The Impact of Chronic Stress Related to COVID-19 on Eating Behaviors and the Risk of Obesity in Children and Adolescents
Abstract
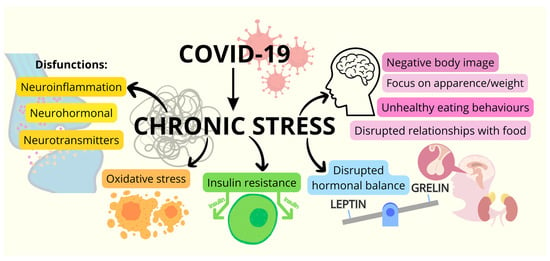
:1. The Impact of Chronic Stress Related to the COVID-19 Pandemic on Eating Behaviors and the Risk of Obesity in Childhood
2. Psychological Aspects of Eating Disorders during the COVID-19 Pandemic
2.1. Mental Health, Pandemic Environment, and Eating Disorders
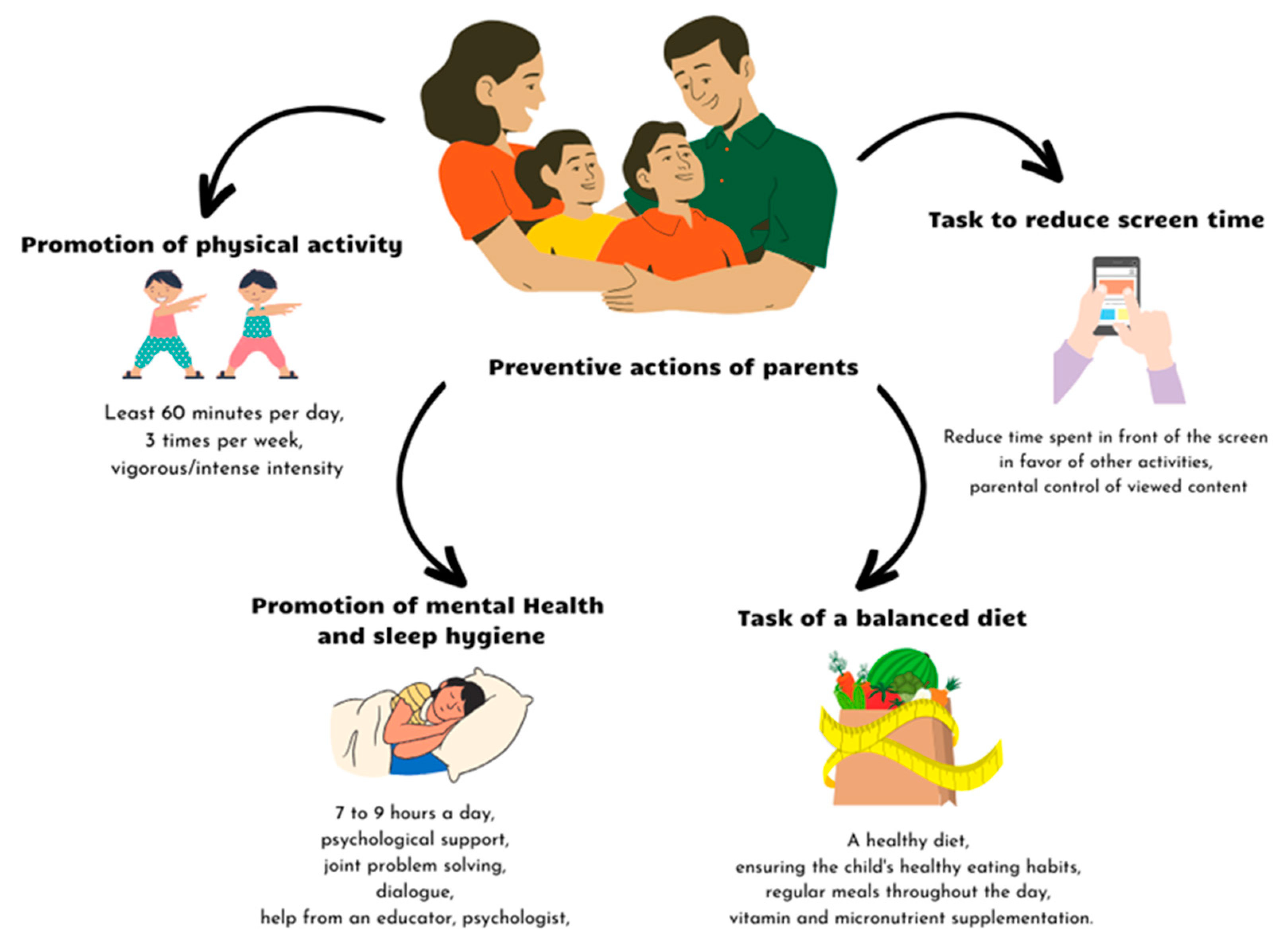
2.2. Prevention of Eating Disorders
3. Medical Aspects of Obesity in Children and Adolescents
3.1. Overview of Obesity in Childhood
3.2. Neurochemical and Molecular Changes Induced by Chronic Stress
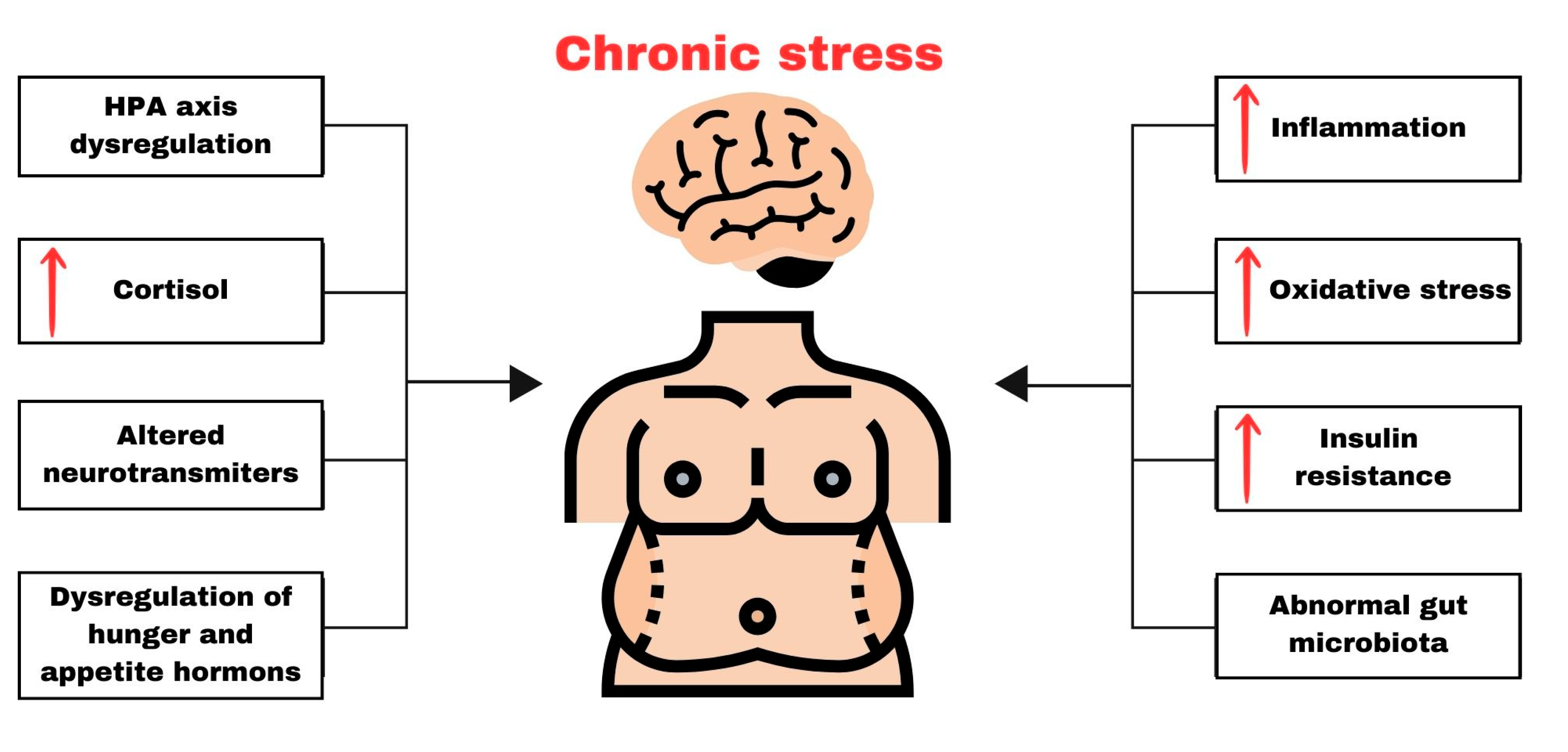
3.2.1. Chronic Activation of the HPA Axis
3.2.2. Chronic Stress, Neurotransmitters, and Obesity
3.2.3. The Impact of Chronic Stress on the Neurohormonal Regulation of Appetite
3.2.4. Chronic Stress, Inflammation, and Obesity
3.2.5. Chronic Stress, Insulin Resistance, and Obesity
3.2.6. Chronic Stress, Oxidative Stress, and Obesity
3.2.7. Chronic Stress, Gut Dysbiosis, and Obesity
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lange, S.J.; Kompaniyets, L.; Freedman, D.S.; Kraus, E.M.; Porter, R.; Blanck, H.M.; Goodman, A.B. Longitudinal Trends in Body Mass Index before and during the COVID-19 Pandemic among Persons Aged 2–19 Years-United States, 2018–2020. Morb. Mortal. Wkly. Rep. 2021, 70, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.H.; Chen, Y.C.; Chen, W.Y.; Chen, C.Y.; Hsu, W.Y.; Chou, Y.; Chang, Y.H. Weight Gain Associated with COVID-19 Lockdown in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3668. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.N.; Yoshida-Montezuma, Y.; Dewart, N.; Jalil, E.; Khattar, J.; De Rubeis, V.; Carsley, S.; Griffith, L.E.; Mbuagbaw, L. Obesity and weight change during the COVID-19 pandemic in children and adults: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13550. [Google Scholar] [CrossRef] [PubMed]
- Woolford, S.J.; Sidell, M.; Li, X.; Else, V.; Young, D.R.; Resnicow, K.; Koebnick, C. Changes in body mass index among children and adolescents during the COVID-19 pandemic. JAMA 2021, 326, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, B.P.; Kelly, M.K.; Powell, M.; Bouchelle, Z.; Mayne, S.L.; Fiks, A.G. COVID-19 and changes in child obesity. Pediatrics 2021, 147, e2021050123. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Geserick, M.; Gausche, R.; Beger, C.; Poulain, T.; Meigen, C.; Körner, A.; Keller, E.; Kiess, W.; Pfäffle, R. Age-and weight group-specific weight gain patterns in children and adolescents during the 15 years before and during the COVID-19 pandemic. Int. J. Obes. 2021, 46, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhang, L.; Yu, W.; Yu, B.; Liu, M.; Zhang, D.; Yang, S. Impact of COVID-19 lockdown on activity patterns and weight status among youths in China: The COVID-19 Impact on Lifestyle Change Survey (COINLICS). Int. J. Obes. 2021, 45, 695–699. [Google Scholar] [CrossRef]
- Torres, S.J.; Nowson, C.A. Relationship between stress, eating behavior, and obesity. Nutrition 2007, 23, 887–894. [Google Scholar] [CrossRef]
- Leigh, S.J.; Uhlig, F.; Wilmes, L.; Sanchez-Diaz, P.; Gheorghe, C.E.; Goodson, M.S.; Kelley-Loughnane, N.; Hyland, N.P.; Cryan, J.F.; Clarke, G. The impact of acute and chronic stress on gastrointestinal physiology and function: A microbiota-gut-brain axis perspective. J. Physiol. 2023, 601, 4491–4538. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Fang, Z.; Hou, G.; Han, M.; Xu, X.; Dong, J.; Zheng, J. The psychological impact of the COVID-19 epidemic on college students in China. Psychiatry Res. 2020, 287, 112934. [Google Scholar] [CrossRef] [PubMed]
- Neville, R.D.; Lakes, K.D.; Hopkins, W.G.; Tarantino, G.; Draper, C.E.; Beck, R.; Madigan, S. Global Changes in Child and Adolescent Physical Activity During the COVID-19 Pandemic: A Systematic Review and Meta-analysis. JAMA Pediatr. 2022, 176, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Warhadpande, M.; Sainz, K.; Jacobson, M.S. Effects of the COVID-19 Pandemic on Pediatric and Adolescent ASCVD Risk Factors. Curr. Atheroscler. Rep. 2023, 25, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. World Obesity Atlas. 2023. Available online: https://data.worldobesity.org/publications/WOF-Obesity-Atlas-V5.pdf (accessed on 29 October 2023).
- Zemrani, B.; Gehri, M.; Masserey, E.; Knob, C.; Pellaton, R. A hidden side of the COVID-19 pandemic in children: The double burden of undernutrition and overnutrition. Int. J. Equity Health 2021, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, M.B.; de Carvalho Padilha, P.; Mantilla-Escalante, D.C.; Ulloa, N.; Brun, P.; Acevedo-Correa, D.; Arantes Ferreira Peres, W.; Martorell, M.; Aires, M.T.; de Oliveira Cardoso, L.; et al. Covid-19 Confinement and Changes of Adolescent’s Dietary Trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients 2020, 12, 1807. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Bekelman, T.A.; Li, M.; Knapp, E.A.; Palmore, M.; Dong, Y.; Elliott, A.J.; Friedman, C.; Galarce, M.; Gilbert-Diamond, D.; et al. Environmental Influences on Child Health Outcomes Program. Trends in Screen Time Use among Children during the COVID-19 Pandemic, July 2019 through August 2021. JAMA Netw Open 2023, 6, e2256157. [Google Scholar] [CrossRef]
- Ouyang, X.; Zhang, X.; Zhang, Q.; Gong, X.; Zhang, R. Preschool children’s screen time during the COVID-19 pandemic: Associations with family characteristics and children’s anxiety/withdrawal and approaches to learning. Curr. Psychol. 2023, 1–15. [Google Scholar] [CrossRef]
- Spence, C. Comfort food: A review. Int. J. Gastron. Food Sci. 2017, 9, 105–109. [Google Scholar] [CrossRef]
- Pourghazi, F.; Eslami, M.; Ehsani, A.; Ejtahed, H.S.; Qorbani, M. Eating habits of children and adolescents during the COVID-19 era: A systematic review. Front. Nutr. 2022, 9, 1004953. [Google Scholar] [CrossRef] [PubMed]
- Trott, M.; Driscoll, R.; Irlado, E.; Pardhan, S. Changes and correlates of screen time in adults and children during the COVID-19 pandemic: A systematic review and meta-analysis. EClinicalMedicine 2022, 48, 101452. [Google Scholar] [CrossRef] [PubMed]
- Branson, V.; Dry, M.J.; Palmer, E.; Turnbull, D. The Adolescent Distress-Eustress Scale: Development and Validation. Sage Open 2019, 9, 2158244019865802. [Google Scholar] [CrossRef]
- Branson, V.; Turnbull, D.; Dry, M.J.; Palmer, E. How Do Young People Experience Stress? A Qualitative Examination of the Indicators of Distress and Eustress in Adolescence. Int. J. Stress. Manag. 2019, 26, 321–329. [Google Scholar] [CrossRef]
- Branson, V.; Palmer, E.; Dry, M.J.; Turnbull, D. A Holistic Understanding of the Effect of Stress on Adolescent Well-Being: A Conditional Process Analysis. Stress. Health 2019, 35, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; McWeeny, R.; Shinkaruk, C.; Baxter, A.; Cao, B.; Greenshaw, A.; Silverstone, P.; Pazderka, H.; Wei, Y. Child and Youth Mental Health and Wellbeing before and after Returning to In-Person Learning in Secondary Schools in the Context of COVID-19. Front. Public. Health 2023, 11, 1212297. [Google Scholar] [CrossRef] [PubMed]
- Dalton, L.; Rapa, E.; Stein, A. Protecting the Psychological Health of Children through Effective Communication about COVID-19. Lancet Child. Adolesc. Health 2020, 4, 346–347. [Google Scholar] [CrossRef]
- Liu, J.J.; Bao, Y.; Huang, X.; Shi, J.; Lu, L. Mental Health Considerations for Children Quarantined Because of COVID-19. Lancet Child. Adolesc. Health 2020, 4, 347–349. [Google Scholar] [CrossRef]
- Imran, N.; Aamer, I.; Sharif, M.I.; Bodla, Z.H.; Naveed, S. Psychological Burden of Quarantine in Children and Adolescents: A Rapid Systematic Review and Proposed Solutions. Pak. J. Med. Sci. 2020, 36, 1106–1116. [Google Scholar] [CrossRef]
- Magson, N.R.; Freeman, J.Y.A.; Rapee, R.M.; Richardson, C.E.; Oar, E.L.; Fardouly, J. Risk and Protective Factors for Prospective Changes in Adolescent Mental Health during the COVID-19 Pandemic. J. Youth Adolesc. 2021, 50, 44. [Google Scholar] [CrossRef]
- Otto, A.K.; Jary, J.M.; Sturza, J.; Miller, C.A.; Prohaska, N.; Bravender, T.; Van Huysse, J. Medical Admissions among Adolescents with Eating Disorders during the COVID-19 Pandemic. Pediatrics 2021, 148, e2021052201. [Google Scholar] [CrossRef] [PubMed]
- Devoe, D.J.; Han, A.; Anderson, A.; Katzman, D.K.; Patten, S.B.; Soumbasis, A.; Flanagan, J.; Paslakis, G.; Vyver, E.; Marcoux, G.; et al. The Impact of the COVID-19 Pandemic on Eating Disorders: A Systematic Review. Int. J. Eat. Disord. 2023, 56, 5–25. [Google Scholar] [CrossRef]
- Paiva, E.D.; da Silva, L.R.; Machado, M.E.D.; de Aguiar, R.C.B.; da Silva Garcia, K.R.; Acioly, P.G.M. Child Behavior during the Social Distancing in the COVID-19 Pandemic. Rev. Bras. Enferm. 2021, 74, e20200762. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.Y.; Wang, L.N.; Liu, J.; Fang, S.F.; Jiao, F.Y.; Pettoello-Mantovani, M.; Somekh, E. Behavioral and Emotional Disorders in Children during the COVID-19 Epidemic. J. Pediatr. 2020, 221, 264. [Google Scholar] [CrossRef] [PubMed]
- Lavigne-Cerván, R.; Costa-López, B.; Juárez-Ruiz de Mier, R.; Real-Fernández, M.; Sánchez-Muñoz de León, M.; Navarro-Soria, I. Consequences of COVID-19 Confinement on Anxiety, Sleep and Executive Functions of Children and Adolescents in Spain. Front. Psychol. 2021, 12, 565516. [Google Scholar] [CrossRef] [PubMed]
- Parent/Caregiver Guide to Helping Families Cope with the Coronavirus Disease 2019 | The National Child Traumatic Stress Network. Available online: https://www.nctsn.org/resources/parent-caregiver-guide-to-helping-families-cope-with-the-coronavirus-disease-2019 (accessed on 31 October 2023).
- Chawla, N.; Tom, A.; Sen, M.S.; Sagar, R. Psychological Impact of COVID-19 on Children and Adolescents: A Systematic Review. Indian J. Psychol. Med. 2021, 43, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Courtney, D.; Watson, P.; Battaglia, M.; Mulsant, B.H.; Szatmari, P. COVID-19 Impacts on Child and Youth Anxiety and Depression: Challenges and Opportunities. Can. J. Psychiatry 2020, 65, 688–691. [Google Scholar] [CrossRef]
- Grover, S.; Venkatesh Raju, V.; Sharma, A.; Shah, R.S. Depression in Children and Adolescents: A Review of Indian Studies. Indian J. Psychol. Med. 2019, 41, 216–227. [Google Scholar] [CrossRef]
- Rice, F.; Riglin, L.; Lomax, T.; Souter, E.; Potter, R.; Smith, D.J.; Thapar, A.K.; Thapar, A. Adolescent and Adult Differences in Major Depression Symptom Profiles. J. Affect. Disord. 2019, 243, 175–181. [Google Scholar] [CrossRef]
- Lindberg, L.; Hagman, E.; Danielsson, P.; Marcus, C.; Persson, M. Anxiety and Depression in Children and Adolescents with Obesity: A Nationwide Study in Sweden. BMC Med. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Mahmood, L.; Flores-Barrantes, P.; Moreno, L.A.; Manios, Y.; Gonzalez-Gil, E.M. The Influence of Parental Dietary Behaviors and Practices on Children’s Eating Habits. Nutrients 2021, 13, 1138. [Google Scholar] [CrossRef] [PubMed]
- Moitra, P.; Madan, J. Impact of Screen Time during COVID-19 on Eating Habits, Physical Activity, Sleep, and Depression Symptoms: A Cross-Sectional Study in Indian Adolescents. PLoS ONE 2022, 17, e0264951. [Google Scholar] [CrossRef] [PubMed]
- Kamaleddine, A.N.; Antar, H.A.; Ali, B.T.A.; Hammoudi, S.F.; Lee, J.; Lee, T.; Bhang, S.Y.; Chung, S.; Salameh, P. Effect of Screen Time on Physical and Mental Health and Eating Habits During COVID-19 Lockdown in Lebanon. Psychiatry Investig. 2022, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.C.; Moss, R.H.; Sykes-Muskett, B.; Conner, M.; O’Connor, D.B. Stress and Eating Behaviors in Children and Adolescents: Systematic Review and Meta-Analysis. Appetite 2018, 123, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.T.; Vitorino, R.S.; da Silva, J.H.; Raposo, L.M.; Aquino, L.A.D.; Ribas, S.A. Eating Habits of Children and Adolescents during the COVID-19 Pandemic: The Impact of Social Isolation. J. Human. Nutr. Diet. 2021, 34, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Muehleman, V. Eating Disorders and Metabolic Diseases. Int. J. Environ. Res. Public Health 2023, 20, 2446. [Google Scholar] [CrossRef]
- Agostino, H.; Burstein, B.; Moubayed, D.; Taddeo, D.; Grady, R.; Vyver, E.; Dimitropoulos, G.; Dominic, A.; Coelho, J.S. Trends in the Incidence of New-Onset Anorexia Nervosa and Atypical Anorexia Nervosa Among Youth During the COVID-19 Pandemic in Canada. JAMA Netw. Open 2021, 4, 2137395. [Google Scholar] [CrossRef]
- Giacomini, G.; Elhadidy, H.S.M.A.; Paladini, G.; Onorati, R.; Sciurpa, E.; Gianino, M.M.; Borraccino, A. Eating Disorders in Hospitalized School-Aged Children and Adolescents during the COVID-19 Pandemic: A Cross-Sectional Study of Discharge Records in Developmental Ages in Italy. Int. J. Environ. Res. Public Health 2022, 19, 12988. [Google Scholar] [CrossRef]
- Mizumoto, Y.; Sasaki, Y.; Sunakawa, H.; Tanese, S.; Shinohara, R.; Kurokouchi, T.; Sugimoto, K.; Seto, M.; Ishida, M.; Itagaki, K.; et al. Current Situation and Clinical Burden of Pediatricians for Children with Eating Disorders during the COVID-19 Pandemic. Glob. Health Med. 2023, 5, 122. [Google Scholar] [CrossRef]
- Asch, D.A.; Buresh, J.; Allison, K.C.; Islam, N.; Sheils, N.E.; Doshi, J.A.; Werner, R.M. Trends in US Patients Receiving Care for Eating Disorders and Other Common Behavioral Health Conditions Before and During the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e2134913. [Google Scholar] [CrossRef]
- Stabouli, S.; Erdine, S.; Suurorg, L.; Jankauskienė, A.; Lurbe, E. Obesity and Eating Disorders in Children and Adolescents: The Bidirectional Link. Nutrients 2021, 13, 4321. [Google Scholar] [CrossRef] [PubMed]
- Breton, E.; Fotso Soh, J.; Booij, L. Immunoinflammatory processes: Overlapping mechanisms between obesity and eating disorders? Neurosci. Biobehav. Rev. 2022, 138, 104688. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, C.; Fernández-Aranda, F.; Jiménez-Murcia, S.; Peñas-Lledó, E.; Granero, R.; Penelo, E.; Tinahones, F.J.; Sancho, C.; Vilarrasa, N.; Montserrat-Gil de Bernabé, M.; et al. Lifetime obesity in patients with eating disorders: Increasing prevalence, clinical and personality correlates. Eur. Eat. Disord. Rev. 2012, 20, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Flament, M.F.; Henderson, K.; Buchholz, A.; Obeid, N.; Nguyen, H.N.; Birmingham, M.; Goldfield, G. Weight Status and DSM-5 Diagnoses of Eating Disorders in Adolescents From the Community. J. Am. Acad. Child. Adolesc. Psychiatry 2015, 54, 403–411.e2. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Lister, N.B.; Baur, L.A.; Garnett, S.P.; Paxton, S.J. Eating disorder risk in adolescents with obesity. Obes. Rev. 2021, 22, e13173. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, J.A.; Jhe, G.; Milliren, C.E.; Lin, J.A.; Spigel, R.; Freizinger, M.; Woods, E.R.; Forman, S.F.; Richmond, T.K. COVID-19 and Eating Disorder and Mental Health Concerns in Patients with Eating Disorders. J. Eat. Disord. 2021, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Spettigue, W.; Obeid, N.; Erbach, M.; Feder, S.; Finner, N.; Harrison, M.E.; Isserlin, L.; Robinson, A.; Norris, M.L. The Impact of COVID-19 on Adolescents with Eating Disorders: A Cohort Study. J. Eat. Disord. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Graell, M.; Morón-Nozaleda, M.G.; Camarneiro, R.; Villaseñor, Á.; Yáñez, S.; Muñoz, R.; Martínez-Núñez, B.; Miguélez-Fernández, C.; Muñoz, M.; Faya, M. Children and Adolescents with Eating Disorders during COVID-19 Confinement: Difficulties and Future Challenges. Eur. Eat. Disord. Rev. 2020, 28, 864–870. [Google Scholar] [CrossRef]
- Castellini, G.; Cassioli, E.; Rossi, E.; Innocenti, M.; Gironi, V.; Sanfilippo, G.; Felciai, F.; Monteleone, A.M.; Ricca, V. The Impact of COVID-19 Epidemic on Eating Disorders: A Longitudinal Observation of Pre versus Post Psychopathological Features in a Sample of Patients with Eating Disorders and a Group of Healthy Controls. Int. J. Eat. Disord. 2020, 53, 1855–1862. [Google Scholar] [CrossRef]
- Shum, M.; Moreno, C.; Kamody, R.; McCollum, S.; Shabanova, V.; Loyal, J. The Evolving Needs of Children Hospitalized for Eating Disorders During the COVID-19 Pandemic. Hosp. Pediatr. 2022, 12, 696–702. [Google Scholar] [CrossRef]
- Toulany, A.; Kurdyak, P.; Guttmann, A.; Stukel, T.A.; Fu, L.; Strauss, R.; Fiksenbaum, L.; Saunders, N.R. Acute Care Visits for Eating Disorders Among Children and Adolescents After the Onset of the COVID-19 Pandemic. J. Adolesc. Health 2022, 70, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Attia, E. Recent Advances in Therapies for Eating Disorders. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Bohon, C. Binge Eating Disorder in Children and Adolescents. Child. Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 549. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, L.L.; Lane, M.A. Identification and Management of Eating Disorders in Children and Adolescents. Pediatrics 2021, 147, e2020040279. [Google Scholar] [CrossRef] [PubMed]
- Mairs, R.; Nicholls, D. Assessment and Treatment of Eating Disorders in Children and Adolescents. Arch. Dis. Child. 2016, 101, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B.; Dahmen, B. Children in Need—Diagnostics, Epidemiology, Treatment and Outcome of Early Onset Anorexia Nervosa. Nutrients 2019, 11, 1932. [Google Scholar] [CrossRef]
- Gorrell, S.; Le Grange, D. Update on Treatments for Adolescent Bulimia Nervosa. Child. Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 537. [Google Scholar] [CrossRef]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The Psychological Impact of Quarantine and How to Reduce It: Rapid Review of the Evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef]
- McCombie, C.; Austin, A.; Dalton, B.; Lawrence, V.; Schmidt, U. “Now It’s Just Old Habits and Misery”-Understanding the Impact of the COVID-19 Pandemic on People With Current or Life-Time Eating Disorders: A Qualitative Study. Front. Psychiatry 2020, 11, 589225. [Google Scholar] [CrossRef]
- Maunder, K.; McNicholas, F. Exploring Carer Burden amongst Those Caring for a Child or Adolescent with an Eating Disorder during COVID-19. J. Eat. Disord. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- PiLaR Program—An Evaluation. HSE National Clinical Program of Eating Disorders National Clinical Programme for Eating Disorders. 2019. Available online: https://www.bodywhys.ie/wp-content/uploads/2019/02/PilarReport_web.pdf (accessed on 31 October 2023).
- Scapaticci, S.; Neri, C.R.; Marseglia, G.L.; Staiano, A.; Chiarelli, F.; Verduci, E. The Impact of the COVID-19 Pandemic on Lifestyle Behaviors in Children and Adolescents: An International Overview. Ital. J. Pediatr. 2022, 48, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.Y.X.; Tam, W.; Shorey, S. Research Review: Effectiveness of Universal Eating Disorder Prevention Interventions in Improving Body Image among Children: A Systematic Review and Meta-Analysis. J. Child. Psychol. Psychiatry 2020, 61, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Roy, D.; Sinha, K.; Parveen, S.; Sharma, G.; Joshi, G. Impact of COVID-19 and Lockdown on Mental Health of Children and Adolescents: A Narrative Review with Recommendations. Psychiatry Res. 2020, 293, 113429. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.N.; Lavender, J.M.; Schvey, N.A.; Tanofsky-Kraff, M. Practical Considerations for Using the Eating Disorder Examination Interview with Adolescents. Adolesc. Health Med. Ther. 2023, 14, 63–85. [Google Scholar] [CrossRef] [PubMed]
- O’Logbon, J.; Newlove-Delgado, T.; McManus, S.; Mathews, F.; Hill, S.; Sadler, K.; Ford, T. How Does the Increase in Eating Difficulties According to the Development and Well-Being Assessment Screening Items Relate to the Population Prevalence of Eating Disorders? An Analysis of the 2017 Mental Health in Children and Young People Survey. Int. J. Eat. Disord. 2022, 55, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- WHO World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 25 October 2023).
- WHO World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 October 2023).
- de Onis, M.; Garza, C.; Onyango, A.W.; Martorell, R. WHO child growth standards. Acta Paediatr. 2006, 95 (Suppl. S450), 3–101. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Defining Child BMI Categories. Available online: https://www.cdc.gov/obesity/basics/childhood-defining.html (accessed on 25 October 2023).
- Grummer-Strawn, L.M.; Reinold, C.; Krebs, N.F. Centers for Disease Control and Prevention (CDC). Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR Recomm. Rep. 2010, 59, 1–15. [Google Scholar]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Avila Edwards, K.C.; Eneli, I.; Hamre, R.; Joseph, M.M.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents With Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Eslami, M.; Pourghazi, F.; Khazdouz, M.; Tian, J.; Pourrostami, K.; Esmaeili-Abdar, Z.; Ejtahed, H.S.; Qorbani, M. Optimal cut-off value of waist circumference-to-height ratio to predict central obesity in children and adolescents: A systematic review and meta-analysis of diagnostic studies. Front. Nutr. 2023, 9, 985319. [Google Scholar] [CrossRef]
- Lister, N.B.; Baur, L.A.; Felix, J.F.; Hill, A.J.; Marcus, C.; Reinehr, T.; Summerbell, C.; Wabitsch, M. Child and adolescent obesity. Nat. Rev. Dis. Primers 2023, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Zong, X.; Kelishadi, R.; Litwin, M.; Hong, Y.M.; Poh, B.K.; Steffen, L.M.; Galcheva, S.V.; Herter-Aeberli, I.; Nawarycz, T.; et al. International Waist Circumference Percentile Cutoffs for Central Obesity in Children and Adolescents Aged 6 to 18 Years. J. Clin. Endocrinol. Metab. 2020, 105, e1569–e1583. [Google Scholar] [CrossRef] [PubMed]
- UNICEF/WHO/World Bank Joint Child Malnutrition Estimates: 2021 Edition Interactive Dashboard. New York (NY): United Nations Children’s Fund. 2021. Available online: https://data.unicef.org/resources/joint-child-malnutrition-estimates-interactive-dashboard-2021/ (accessed on 29 October 2023).
- World Health Organization (WHO). Regional Office for Europe. WHO European Regional Obesity Report. 2022. Available online: https://iris.who.int/bitstream/handle/10665/353747/9789289057738-eng.pdf?sequence=1&isAllowed=y (accessed on 29 October 2023).
- Fryar, C.D.; Carroll, M.D.; Afful, J. Prevalence of Overweight, Obesity, and Severe Obesity among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 through 2017–2018. NCHS Health E-Stats. 2020. Available online: https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf (accessed on 29 October 2023).
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128 9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Skelton, J.A.; Klish, W.J. Overview of the Health Consequences of Obesity in Children and Adolescents. Available online: https://www.uptodate.com/contents/overview-of-the-health-consequences-of-obesity-in-children-and-adolescents?topicRef=5874&source=see_link#H1 (accessed on 29 October 2023).
- Chen, Y.; Qian, L. Association between lifetime stress and obesity in Canadians. Prev. Med. 2012, 55, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Chida, Y.; Gibson, E.L.; Whitaker, K.L.; Steptoe, A. Stress and adiposity: A meta-analysis of longitudinal studies. Obesity 2011, 19, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Grilo, C.M.; White, M.A.; Sinha, R. Food cravings mediate the relationship between chronic stress and body mass index. J. Health Psychol. 2015, 20, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Rizvi, M.R.; Saraswat, S. Obesity and Stress: A Contingent Paralysis. Int. J. Prev. Med. 2022, 13, 95. [Google Scholar]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic stress and obesity: A new view of “comfort food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef]
- Fardet, L.; Fève, B. Systemic glucocorticoid therapy: A review of its metabolic and cardiovascular adverse events. Drugs 2014, 74, 1731–1745. [Google Scholar] [CrossRef]
- Adam, T.C.; Epel, E.S. Stress, eating and the reward system. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef]
- Herbet, M.; Korga, A.; Gawrońska-Grzywacz, M.; Izdebska, M.; Piątkowska-Chmiel, I.; Poleszak, E.; Wróbel, A.; Matysiak, W.; Jodłowska-Jędrych, B.; Dudka, J. Chronic Variable Stress Is Responsible for Lipid and DNA Oxidative Disorders and Activation of Oxidative Stress Response Genes in the Brain of Rats. Oxidative Med. Cell. Longev. 2017, 2017, 7313090. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar] [PubMed]
- Warne, J.P. Shaping the stress response: Interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol. Cell. Endocrinol. 2009, 300, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Davy, K.P. The global epidemic obesity: Are we becoming more sympathetic? Curr. Hypertens. Rep. 2004, 6, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, A.G.; Rutters, F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol. Behav. 2008, 94, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.M.; Jastreboff, A.M.; White, M.A.; Grilo, C.M.; Sinha, R. Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity 2017, 25, 713–720. [Google Scholar] [CrossRef]
- Raber, J. Detrimental effects of chronic hypothalamic-pituitary-adrenal axis activation. From obesity to memory deficits. Mol. Neurobiol. 1998, 18, 1–22. [Google Scholar] [CrossRef]
- Rebuffé-Scrive, M.; Krotkiewski, M.; Elfverson, J.; Björntorp, P. Muscle and adipose tissue morphology and metabolism in Cushing’s syndrome. J. Clin. Endocrinol. Metab. 1988, 67, 1122–1128. [Google Scholar] [CrossRef]
- Rosmond, R.; Dallman, M.F.; Björntorp, P. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J. Clin. Endocrinol. Metab. 1998, 83, 1853–1859. [Google Scholar] [CrossRef]
- Caron, A.; Jane Michael, N. New Horizons: Is Obesity a Disorder of Neurotransmission? J. Clin. Endocrinol. Metab. 2021, 106, e4872–e4886. [Google Scholar] [CrossRef] [PubMed]
- Page, K.A.; Seo, D.; Belfort-DeAguiar, R.; Lacadie, C.; Dzuira, J.; Naik, S.; Amarnath, S.; Constable, R.T.; Sherwin, R.S.; Sinha, R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J. Clin. Investig. 2011, 121, 4161–4169. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Military Nutrition Research; Marriott, B.M. (Eds.) Food Components to Enhance Performance: An Evaluation of Potential Performance-Enhancing Food Components for Operational Rations; National Academies Press (US): Cambridge, MA, USA, 1994. [Google Scholar]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Lemoine-Morel, S.; Mathieu, M.E.; Casazza, G.A.; Jabbour, G. Catecholamines and obesity: Effects of exercise and training. Sports Med. (Auckl. N.Z.) 2013, 43, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rinwa, P.; Kaur, G.; Machawal, L. Stress: Neurobiology, consequences and management. J. Pharm. Bioallied Sci. 2013, 5, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.W.; Lowry, C.A. Functional topography of midbrain and pontine serotonergic systems: Implications for synaptic regulation of serotonergic circuits. Psychopharmacology 2011, 213, 243–264. [Google Scholar] [CrossRef]
- Natarajan, R.; Northrop, N.A.; Yamamoto, B.K. Protracted effects of chronic stress on serotonin-dependent thermoregulation. Stress (Amst. Neth.) 2015, 18, 668–676. [Google Scholar] [CrossRef]
- Berthoud, H.R. The neurobiology of food intake in an obesogenic environment. Proc. Nutr. Soc. 2012, 71, 478–487. [Google Scholar] [CrossRef]
- Sinha, R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008, 1141, 105–130. [Google Scholar] [CrossRef]
- Bloom, S. Hormonal regulation of appetite. Obes. Rev. 2007, 8, 63–65. [Google Scholar] [CrossRef]
- Takeda, E.; Terao, J.; Nakaya, Y.; Miyamoto, K.; Baba, Y.; Chuman, H.; Kaji, R.; Ohmori, T.; Rokutan, K. Stress control and human nutrition. J. Med. Investig. 2004, 51, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zahorska-Markiewicz, B.; Obuchowicz, E.; Waluga, M.; Tkacz, E.; Herman, Z.S. Neuropeptide Y in obese women during treatment with adrenergic modulation drugs. Med. Sci. Monit. 2001, 7, 403–408. [Google Scholar] [PubMed]
- Baver, S.B.; Hope, K.; Guyot, S.; Bjørbaek, C.; Kaczorowski, C.; O’Connell, K.M. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 5486–5496. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metabolism 2015, 64, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Klok, M.; Jakobsdottir, S.; Drent, M. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Bouillon-Minois, J.B.; Trousselard, M.; Thivel, D.; Benson, A.C.; Schmidt, J.; Moustafa, F.; Bouvier, D.; Dutheil, F. Leptin as a Biomarker of Stress: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3350. [Google Scholar] [CrossRef] [PubMed]
- Heiman, M.L.; Ahima, R.S.; Craft, L.S.; Schoner, B.; Stephens, T.W.; Flier, J.S. Leptin inhibition of the hypothalamic-pituitaryadrenal axis in response to stress. Endocrinology 1997, 138, 3859–3863. [Google Scholar] [CrossRef] [PubMed]
- Benomar, Y.; Wetzler, S.; Larue-Achagiotis, C.; Djiane, J.; Tome, D.; Taouis, M. In vivo leptin infusion impairs insulin and leptin signalling in liver and hypothalamus. Mol. Cell Endocrinol. 2005, 242, 59–66. [Google Scholar] [CrossRef]
- le Roux, C.W.; Patterson, M.; Vincent, R.P.; Hunt, C.; Ghatei, M.A.; Bloom, S.R. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J. Clin. Endocrinol. Metab. 2005, 90, 1068–1071. [Google Scholar] [CrossRef]
- Sarker, M.R.; Franks, S.; Caffrey, J. Direction of post-prandial ghrelin response associated with cortisol response, perceived stress and anxiety, and self-reported coping and hunger in obese women. Behav. Brain Res. 2013, 257, 197–200. [Google Scholar] [CrossRef]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Karin, M. JNK1 and IKKbeta: Molecular links between obesity and metabolic dysfunction. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 2596–2611. [Google Scholar] [CrossRef]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.-F. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.Z.; Peter, A.; Machicao, F.; Lamprinou, A.; Machann, J.; Schick, F.; Königsrainer, I.; Königsrainer, A.; Fritsche, A.; Staiger, H.; et al. Genetic Variation in the 11β-hydroxysteroid-dehydrogenase 1 Gene Determines NAFLD and Visceral Obesity. J. Clin. Endocrinol. Metab. 2016, 101, 4743–4751. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Whincup, P.H.; Rumley, A.; Lowe, G.D. Inter-relationships of interleukin-6, cardiovascular risk factors and the metabolic syndrome among older men. J. Thromb. Haemost. 2007, 5, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Illán-Gómez, F.; Gonzálvez-Ortega, M.; Orea-Soler, I.; Alcaraz-Tafalla, M.S.; Aragón-Alonso, A.; Pascual-Díaz, M.; Pérez-Paredes, M.; Lozano-Almela, M.L. Obesity and inflammation: Change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes. Surg. 2012, 22, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, E.S.; Maislin, G.; Schwab, R.J.; Staley, B.; Benediktsdottir, B.; Olafsson, I.; Juliusson, S.; Romer, M.; Gislason, T.; Pack, A.I. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: The Icelandic Sleep Apnea Cohort. Sleep 2012, 35, 921–932. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Jaleel, F.; Jaleel, A.; Rahman, M.; Alam, E. Comparison of adiponectin, leptin and blood lipid levels in normal and obese postmenopause women. J. Pak. Med. Assoc. 2006, 56, 391–394. [Google Scholar]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and Diabetes mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Saad, M.; Santos, A.; Prada, P. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.S.; Schulman, I.H.; Zeng, Q. Związek między układem renina-angiotensyna a insulinoopornością: Implikacje dla chorób sercowo-naczyniowych. Vasc. Med. 2012, 17, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.O.; Brechtel, G.; Johnson, A.; Fineberg, N.; Baron, A.D. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J. Clin. Investig. 1994, 94, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.A.; Steptoe, A. Type 2 diabetes mellitus and psychological stress—A modifiable risk factor. Nat. Rev. Endocrinol. 2017, 13, 547. [Google Scholar] [CrossRef] [PubMed]
- Parkulo, T. The Effects of Chronic Stress and Exercise on Mouse Pancreatic Islet of Langerhans Morphology and Muscle Atrophy Gene Expression. Graduate Thesis, West Virginia University, Morgantown, WV, USA, 2014. [Google Scholar]
- Huffman, F.G.; Vallasciani, M.; Vaccaro, J.A.; Exebio, J.C.; Zarini, G.G.; Nayer, A.; Ajabshir, S. The association of depression and perceived stress with beta cell function between African and Haitian Americans with and without type 2 diabetes. J. Diabetes Mellit. 2013, 3, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Ghanim, H.; Chaudhuri, A.; Dhindsa, S.; Kim, S.S. Macronutrient intake induces oxidative and inflammatory stress: Potential relevance to atherosclerosis and insulin resistance. Exp. Mol. Med. 2010, 42, 245. [Google Scholar] [CrossRef]
- Čolak, E. New markers of oxidative damage to macromolecules. J. Med. Biochem. 2008, 27, 1. [Google Scholar] [CrossRef]
- Martyn, J.A.; Kaneki, M.; Yasuhara, S. Obesity-induced insulin resistance and hyperglycemia: Etiologic factors and molecular mechanisms. Anesthesiology 2008, 109, 137–148. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Siassi, F.; Minaie, S.; Djalali, M.; Rahimi, A.; Chamari, M. Is obesity associated with increased plasma lipid peroxidación and oxidative stress in women. ARYA Atheroscler. J. 2007, 2, 189–192. [Google Scholar]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Frossi, B.; de Carli, M.; Daniel, K.C.; Rivera, J.; Pucillo, C. Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur. J. Immunol. 2003, 33, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sharma, P.; Sahakyan, K.R.; Davison, D.E.; Sert-Kuniyoshi, F.H.; Romero-Corral, A.; Swain, J.M.; Jensen, M.D.; Lopez-Jimenez, F.; Kara, T.; et al. Differential effects of leptin on adiponectin expression with weight gain versus obesity. Int. J. Obes. 2016, 40, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Iwan-Zietek, I.; Ruszkowska-Ciastek, B.; Michalska, M.; Overskaug, E.; Goralczyk, K.; Dabrowiecki, S.; Rosc, D. Association of adiponectin and leptin-to-adiponectin ratio with the function of platelets in morbidly obese patients. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2016, 67c, 555–561. [Google Scholar]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, J.; Lü, J.M.; Chai, H.; Wang, X.; Lin, P.H.; Yao, Q. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am. J. Physiol. HeartCirc. Physiol. 2010, 299, 193–201. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
| Psychological and Pedagogical Interventions Used in Eating Disorder Treatment | |||
|---|---|---|---|
| Anorexia Nervosa | Bulimia Nervosa | Binge Eating Disorder | References |
|
|
| [64,65,66,67,68,69] |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
| ||
| |||
| |||
| |||
| |||
| Organization | Diagnostic Criteria for Obesity |
|---|---|
| World Health Organization [80,81] | Children under 5 years of age Overweight: age- and sex-adjusted weight-for-height >2 standard deviations above WHO Child Growth Standards median Obesity: age- and sex-adjusted weight-for-height >3 standard deviations above the WHO Child Growth Standards median Children between 5 and 19 years of age Overweight: age- and sex-adjusted BMI >1 standard deviation above the WHO Growth Reference median Obesity: age- and sex-adjusted BMI-for-age >2 standard deviations above the WHO Growth Reference median |
| U.S. Centers for Disease Control and Prevention (CDC) [82,83] | Children between 2 and 19 years of age Overweight: age- and sex-adjusted BMI ≥85th to <95th percentile on CDC Growth Charts Obesity: age- and sex-adjusted BMI ≥95th percentile on CDC Growth Charts Severe obesity: 120% of the 95th percentile of the age- and sex-adjusted BMI or greater, or BMI ≥35 kg/m2 Children under 2 years of age CDC recommends the use of the WHO criteria |
| American Academy of Pediatrics (AAP) [84] | Severe obesity Class 2 obesity: age- and sex-adjusted BMI ≥ 35 to <40 kg/m2 or ≥120 to 140% of the 95th percentile, whichever is lower Class 3 obesity: age- and sex-adjusted BMI ≥ 40 kg/m2 or ≥140% of the 95th percentile, whichever is lower |
| International Obesity Task Force (IOTF) [85] | Overweight International age- and sex-specific BMI percentile cutoffs equivalent to BMI 25 kg/m2 at age 18 years Obesity International age- and sex-specific BMI percentile cutoffs equivalent to BMI 30 kg/m2 at age 18 years |
| System | Disorder |
|---|---|
| Cardiovascular | Hypertension Left ventricle hypertrophy Premature atherosclerotic cardiovascular disease |
| Respiratory | Asthma Obstructive sleep apnea Sleep disorders Hypoventilation syndrome |
| Endocrine | Dyslipidemia Impaired glucose tolerance Type 2 diabetes Metabolic syndrome Polycystic ovary syndrome Impaired growth and puberty |
| Gastrointestinal | Metabolic dysfunction-associated steatotic liver disease Gastroesophageal reflux disease Cholelithiasis Constipation Micronutrient deficiencies |
| Musculoskeletal | Slipped capital femoral epiphysis Blount disease Fractures Pes planus |
| Skin | Acanthosis nigricans Striae Intertrigo Hidradenitis suppurativa Furunculosis |
| Renal | Enuresis Glomerulosclerosis |
| Dental | Dental caries Periodontal disease |
| Nervous system | Idiopathic intracranial hypertension |
| Psychosocial | Reduced self-esteem Depression Anxiety Disordered eating Internalizing disorders |
| Long-term complications | Adult obesity Coronary artery disease Type 2 diabetes Certain cancers Infertility Osteoarthritis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piątkowska-Chmiel, I.; Krawiec, P.; Ziętara, K.J.; Pawłowski, P.; Samardakiewicz, M.; Pac-Kożuchowska, E.; Herbet, M. The Impact of Chronic Stress Related to COVID-19 on Eating Behaviors and the Risk of Obesity in Children and Adolescents. Nutrients 2024, 16, 54. https://doi.org/10.3390/nu16010054
Piątkowska-Chmiel I, Krawiec P, Ziętara KJ, Pawłowski P, Samardakiewicz M, Pac-Kożuchowska E, Herbet M. The Impact of Chronic Stress Related to COVID-19 on Eating Behaviors and the Risk of Obesity in Children and Adolescents. Nutrients. 2024; 16(1):54. https://doi.org/10.3390/nu16010054
Chicago/Turabian StylePiątkowska-Chmiel, Iwona, Paulina Krawiec, Karolina Joanna Ziętara, Piotr Pawłowski, Marzena Samardakiewicz, Elżbieta Pac-Kożuchowska, and Mariola Herbet. 2024. "The Impact of Chronic Stress Related to COVID-19 on Eating Behaviors and the Risk of Obesity in Children and Adolescents" Nutrients 16, no. 1: 54. https://doi.org/10.3390/nu16010054
APA StylePiątkowska-Chmiel, I., Krawiec, P., Ziętara, K. J., Pawłowski, P., Samardakiewicz, M., Pac-Kożuchowska, E., & Herbet, M. (2024). The Impact of Chronic Stress Related to COVID-19 on Eating Behaviors and the Risk of Obesity in Children and Adolescents. Nutrients, 16(1), 54. https://doi.org/10.3390/nu16010054