615. [Google Scholar] [CrossRef]
- Agostoni, C.; Nobile, M.; Ciappolino, V.; Delvecchio, G.; Tesei, A.; Turolo, S.; Crippa, A.; Mazzocchi, A.; Altamura, C.A.; Brambilla, P. The Role of Omega-3 Fatty Acids in Developmental Psychopathology: A Systematic Review on Early Psychosis, Autism, and ADHD. Int. J. Mol. Sci. 2017, 18, 2608. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.P.; Bandarra, N.M.; Figueiredo-Braga, M. The role of marine omega-3 in human neurodevelopment, including Autism Spectrum Disorders and Attention-Deficit/Hyperactivity Disorder—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Irvine, N.; England-Mason, G.; Field, C.J.; Dewey, D.; Aghajafari, F. Prenatal Folate and Choline Levels and Brain and Cognitive Development in Children: A Critical Narrative Review. Nutrients 2022, 14, 364. [Google Scholar] [CrossRef]
- Obeid, R.; Derbyshire, E.; Schon, C. Association between Maternal Choline, Fetal Brain Development, and Child Neurocognition: Systematic Review and Meta-Analysis of Human Studies. Adv. Nutr. 2022, 13, 2445–2457. [Google Scholar] [CrossRef]
- Derbyshire, E.; Obeid, R. Choline, Neurological Development and Brain Function: A Systematic Review Focusing on the First 1000 Days. Nutrients 2020, 12, 1731. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; West, A.A.; Caudill, M.A. Maternal choline supplementation: A nutritional approach for improving offspring health? Trends Endocrinol. Metab. 2014, 25, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y. The brain-from neurodevelopment to neurodegeneration. FEBS J. 2022, 289, 2010–2012. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal development of brain circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- Sydnor, V.J.; Larsen, B.; Bassett, D.S.; Alexander-Bloch, A.; Fair, D.A.; Liston, C.; Mackey, A.P.; Milham, M.P.; Pines, A.; Roalf, D.R.; et al. Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology. Neuron 2021, 109, 2820–2846. [Google Scholar] [CrossRef]
- Barker, D.J. In utero programming of chronic disease. Clin. Sci. 1998, 95, 115–128. [Google Scholar] [CrossRef]
- Amgalan, A.; Andescavage, N.; Limperopoulos, C. Prenatal origins of neuropsychiatric diseases. Acta Paediatr. 2021, 110, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients 2019, 11, 2995. [Google Scholar] [CrossRef] [PubMed]
- Heland, S.; Fields, N.; Ellery, S.J.; Fahey, M.; Palmer, K.R. The role of nutrients in human neurodevelopment and their potential to prevent neurodevelopmental adversity. Front. Nutr. 2022, 9, 992120. [Google Scholar] [CrossRef]
- Zeisel, S.H. The fetal origins of memory: The role of dietary choline in optimal brain development. J. Pediatr. 2006, 149, S131–S136. [Google Scholar] [CrossRef]
- Bernhard, W.; Full, A.; Arand, J.; Maas, C.; Poets, C.F.; Franz, A.R. Choline supply of preterm infants: Assessment of dietary intake and pathophysiological considerations. Eur. J. Nutr. 2013, 52, 1269–1278. [Google Scholar] [CrossRef]
- Strain, J.J.; Bonham, M.P.; Duffy, E.M.; Wallace, J.M.W.; Robson, P.J.; Clarkson, T.W.; Shamlaye, C. Nutrition and neurodevelopment: The search for candidate nutrients in the Seychelles Child Development Nutrition Study. Neurotoxicology 2020, 81, 300–306. [Google Scholar] [CrossRef]
- Christifano, D.N.; Chollet-Hinton, L.; Hoyer, D.; Schmidt, A.; Gustafson, K.M. Intake of eggs, choline, lutein, zeaxanthin, and DHA during pregnancy and their relationship to fetal neurodevelopment. Nutr. Neurosci. 2022, 26, 749–755. [Google Scholar] [CrossRef]
- Trujillo-Gonzalez, I.; Friday, W.B.; Munson, C.A.; Bachleda, A.; Weiss, E.R.; Alam, N.M.; Sha, W.; Zeisel, S.H.; Surzenko, N. Low availability of choline in utero disrupts development and function of the retina. FASEB J. 2019, 33, 9194–9209. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients 2019, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef]
- Derbyshire, E.; Obeid, R.; Schon, C. Habitual Choline Intakes across the Childbearing Years: A Review. Nutrients 2021, 13, 4390. [Google Scholar] [CrossRef] [PubMed]
- EFSA. EFSA Dietary Reference Values for Choline. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J. 2016, 14, 4484. [Google Scholar]
- Roeren, M.; Kordowski, A.; Sina, C.; Smollich, M. Inadequate Choline Intake in Pregnant Women in Germany. Nutrients 2022, 14, 4862. [Google Scholar] [CrossRef]
- Probst, Y.; Sulistyoningrum, D.C.; Netting, M.J.; Gould, J.F.; Wood, S.; Makrides, M.; Best, K.P.; Green, T.J. Estimated Choline Intakes and Dietary Sources of Choline in Pregnant Australian Women. Nutrients 2022, 14, 3819. [Google Scholar] [CrossRef]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef]
- Loffelholz, K.; Klein, J.; Koppen, A. Choline, a precursor of acetylcholine and phospholipids in the brain. Prog. Brain Res. 1993, 98, 197–200. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Mellott, T.J. Choline nutrition programs brain development via DNA and histone methylation. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 82–94. [Google Scholar] [CrossRef]
- Bekdash, R.A. Choline and the Brain: An Epigenetic Perspective. Adv. Neurobiol. 2016, 12, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline: Needed for normal development of memory. J. Am. Coll. Nutr. 2000, 19, 528S–531S. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Niculescu, M.D. Perinatal choline influences brain structure and function. Nutr. Rev. 2006, 64, 197–203. [Google Scholar] [CrossRef]
- Chin, E.W.M.; Lim, W.M.; Ma, D.; Rosales, F.J.; Goh, E.L.K. Choline Rescues Behavioural Deficits in a Mouse Model of Rett Syndrome by Modulating Neuronal Plasticity. Mol. Neurobiol. 2019, 56, 3882–3896. [Google Scholar] [CrossRef] [PubMed]
- Bastian, T.W.; von Hohenberg, W.C.; Kaus, O.R.; Lanier, L.M.; Georgieff, M.K. Choline Supplementation Partially Restores Dendrite Structural Complexity in Developing Iron-Deficient Mouse Hippocampal Neurons. J. Nutr. 2022, 152, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Surzenko, N.; Friday, W.B.; Zeisel, S.H. Maternal dietary intake of choline in mice regulates development of the cerebral cortex in the offspring. FASEB J. 2016, 30, 1566–1578. [Google Scholar] [CrossRef]
- Agam, G.; Taylor, Z.; Vainer, E.; Golan, H.M. The influence of choline treatment on behavioral and neurochemical autistic-like phenotype in Mthfr-deficient mice. Transl. Psychiatry 2020, 10, 316. [Google Scholar] [CrossRef]
- Langley, E.A.; Krykbaeva, M.; Blusztajn, J.K.; Mellott, T.J. High maternal choline consumption during pregnancy and nursing alleviates deficits in social interaction and improves anxiety-like behaviors in the BTBR T+Itpr3tf/J mouse model of autism. Behav. Brain Res. 2015, 278, 210–220. [Google Scholar] [CrossRef]
- Bekdash, R.A. Choline, the brain and neurodegeneration: Insights from epigenetics. Front. Biosci. 2018, 23, 1113–1143. [Google Scholar] [CrossRef]
- Zeisel, S. Choline, Other Methyl-Donors and Epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: Essential for brain development and function. Adv. Pediatr. 1997, 44, 263–295. [Google Scholar] [PubMed]
- Zeisel, S.H. The supply of choline is important for fetal progenitor cells. Semin. Cell. Dev. Biol. 2011, 22, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Getty, C.M.; Sutton, B.P.; Dilger, R.N. Perinatal choline deficiency delays brain development and alters metabolite concentrations in the young pig. Nutr. Neurosci. 2016, 19, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Getty, C.M.; Dilger, R.N. Maternal Dietary Choline Status Influences Brain Gray and White Matter Development in Young Pigs. Curr. Dev. Nutr. 2018, 2, nzy015. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.; Basiri, R. Amino Acids, B Vitamins, and Choline May Independently and Collaboratively Influence the Incidence and Core Symptoms of Autism Spectrum Disorder. Nutrients 2022, 14, 2896. [Google Scholar] [CrossRef]
- Friedman, S.D.; Shaw, D.W.; Artru, A.A.; Dawson, G.; Petropoulos, H.; Dager, S.R. Gray and white matter brain chemistry in young children with autism. Arch. Gen. Psychiatry 2006, 63, 786–794. [Google Scholar] [CrossRef]
- Corrigan, N.M.; Shaw, D.W.; Estes, A.M.; Richards, T.L.; Munson, J.; Friedman, S.D.; Dawson, G.; Artru, A.A.; Dager, S.R. Atypical developmental patterns of brain chemistry in children with autism spectrum disorder. JAMA Psychiatry 2013, 70, 964–974. [Google Scholar] [CrossRef]
- O’Neill, J.; Bansal, R.; Goh, S.; Rodie, M.; Sawardekar, S.; Peterson, B.S. Parsing the Heterogeneity of Brain Metabolic Disturbances in Autism Spectrum Disorder. Biol. Psychiatry 2020, 87, 174–184. [Google Scholar] [CrossRef]
- Margari, L.; De Giacomo, A.; Craig, F.; Palumbi, R.; Peschechera, A.; Margari, M.; Picardi, F.; Caldarola, M.; Maghenzani, M.A.; Dicuonzo, F. Frontal lobe metabolic alterations in autism spectrum disorder: A (1)H-magnetic resonance spectroscopy study. Neuropsychiatr. Dis. Treat. 2018, 14, 1871–1876. [Google Scholar] [CrossRef]
- Hardan, A.Y.; Minshew, N.J.; Melhem, N.M.; Srihari, S.; Jo, B.; Bansal, R.; Keshavan, M.S.; Stanley, J.A. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Res. 2008, 163, 97–105. [Google Scholar] [CrossRef]
- Doyle-Thomas, K.A.; Card, D.; Soorya, L.V.; Wang, A.T.; Fan, J.; Anagnostou, E. Metabolic mapping of deep brain structures and associations with symptomatology in autism spectrum disorders. Res. Autism Spectr. Disord. 2014, 8, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, R.; Xu, Y.; Zhou, Z.; Guan, P.; Wu, Y.; Zhou, J.; Cheng, Z.; Zhang, L. Altered Metabolic Characteristics in Plasma of Young Boys with Autism Spectrum Disorder. J. Autism Dev. Disord. 2022, 52, 4897–4907. [Google Scholar] [CrossRef] [PubMed]
- Gabis, L.V.; Ben-Hur, R.; Shefer, S.; Jokel, A.; Shalom, D.B. Improvement of Language in Children with Autism with Combined Donepezil and Choline Treatment. J. Mol. Neurosci. 2019, 69, 224–234. [Google Scholar] [CrossRef]
- Hamlin, J.C.; Pauly, M.; Melnyk, S.; Pavliv, O.; Starrett, W.; Crook, T.A.; James, S.J. Dietary intake and plasma levels of choline and betaine in children with autism spectrum disorders. Autism Res. Treat. 2013, 2013, 578429. [Google Scholar] [CrossRef]
- Hyman, S.L.; Stewart, P.A.; Schmidt, B.; Cain, U.; Lemcke, N.; Foley, J.T.; Peck, R.; Clemons, T.; Reynolds, A.; Johnson, C.; et al. Nutrient intake from food in children with autism. Pediatrics 2012, 130 (Suppl. S2), S145–S153. [Google Scholar] [CrossRef]
- Alger, J.R.; O’Neill, J.; O’Connor, M.J.; Kalender, G.; Ly, R.; Ng, A.; Dillon, A.; Narr, K.L.; Loo, S.K.; Levitt, J.G. Neuroimaging of Supraventricular Frontal White Matter in Children with Familial Attention-Deficit Hyperactivity Disorder and Attention-Deficit Hyperactivity Disorder Due to Prenatal Alcohol Exposure. Neurotox. Res. 2021, 39, 1054–1075. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; O’Connor, M.J.; Yee, V.; Ly, R.; Narr, K.; Alger, J.R.; Levitt, J.G. Differential neuroimaging indices in prefrontal white matter in prenatal alcohol-associated ADHD versus idiopathic ADHD. Birth Defects Res. 2019, 111, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zang, Y.F.; Zeng, Y.W.; Zhang, L.; Wang, Y.F. Striatal neuronal loss or dysfunction and choline rise in children with attention-deficit hyperactivity disorder: A 1H-magnetic resonance spectroscopy study. Neurosci. Lett. 2001, 315, 45–48. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Fink, B.A.; Fuglestad, A.J.; Eckerle, J.K.; Boys, C.J.; Sandness, K.E.; Radke, J.P.; Miller, N.C.; Lindgren, C.; Brearley, A.M.; et al. Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J. Neurodev. Disord. 2020, 12, 9. [Google Scholar] [CrossRef]
- Borlase, N.; Melzer, T.R.; Eggleston, M.J.F.; Darling, K.A.; Rucklidge, J.J. Resting-state networks and neurometabolites in children with ADHD after 10 weeks of treatment with micronutrients: Results of a randomised placebo-controlled trial. Nutr. Neurosci. 2020, 23, 876–886. [Google Scholar] [CrossRef]
- Carrey, N.; MacMaster, F.P.; Fogel, J.; Sparkes, S.; Waschbusch, D.; Sullivan, S.; Schmidt, M. Metabolite changes resulting from treatment in children with ADHD: A 1H-MRS study. Clin. Neuropharmacol. 2003, 26, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, G.; Ende, G.; Alm, B.; Deuschle, M.; Heuser, I.; Colla, M. Increased NAA and reduced choline levels in the anterior cingulum following chronic methylphenidate. A spectroscopic test-retest study in adult ADHD. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Russell, V.A.; Oades, R.D.; Tannock, R.; Killeen, P.R.; Auerbach, J.G.; Johansen, E.B.; Sagvolden, T. Response variability in Attention-Deficit/Hyperactivity Disorder: A neuronal and glial energetics hypothesis. Behav. Brain Funct. 2006, 2, 30. [Google Scholar] [CrossRef]
- Wiguna, T.; Guerrero, A.P.; Wibisono, S.; Sastroasmoro, S. Effect of 12-week administration of 20-mg long-acting methylphenidate on Glu/Cr, NAA/Cr, Cho/Cr, and mI/Cr ratios in the prefrontal cortices of school-age children in Indonesia: A study using 1H magnetic resonance spectroscopy (MRS). Clin. Neuropharmacol. 2012, 35, 81–85. [Google Scholar] [CrossRef]
- Horowitz-Kraus, T.; Brunst, K.J.; Cecil, K.M. Children With Dyslexia and Typical Readers: Sex-Based Choline Differences Revealed Using Proton Magnetic Resonance Spectroscopy Acquired Within Anterior Cingulate Cortex. Front. Hum. Neurosci. 2018, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Pugh, K.R.; Frost, S.J.; Rothman, D.L.; Hoeft, F.; Del Tufo, S.N.; Mason, G.F.; Molfese, P.J.; Mencl, W.E.; Grigorenko, E.L.; Landi, N.; et al. Glutamate and choline levels predict individual differences in reading ability in emergent readers. J. Neurosci. 2014, 34, 4082–4089. [Google Scholar] [CrossRef] [PubMed]
- Del Tufo, S.N.; Frost, S.J.; Hoeft, F.; Cutting, L.E.; Molfese, P.J.; Mason, G.F.; Rothman, D.L.; Fulbright, R.K.; Pugh, K.R. Neurochemistry Predicts Convergence of Written and Spoken Language: A Proton Magnetic Resonance Spectroscopy Study of Cross-Modal Language Integration. Front. Psychol. 2018, 9, 1507. [Google Scholar] [CrossRef]
- Bruno, J.L.; Lu, Z.L.; Manis, F.R. Phonological processing is uniquely associated with neuro-metabolic concentration. Neuroimage 2013, 67, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Laycock, S.K.; Wilkinson, I.D.; Wallis, L.I.; Darwent, G.; Wonders, S.H.; Fawcett, A.J.; Griffiths, P.D.; Nicolson, R.I. Cerebellar volume and cerebellar metabolic characteristics in adults with dyslexia. Ann. N. Y. Acad. Sci. 2008, 1145, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, J.D.; Dougherty, R.F.; Ben-Shachar, M.; Wandell, B.A. Development of white matter and reading skills. Proc. Natl. Acad. Sci. USA 2012, 109, E3045–E3053. [Google Scholar] [CrossRef]
- Wandell, B.A.; Yeatman, J.D. Biological development of reading circuits. Curr. Opin. Neurobiol. 2013, 23, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kossowski, B.; Chyl, K.; Kacprzak, A.; Bogorodzki, P.; Jednorog, K. Dyslexia and age related effects in the neurometabolites concentration in the visual and temporo-parietal cortex. Sci. Rep. 2019, 9, 5096. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.J.; Brown, F.C.; Roth, R.M.; Beers, S.R. Processing speed and working memory performance in those with both ADHD and a reading disorder compared with those with ADHD alone. Arch. Clin. Neuropsychol. 2011, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef]
- Bahnfleth, C.L.; Strupp, B.J.; Caudill, M.A.; Canfield, R.L. Prenatal choline supplementation improves child sustained attention: A 7-year follow-up of a randomized controlled feeding trial. FASEB J. 2022, 36, e22054. [Google Scholar] [CrossRef]
- Nevins, J.E.H.; Donovan, S.M.; Snetselaar, L.; Dewey, K.G.; Novotny, R.; Stang, J.; Taveras, E.M.; Kleinman, R.E.; Bailey, R.L.; Raghavan, R.; et al. Omega-3 Fatty Acid Dietary Supplements Consumed During Pregnancy and Lactation and Child Neurodevelopment: A Systematic Review. J. Nutr. 2021, 151, 3483–3494. [Google Scholar] [CrossRef]
- Davis-Bruno, K.; Tassinari, M.S. Essential fatty acid supplementation of DHA and ARA and effects on neurodevelopment across animal species: A review of the literature. Birth Defects Res. B Dev. Reprod. Toxicol. 2011, 92, 240–250. [Google Scholar] [CrossRef]
- Richardson, A.J. Omega-3 fatty acids in ADHD and related neurodevelopmental disorders. Int. Rev. Psychiatry 2006, 18, 155–172. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Mayr, J.A. Choline-related-inherited metabolic diseases-A mini review. J. Inherit. Metab. Dis. 2019, 42, 237–242. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Amenta, F. Choline-containing phospholipids: Relevance to brain functional pathways. Clin. Chem. Lab. Med. 2013, 51, 513–521. [Google Scholar] [CrossRef]
- Lebel, C.; MacMaster, F.P.; Dewey, D. Brain metabolite levels and language abilities in preschool children. Brain Behav. 2016, 6, e00547. [Google Scholar] [CrossRef] [PubMed]
- Benamor, L. (1)H-Magnetic resonance spectroscopy study of stimulant medication effect on brain metabolites in French Canadian children with attention deficit hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2014, 10, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Mirizzi, P.; Fadda, R.; Pirollo, C.; Ricciardi, O.; Mazza, M.; Valenti, M. Food Selectivity in Children with Autism: Guidelines for Assessment and Clinical Interventions. Int. J. Environ. Res. Public. Health 2023, 20, 5092. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Olsen, A.; Olafsdottir, A.S. Fussy Eating among Children and Their Parents: Associations in Parent-Child Dyads, in a Sample of Children with and without Neurodevelopmental Disorders. Nutrients 2021, 13, 2196. [Google Scholar] [CrossRef]
- Stein, J. The current status of the magnocellular theory of developmental dyslexia. Neuropsychologia 2019, 130, 66–77. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Zeisel, S.H. Choline: The Neurocognitive Essential Nutrient of Interest to Obstetricians and Gynecologists. J. Diet. Suppl. 2020, 17, 733–752. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Natker, E.; Zeisel, S.H.; Zelman, K.M. Choline: The Underconsumed and Underappreciated Essential Nutrient. Nutr. Today 2018, 53, 240–253. [Google Scholar] [CrossRef]
- AMA. AMA Backs Global Health Experts in Calling Infertility a Disease. Available online: https://www.ama-assn.org/delivering-care/public-health/ama-backs-global-health-experts-calling-infertility-disease#:~:text=Experts%20at%20the%20World%20Health%20Organization%20%28WHO%29%20and,of%20WHO%E2%80%99s%20designation%20of%20infertility%20as%20a%20disease (accessed on 17 April 2023).
- AAP. Food for Thought: American Academy of Pediatrics Aims to Ensure Kids Get Key Nutrients for Brain Development during First 1000 Days of Life. Available online: https://www.healthychildren.org/English/news/Pages/AAP-aims-to-ensure-kids-get-nutrients-for-brain-development-policy.aspx (accessed on 17 April 2023).
- Vennemann, F.B.; Ioannidou, S.; Valsta, L.M.; Dumas, C.; Ocke, M.C.; Mensink, G.B.; Lindtner, O.; Virtanen, S.M.; Tlustos, C.; D’Addezio, L.; et al. Dietary intake and food sources of choline in European populations. Br. J. Nutr. 2015, 114, 2046–2055. [Google Scholar] [CrossRef]
- Adams, J.B.; Kirby, J.K.; Sorensen, J.C.; Pollard, E.L.; Audhya, T. Evidence based recommendations for an optimal prenatal supplement for women in the US: Vitamins and related nutrients. Matern. Health Neonatol. Perinatol. 2022, 8, 4. [Google Scholar] [CrossRef]
- Growdon, J.H.; Cohen, E.L.; Wurtman, R.J. Huntington’s disease: Clinical and chemical effects of choline administration. Ann. Neurol. 1977, 1, 418–422. [Google Scholar] [CrossRef]
- Gelenberg, A.J.; Doller-Wojcik, J.C.; Growdon, J.H. Choline and lecithin in the treatment of tardive dyskinesia: Preliminary results from a pilot study. Am. J. Psychiatry 1979, 136, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.M.; Millac, P.; Stout, G.S.; Ward, J.W. The use of choline chloride in ataxic disorders. J. Neurol. Neurosurg. Psychiatry 1980, 43, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Could we be overlooking a potential choline crisis in the United Kingdom? BMJ Nutr. Prev. Health 2019, 2, 86–89. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
