The Effect of Hyperbaric Storage on the Nutritional Value and Retention of Certain Bioactive Proteins in Human Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
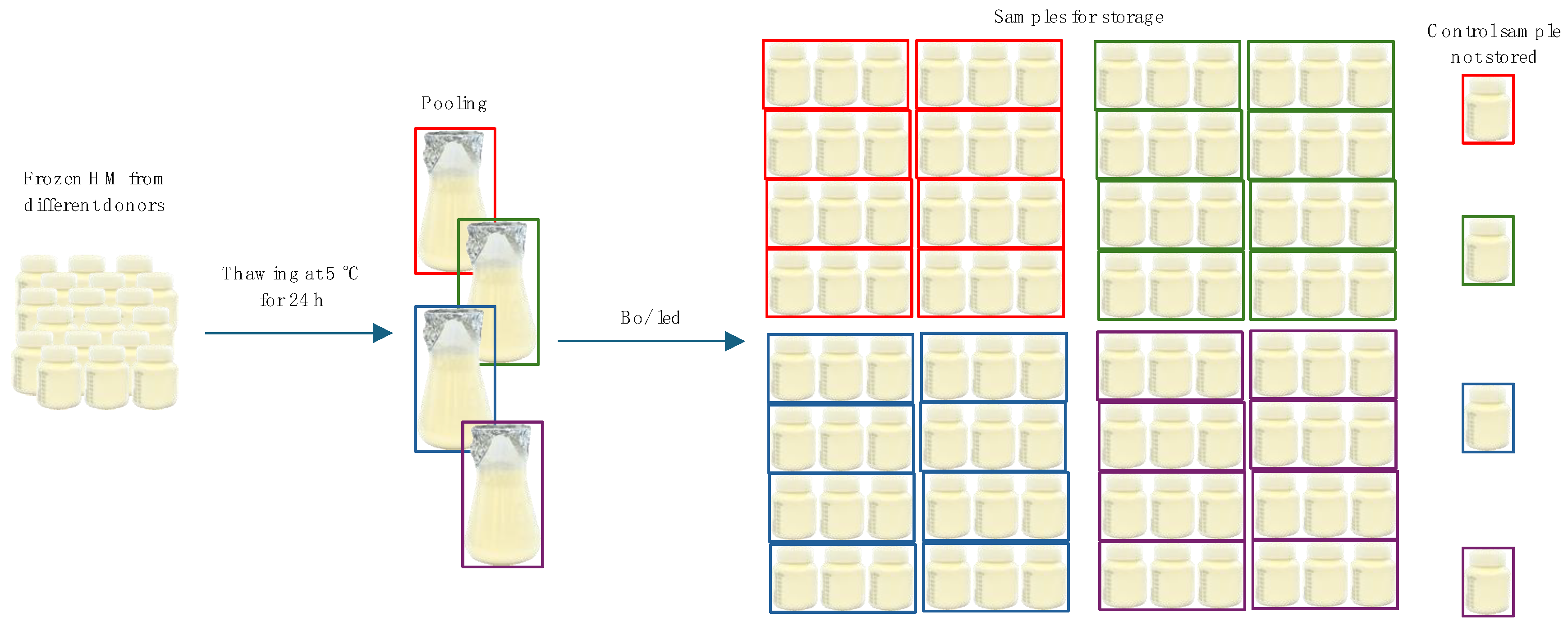
2.2. Sample Preparation for Storage
2.3. Storage
2.4. Determination of Essential Macronutrients and the Energy Value
2.5. Determination of α-LAC, LYZ and LF Content Using High-Performance Liquid Chromatography (HPLC)
2.6. Determination of sIgA Content
2.7. Statistical Analysis
3. Results and Discussion
3.1. Macronutrient Content and Energy Value of HM
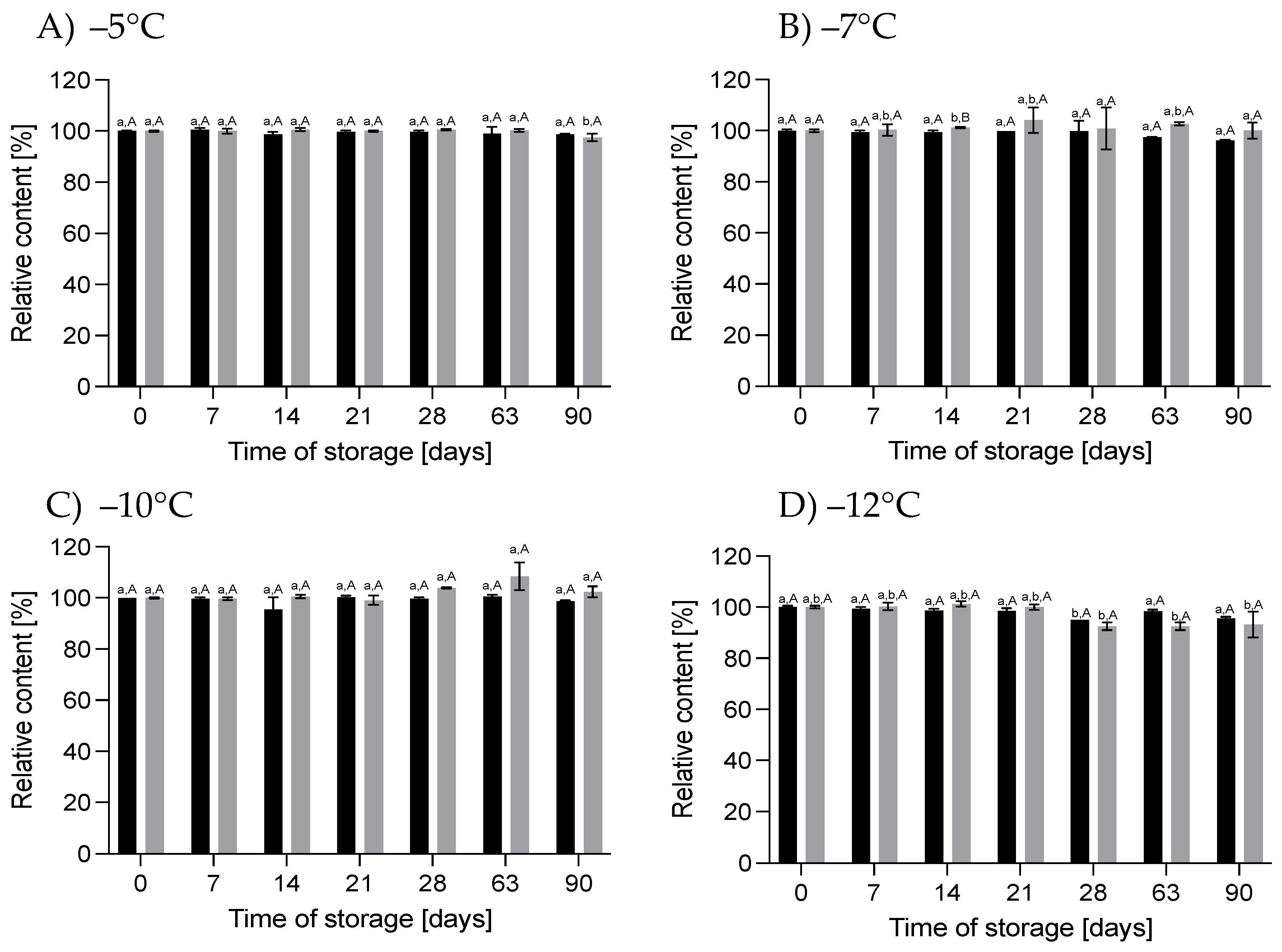
3.1.1. Effect of HS-ST on Proteins
3.1.2. Effect of HS-ST on Carbohydrates
3.1.3. Effect of HS-ST on Fat and the Energy Value
3.2. Bioactive Protein Content of HM
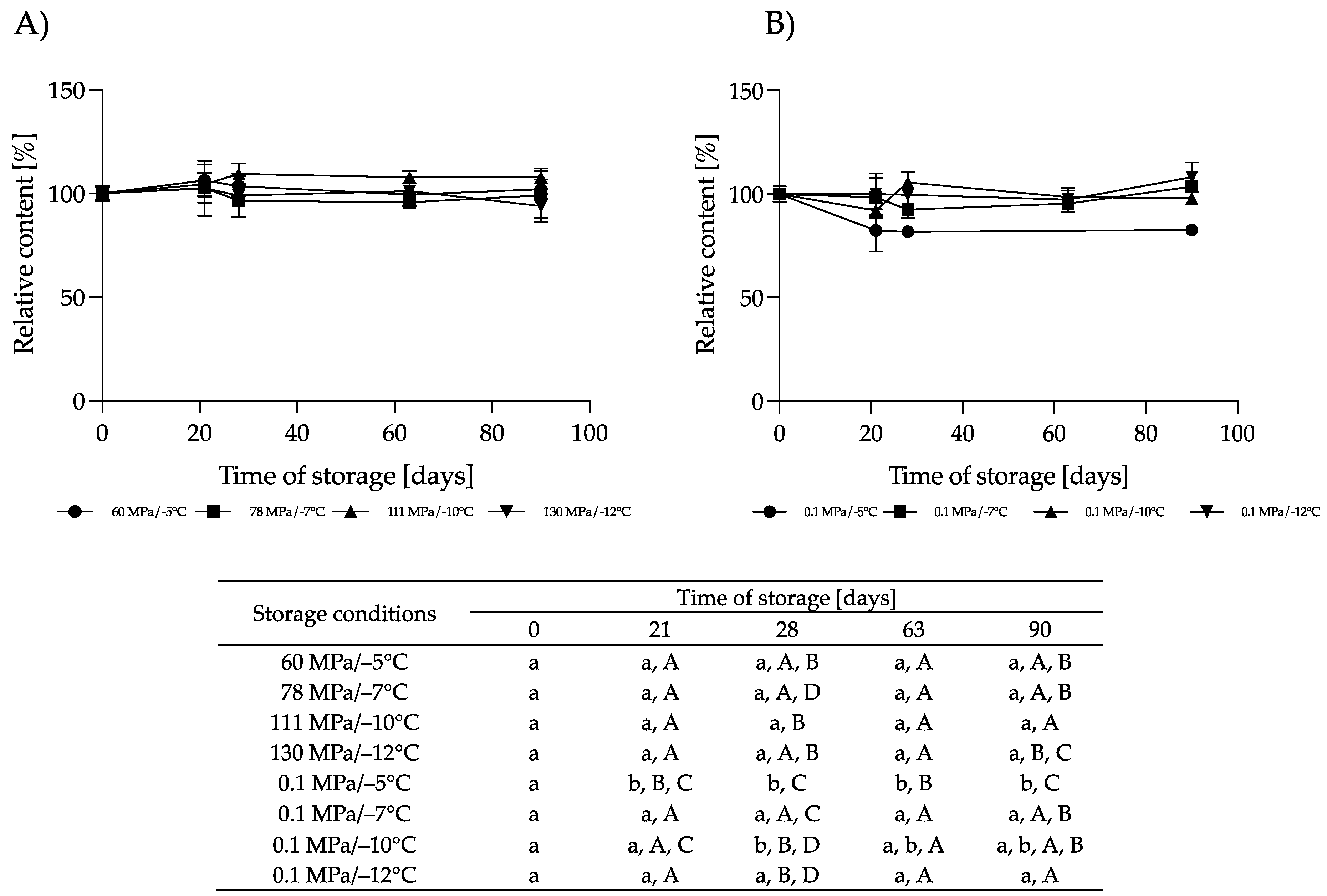
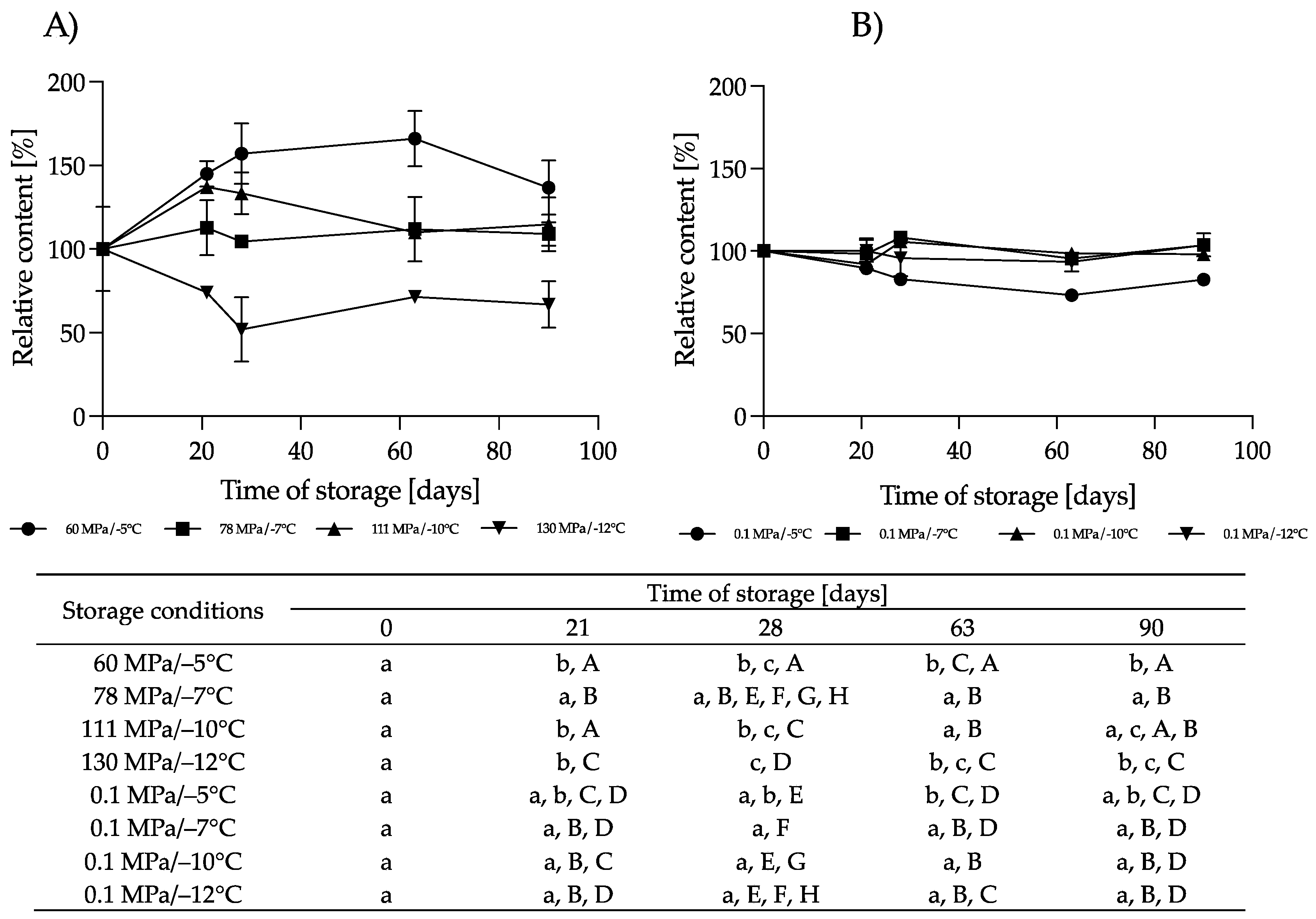
3.2.1. Effect of HS-ST on α-LAC
3.2.2. Effect of HS-ST on LF
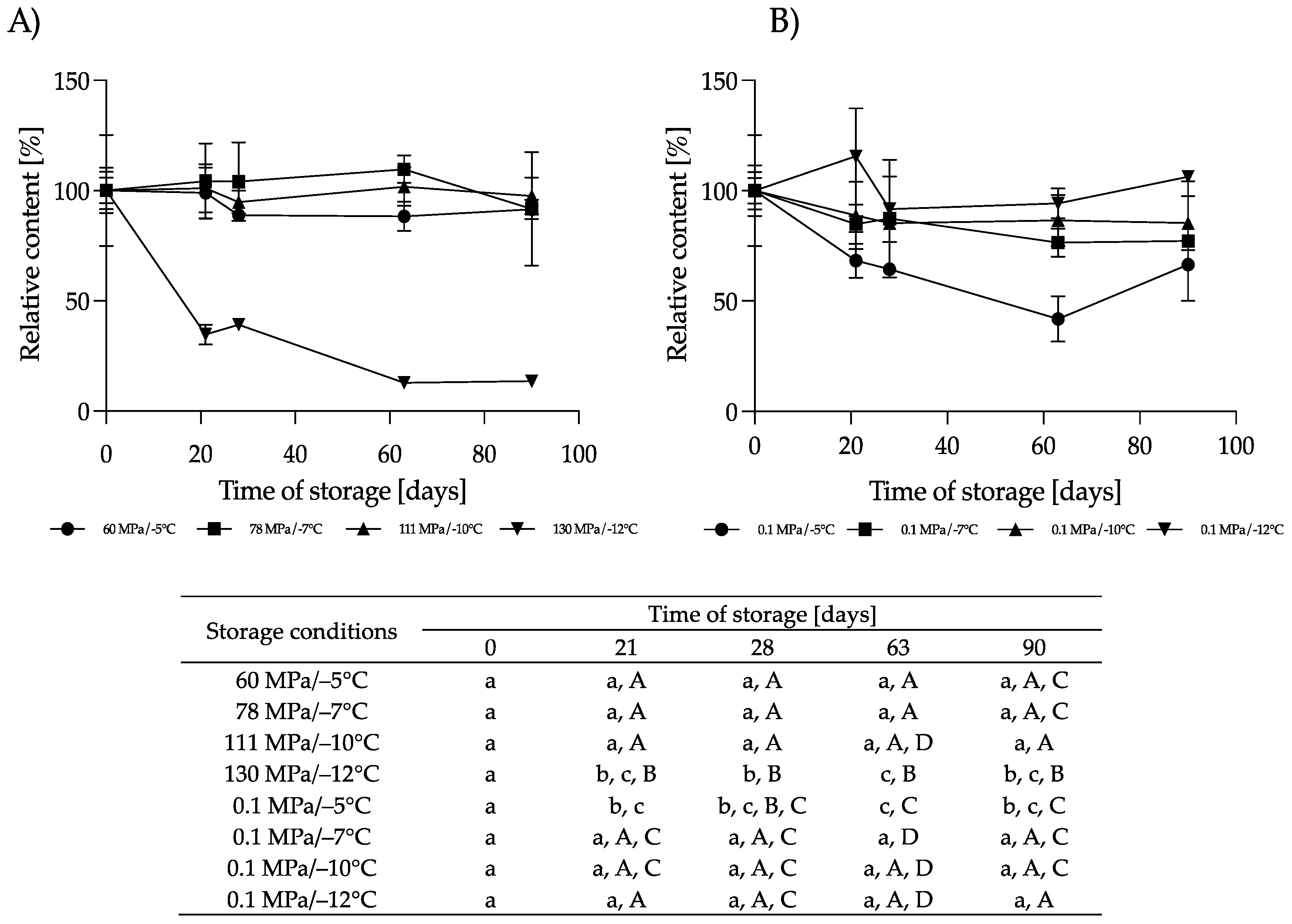
3.2.3. Effect of HS-ST on LYZ
3.2.4. Effect of HS-ST on sIgA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samuel, T.M.; Zhou, Q.; Giuffrida, F.; Munblit, D.; Verhasselt, V.; Thakkar, S.K. Nutritional and Non-Nutritional Composition of Human Milk Is Modulated by Maternal, Infant, and Methodological Factors. Front. Nutr. 2020, 7, 576133. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st Century: Epidemiology, Mechanisms, and Lifelong Effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Rollo, D.E.; Radmacher, P.G.; Turcu, R.M.; Myers, S.R.; Adamkin, D.H. Stability of Lactoferrin in Stored Human Milk. J. Perinatol. 2014, 34, 284–286. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, N.R.; Vieco, D.E.; De La Cruz-Bértolo, J.; Lora-Pablos, D.; Velasco, N.U.; Pallás-Alonso, C.R. Effect of Holder Pasteurization and Frozen Storage on Macronutrients and Energy Content of Breast Milk. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Friend, B.A.; Shahani, K.M.; Long, C.A.; Vaughn, L.A. The Effect of Processing and Storage on Key Enzymes, B Vitamins, and Lipids of Mature Human Milk. I. Evaluation of Fresh Samples and Effects of Freezing and Frozen Storage. Pediatr. Res. 1983, 17, 61–64. [Google Scholar] [CrossRef]
- Dussault, N.; Cayer, M.P.; Landry, P.; De Grandmont, M.J.; Cloutier, M.; Thibault, L.; Girard, M. Comparison of the Effect of Holder Pasteurization and High-Pressure Processing on Human Milk Bacterial Load and Bioactive Factors Preservation. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 756–762. [Google Scholar] [CrossRef]
- Malinowska-Pańczyk, E. Can High Hydrostatic Pressure Processing Be the Best Way to Preserve Human Milk? Trends Food Sci. Technol. 2020, 101, 133–138. [Google Scholar] [CrossRef]
- Santos, M.D.; Fidalgo, L.G.; Pinto, C.A.; Duarte, R.V.; Lemos, Á.T.; Delgadillo, I.; Saraiva, J.A. Hyperbaric Storage at Room like Temperatures as a Possible Alternative to Refrigeration: Evolution and Recent Advances. Crit. Rev. Food Sci. Nutr. 2021, 61, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Malinowska-Pańczyk, E.; Mazur, K.; Martysiak-Żurowska, D. A New Method of Human Milk Preservation: Storage in Unfrozen State under High Pressure-Subzero Temperature Conditions. In Proceedings of the 7th International Congress og the European Milk Bank Association (EMBA), Madrid, Spain, 25–27 October 2023; p. 100. [Google Scholar]
- Bridgman, P.W. Water, in the Liquid and Five Solid Forms, under Pressure. Available online: https://www.jstor.org/stable/pdf/20022754.pdf (accessed on 22 April 2024).
- Malinowska-Pańczyk, E.; Mazur, K.; Martysiak-Żurowska, D.; Kusznierewicz, B. Storage of Human Milk in a Unfrozen State under Moderate Pressure at Subzero Temperature- a New Method of Preservation. PL Patent Application P.447629, 29 January 2024. [Google Scholar]
- Miris, A.B. Available online: https://www.mirissolutions.com/support/user-manuals (accessed on 22 April 2024).
- Bakar, S.Y.B.A.; Salim, M.; Clulow, A.J.; Nicholas, K.R.; Boyd, B.J. Human Milk Composition and the Effects of Pasteurisation on the Activity of Its Components. Trends Food Sci. Technol. 2021, 111, 166–174. [Google Scholar] [CrossRef]
- Goóes, H.C.A.; Torres, A.G.; Donangelo, C.M.; Trugo, N.M.F. Nutrient Composition of Banked Human Milk in Brazil and Influence of Processing on Zinc Distribution in Milk Fractions. Nutrition 2002, 18, 590–594. [Google Scholar] [CrossRef]
- Quinn, E.A.; Bista, K.D.; Childs, G. Milk at Altitude: Human Milk Macronutrient Composition in a High-Altitude Adapted Population of Tibetans. Am. J. Phys. Anthropol. 2016, 159, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Sever, O.; Mandel, D.; Mimouni, F.B.; Marom, R.; Cohen, S.; Lubetzky, R. Macronutrients in Human Milk: Colostrum Lactose but Not Fat or Protein Predicts Mature Human Milk Content. Infant. Child. Adolesc. Nutr. 2015, 7, 162–165. [Google Scholar] [CrossRef]
- Binder, C.; Baumgartner-Parzer, S.; Gard, L.I.; Berger, A.; Thajer, A. Human Milk Processing and Its Effect on Protein and Leptin Concentrations. Nutrients 2023, 15, 347. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, K.Y.; Rechtman, D.J.; Lee, M.L.; Montoya, A.; Medo, E.T. Macronutrient Analysis of a Nationwide Sample of Donor Breast Milk. J. Am. Diet. Assoc. 2009, 109, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, P.; Mallardi, D.; Liotto, N.; Tabasso, C.; Menis, C.; Perrone, M.; Roggero, P.; Mosca, F. Macronutrient Content of Pooled Donor Human Milk before and after Holder Pasteurization. BMC Pediatr. 2019, 19, 58. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, Z.; Ren, Y.; Duan, Y.; Gao, H.; Liu, B.; Ye, W.; Wang, J.; Yin, S. Comparison of Macronutrient Contents in Human Milk Measured Using Mid-Infrared Human Milk Analyser in a Field Study vs. Chemical Reference Methods. Matern. Child Nutr. 2017, 13, e12248. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.A.; Soares, F.V.M.; Pimenta, H.P.; Abranches, A.D.; Moreira, M.E.L. Analysis of the Influence of Pasteurization, Freezing/Thawing, and Offer Processes on Human Milk’s Macronutrient Concentrations. Early Hum. Dev. 2011, 87, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Mandel, D.; Mangel, L.; Marom, R.; Lubetzky, R. The Effect of Deep Freezing on Human Milk Macronutrients Content. Breastfeed. Med. 2019, 14, 172–176. [Google Scholar] [CrossRef]
- Sousa, S.G.; Delgadillo, I.; Saraiva, J.A. Human Milk Composition and Preservation: Evaluation of High-Pressure Processing as a Nonthermal Pasteurization Technology. Crit. Rev. Food Sci. Nutr. 2016, 56, 1043–1060. [Google Scholar] [CrossRef]
- Manin, L.P.; Rydlewski, A.A.; Pizzo, J.S.; da Cruz, V.H.M.; da Silva Alves, E.; Santos, P.D.S.; Mikcha, J.M.G.; Cristianini, M.; Santos, O.O.; Visentainer, J.V. Effects of Pasteurization and High-Pressure Processing on the Fatty Acids, Triacylglycerol Profile, Dornic Acidity, and Macronutrients in Mature Human Milk. J. Food Compos. Anal. 2023, 115, 104918. [Google Scholar] [CrossRef]
- Pitino, M.A.; Unger, S.; Doyen, A.; Pouliot, Y.; Aufreiter, S.; Stone, D.; Kiss, A.; O’Connor, D.L. High Hydrostatic Pressure Processing Better Preserves the Nutrient and Bioactive Compound Composition of Human Donor Milk. J. Nutr. 2019, 149, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chen, C.H.; Lin, M.C. The Macronutrients in Human Milk Change after Storage in Various Containers. Pediatr. Neonatol. 2012, 53, 205–209. [Google Scholar] [CrossRef] [PubMed]
- De Waard, M.; Mank, E.; Van Dijk, K.; Schoonderwoerd, A.; Van Goudoever, J.B. Holder-Pasteurized Human Donor Milk: How Long Can It Be Preserved? J. Pediatr. Gastroenterol. Nutr. 2018, 66, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.Y.; Fang, L.J.; Chang, C.S.; Wu, T.Z. The Effect of Processing Donor Milk on Its Nutrient and Energy Content. Breastfeed. Med. 2020, 15, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Berkow, S.E.; Freed, L.M.; Hamosh, M.; Bitman, J.; Wood, D.L.; Happ, B.; Hamosh, P. Lipases and Lipids in Human Milk: Effect of Freeze-Thawing and Storage. Pediatr. Res. 1984, 18, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Dill, C.W.; Chen, C.T.; Alford, E.S.; Edwards, R.L.; Richter, R.L.; Garza, C. Lipolytic Activity During Storage of Human Milk: Accumulation of Free Fatty Acids. J. Food Prot. 1984, 47, 690–693. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive Proteins in Human Milk: Health, Nutrition, and Implications for Infant Formulas. J. Pediatr. 2016, 173, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Forsum, E. Determination of α-Lactalbumin in Human Milk. J. Dairy. Sci. 1976, 59, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-Lactalbumin in Human Nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Montagne, P.; Cuillière, M.L.; Molé, C.; Béné, M.C.; Faure, G. Changes in Lactoferrin and Lysozyme Levels in Human Milk during the First Twelve Weeks of Lactation. Adv. Exp. Med. Biol. 2001, 501, 241–247. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Lis-Kuberka, J.; Królak-Olejnik, B.; Orczyk-Pawiłowicz, M. Changes in Human Milk Immunoglobulin Profile During Prolonged Lactation. Front. Pediatr. 2020, 8, 524094. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Malinowska-Pańczyk, E.; Orzołek, M.; Kusznierewicz, B.; Kiełbratowska, B. Effect of Microwave and Convection Heating on Selected Nutrients of Human Milk. Food Chem. 2022, 369, 130958. [Google Scholar] [CrossRef]
- Vigolo, V.; Niero, G.; Penasa, M.; De Marchi, M. Effects of Preservative, Storage Time, and Temperature of Analysis on Detailed Milk Protein Composition Determined by Reversed-Phase High-Performance Liquid Chromatography. J. Dairy Sci. 2022, 105, 7917–7925. [Google Scholar] [CrossRef]
- Mazri, C.; Sánchez, L.; Ramos, S.J.; Calvo, M.; Pérez, M.D. Effect of High-Pressure Treatment on Denaturation of Bovine Lactoferrin and Lactoperoxidase. J. Dairy Sci. 2012, 95, 549–557. [Google Scholar] [CrossRef]
- Czank, C.; Prime, D.K.; Hartmann, B.; Simmer, K.; Hartmann, P.E. Retention of the Immunological Proteins of Pasteurized Human Milk in Relation to Pasteurizer Design and Practice. Pediatr. Res. 2009, 66, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.E.; Law, B.A.; Marshall, V.M.E.; Reiter, B. Influence of the Heat Treatment of Human Milk on Some of Its Protective Constituents. J. Pediatr. 1977, 90, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Martysiak-Żurowska, D.; Malinowska-Pańczyk, E.; Orzołek, M.; Kiełbratowska, B.; Sinkiewicz–Darol, E. Effect of Convection and Microwave Heating on the Retention of Bioactive Components in Human Milk. Food Chem. 2022, 374, 131772. [Google Scholar] [CrossRef]
- Raoof, N.A.; Adamkin, D.H.; Radmacher, P.G.; Telang, S. Comparison of Lactoferrin Activity in Fresh and Stored Human Milk. J. Perinatol. 2015, 36, 207–209. [Google Scholar] [CrossRef]
- Hamosh, M. Protective Function of Proteins and Lipids in Human Milk. Biol. Neonate 1998, 74, 163–176. [Google Scholar] [CrossRef]
- Pacheco Golinelli, L.; Mere Del Aguila, E.; Margaret Flosi Paschoalin, V.; Trajano Silva, J.; Adam Conte-Junior, C. Functional Aspect of Colostrum and Whey Proteins in Human Milk. J. Hum. Nutr. Food Sci. 2014, 2, 1035. [Google Scholar]
- Lima, H.K.; Wagner-Gillespie, M.; Hubble, C.; Vogel, K.; Perrin, M.; Fogleman, A.D. Effect of Holder Pasteurization and Retort Processing on Bioactive Components and Nutritional Content of Human Milk. FASEB J. 2017, 31, 958. [Google Scholar] [CrossRef]
- Adhisivam, B.; Vishnu Bhat, B.; Rao, K.; Kingsley, S.M.; Plakkal, N.; Palanivel, C. Effect of Holder Pasteurization on Macronutrients and Immunoglobulin Profile of Pooled Donor Human Milk. J. Matern. Fetal Neonatal Med. 2019, 32, 3016–3019. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Value * |
|---|---|
| No. of donors | 25 |
| Age (years) | 30.68 ± 5.14 (19–39) |
| Gestation number (n) | 1.64 ± 0.99 (1–4) |
| Gestation lenght (weeks) | 37.3 ± 4.1 (28–41) |
| Birthweight (g) | 3053.4 ± 1093.8 (850–4900) |
| Apgar scores (Apgar scale) | 8.9 ± 1.9 (1–10) |
| Child’s gender | female 8, male 17 |
| Lactation period (days) | 138.2 ± 158.7 (3–803) |
| Macronutrient | Content |
|---|---|
| Crude protein [g/100 mL] | 1.25 ± 0.155 |
| True protein [g/100 mL] | 1.01 ± 0.131 |
| Carbohydrates [g/100 mL] | 8.36 ± 0.072 |
| Fat [g/100 mL] | 3.96 ± 0.416 |
| Energy value [kcal/100 mL] | 75.375 ± 4.256 |
| Bioactive Proteins | |
| Lactoferrine [g/100 mL] | 0.305 ± 0.0147 |
| α-lactalbumin [g/100 mL] | 0.272 ± 0.0597 |
| Lysozyme [g/100 mL] | 0.013 ± 0.0008 |
| sIgA [g/100 mL] | 0.107 ± 0.0486 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, K.; Kusznierewicz, B.; Martysiak-Żurowska, D.; Drążkowska, I.; Malinowska-Pańczyk, E. The Effect of Hyperbaric Storage on the Nutritional Value and Retention of Certain Bioactive Proteins in Human Milk. Nutrients 2024, 16, 1455. https://doi.org/10.3390/nu16101455
Mazur K, Kusznierewicz B, Martysiak-Żurowska D, Drążkowska I, Malinowska-Pańczyk E. The Effect of Hyperbaric Storage on the Nutritional Value and Retention of Certain Bioactive Proteins in Human Milk. Nutrients. 2024; 16(10):1455. https://doi.org/10.3390/nu16101455
Chicago/Turabian StyleMazur, Katarzyna, Barbara Kusznierewicz, Dorota Martysiak-Żurowska, Izabela Drążkowska, and Edyta Malinowska-Pańczyk. 2024. "The Effect of Hyperbaric Storage on the Nutritional Value and Retention of Certain Bioactive Proteins in Human Milk" Nutrients 16, no. 10: 1455. https://doi.org/10.3390/nu16101455
APA StyleMazur, K., Kusznierewicz, B., Martysiak-Żurowska, D., Drążkowska, I., & Malinowska-Pańczyk, E. (2024). The Effect of Hyperbaric Storage on the Nutritional Value and Retention of Certain Bioactive Proteins in Human Milk. Nutrients, 16(10), 1455. https://doi.org/10.3390/nu16101455






