The Longitudinal Association of Egg Consumption with Cognitive Function in Older Men and Women: The Rancho Bernardo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Statistical Analysis
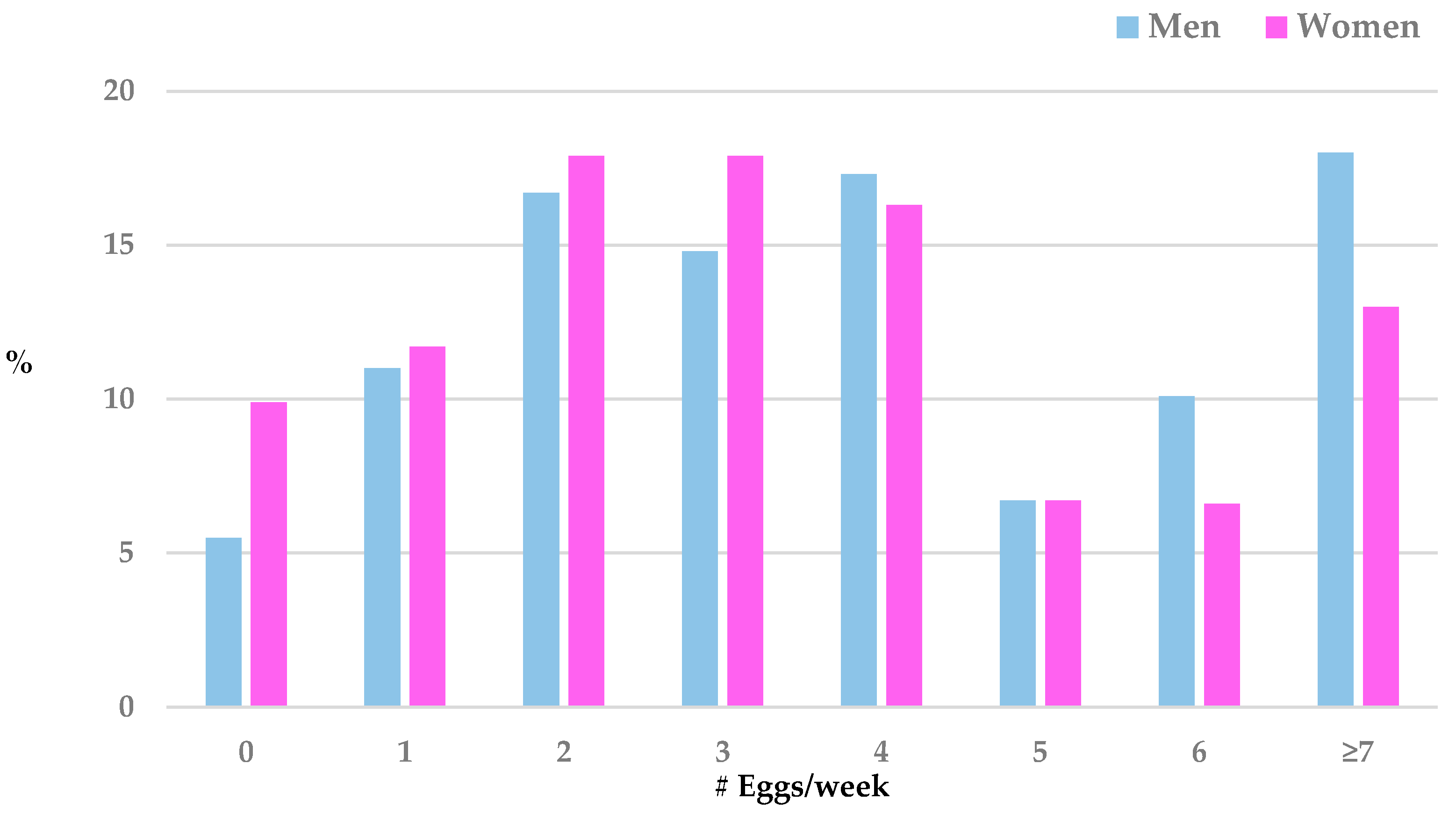
3. Results
4. Discussion
4.1. Study Outcomes
4.2. Biologic Plausibility
4.3. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2022 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2022, 18, 700–789. Available online: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf (accessed on 10 February 2023). [CrossRef]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Diment. 2021, 17, 1965–1966. [Google Scholar] [CrossRef]
- Daviglus, M.L.; Bell, C.C.; Berrettini, W.; Bowen, P.E.; Connolly, E.S.; Cox, N.J.; Dunbar-Jacob, J.M.; Granieri, E.C.; Hunt, G.; McGarry, K.; et al. National Institutes of Health State-of-the Science Conference statement: Preventing Alzheimer disease and cognitive decline. Ann. Intern. Med. 2010, 153, 176–181. [Google Scholar] [CrossRef]
- Hersi, M.; Irvine, B.; Gupta, P.; Gomes, J.; Birkett, N.; Krewski, D. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology 2017, 61, 143–187. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Yu, W.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol Consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Xu, W.; Zhang, W.; Wang, H.-F.; Ou, Y.-N.; Qu, Y.; Shen, X.-N.; Chen, S.-D.; Wu, K.-M.; Zhao, Q.-H.; et al. Modifiable risk factors for incident dementia and cognitive impairment: An umbrella review of evidence. J. Affect. Disord. 2022, 314, 160–167. [Google Scholar] [CrossRef]
- Reas, E.T.; Laughlin, G.A.; Bergstrom, J.; Kritz-Silverstein, D.; McEvoy, L.K. Physical activity and trajectories of cognitive change in community-dwelling older adults: The Rancho Bernardo Study. J. Alzheimer’s Dis. 2019, 71, 109–118. [Google Scholar] [CrossRef]
- Reas, E.T.; Laughlin, G.A.; Bergstrom, J.; Kritz-Silverstein, D.; Richard, E.L.; Barrett-Connor, E.; McEvoy, L.K. Lifetime physical activity and late-life cognitive function: The Rancho Bernardo Study. Age Aging 2019, 48, 241–246. [Google Scholar] [CrossRef]
- Reas, E.T.; Laughlin, G.A.; Bergstrom, J.; Kritz-Silverstein, D.; Barrett-Connor, E.; McEvoy, L.K. Effects of sex and education on cognitive change over a 27-year period in older adults: The Rancho Bernardo Study. Am. J. Geriatr. Psychiatry 2017, 25, 889–899. [Google Scholar] [CrossRef]
- Penninkilampi, R.; Casey, A.-N.; Singh, M.F.; Brodaty, H. The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2018, 66, 1619–1633. [Google Scholar] [CrossRef]
- Cao, G.-Y.; Li, M.; Han, L.; Tayie, F.; Yao, S.-S.; Huang, Z.; Ai, P.; Liu, Y.-Z.; Hu, Y.-H.; Xu, B. Dietary fat intake and cognitive function among older populations: A systematic review and meta-analysis. J. Prev. Alzheimers Dis. 2019, 6, 204–211. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of dietary patterns, foods and micro- and macronutrients with Alzheimer’s Disease and late-life cognitive disorders. J. Alzheimer’s Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef]
- Liu, L.; Qiao, S.; Zhuang, L.; Xu, S.; Chen, L.; Lai, Q.; Wang, W. Choline intake correlates with cognitive performance among elder adults in the United States. Behav. Neurol. 2021, 2021, 2962245. [Google Scholar] [CrossRef]
- Ylilauri, M.P.; Voutilainen, S.; Lönnroos, E.; Virtanen, H.E.; Tuomainen, T.-P.; Salonen, J.T.; Virtanen, J.K. Associations of dietary choline intake with risk of incident dementia and with cognitive performance: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2019, 110, 1416–1423. [Google Scholar] [CrossRef]
- Nakazaki, E.; Mah, E.; Sanoshy, K.; Citrolo, D.; Watanabe, F. Citicoline and memory function in older adults: A randomized, double-blind, placebo-controlled clinical trial. J. Nutr. 2021, 151, 2153–2160. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. The effects of Lutein and zeaxanthin supplementation on cognitive function in adults with self-reported mild cognitive complaints: A randomized, double-blind, placebo-controlled study. Front. Nutr. 2022, 9, 843512. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, H.; Wang, Y.; Schneider, J.A.; Willett, W.C.; Morris, M.C. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: A community-based cohort of older adults. Am. J. Clin. Nutr. 2021, 113, 200–208. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Joffe, S.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between serum and brain carotenoids, α-tacopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, S.W. Relationships between dietary intake and cognitive function in healthy Korean children and adolescents. J Lifestyle Med. 2017, 7, 10–17. [Google Scholar] [CrossRef]
- Vizuete, A.A.; Robles, F.; Rodriguez-Rodriguez, E.; Lopez-Sobaler, A.M.; Ortega, R.M. Association between food and nutrient intakes and cognitive capacity in a group of institutionalized elderly people. Eur. J. Nutr. 2010, 49, 293–300. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, L.; Feng, L.; Xi, Y.; Yu, H.; Ma, W.; Zhang, D.; Xiao, R. Association of dietary intake and lifestyle pattern with mild cognitive impairment in the elderly. J. Nutr. Health Aging 2015, 19, 164–168. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, B.; Zeng, G.; Li, J.; Wang, W.; Wang, B.; Yuan, Q. Is there an association between mild cognitive impairment and dietary pattern in Chinese elderly? Results from a cross-sectional population study. BMC Public Health 2010, 10, 595. [Google Scholar] [CrossRef]
- An, R.; Li, D.; McCaffrey, J.; Khan, N. Whole egg consumption and cognitive function among US older adults. J. Hum. Nutr. Diet. 2022, 35, 554–565. [Google Scholar] [CrossRef]
- Ylilauri, M.P.; Voutilainen, S.; Lönnroos, E.; Mursu, J.; Virtanen, H.E.; Koskinen, T.T.; Salonen, J.T.; Tuomainen, T.P.; Virtanen, J.K. Association of dietary cholesterol and egg intakes with the risk of incident dementia or Azheimer disease: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2017, 105, 476–484. [Google Scholar] [CrossRef]
- Bishop, N.J.; Zuniga, K.E. Egg consumption, multi-domain cognitive performance, and short-term cognitive change in a representative sample of older U.S. adults. J. Am. Coll. Nutr. 2019, 38, 537–546. [Google Scholar] [CrossRef]
- Spreen, O.; Strauss, E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Blessed, G.; Tomlinson, B.E.; Roth, M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br. J. Psychiatry 1968, 114, 797–811. [Google Scholar] [CrossRef]
- Buschke, H.; Fuld, P.A. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974, 24, 1019–1025. [Google Scholar] [CrossRef]
- Russell, E.W. A multiple scoring method for the assessment of complex memory functions. J. Consult. Clin. Psychol. 1975, 43, 800–809. [Google Scholar] [CrossRef]
- Wechsler, D. A standardized memory scale for clinical use. J. Psychol. 1945, 19, 87–95. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Reitan, R. Validity of the Trail-Making Test as an indicator of organic brain disease. Percept. Mot Skills 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Borkowski, J.B.; Benton, A.L.; Spreen, O. Word fluency and brain damage. Neuropsychologia 1967, 5, 135–140. [Google Scholar] [CrossRef]
- McNamara, D.J. The fifty-year rehabilitation of the egg. Nutrients 2015, 7, 8716–8722. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Laye, S.; Pierantoni, R.; et al. Impact of dietary fats on brain function. Curr. Neuropharmacol. 2018, 16, 1059–1085. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Corbin, K.D. Choline. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., MacDonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012; pp. 405–418. [Google Scholar]
- Poly, C.; Massaro, J.M.; Seshadri, S.; Sheshadri, S.; Wolf, P.A.; Cho, E.; Krall, E.; Jacques, P.F.; Au, R. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2011, 94, 1584–1591. [Google Scholar] [CrossRef]
- Mewborn, C.M.; Lindbergh, C.A.; Robinson, T.L.; Gogniat, M.A.; Terry, D.P.; Jean, K.R.; Hammond, B.R.; Renzi-Hammond, L.M.; Miller, L.S. Lutein and zeaxanthin are positively associated with visual-spatial functioning in older adults: An fMRI Study. Nutrients 2018, 10, 458. [Google Scholar] [CrossRef]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional intervention to prevent Alzheimer’s Disease: Potential benefits of xanthophyll carotenoids and omega-3 fatty acids combined. J. Alzheimer’s Dis. 2018, 64, 367–378. [Google Scholar] [CrossRef]
- Hammond, B.R.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of Lutein/zeaxanthin supplementation on the cognitive function of community dwelling older adults: A randomized, double-masked, placebo-controlled trial. Front. Aging Neurosci. 2017, 9, 254. [Google Scholar] [CrossRef]
| Overall | Men (N = 617) | Women (N = 898) | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | N | (%) | N | (%) | p-Value a |
| Education (some college) | 1502 | 476 | (78.3) | 564 | (63.1) | <0.0001 |
| Current Smoking | 1515 | 115 | (18.6) | 221 | (24.6) | 0.0060 |
| Cholesterol-lowering Meds | 1515 | 18 | (2.9) | 25 | (2.8) | 0.8779 |
| Diabetes (self-report) | 1510 | 23 | (3.7) | 14 | (1.6) | 0.0072 |
| Heart attack (self-report) | 1505 | 27 | (4.4 | 17 | (1.9) | 0.0085 |
| Stroke (self-report) | 1505 | 5 | (0.008) | 2 | (0.002) | 0.2088 |
| Hypertension (self-report) | 1505 | 119 | (19.3) | 181 | (20.4) | 0.6468 |
| Mean | (SD) | Mean | (SD) | p-Value a | ||
| Age (years) | 1515 | 59.2 | (8.3) | 59.0 | (7.8) | 0.5626 |
| Body Mass Index (BMI) | 1515 | 25.8 | (2.8) | 23.4 | (3.1) | <0.0001 |
| Total Cholesterol | 1512 | 210.5 | (33.5) | 221.1 | (37.6) | <0.0001 |
| Glucose | 1448 | 107.9 | (19.4) | 103.1 | (18.1) | <0.0001 |
| Triglycerides b | 1512 | 109.0 | (78.0) | 102.1 | (60.0) | 0.0020 |
| Systolic Blood Pressure | 1515 | 133.0 | (24.0) | 128.1 | (24.6) | <0.0001 |
| Diastolic Blood Pressure | 1515 | 79.2 | (12.0) | 77.2 | (11.7) | <0.0001 |
| # eggs/week | 1515 | 4.2 | (3.2) | 3.5 | (2.7) | <0.0001 |
| Cognitive Function Tests | ||||||
| Buschke | 1464 | |||||
| Total Recall | 34.3 | (9.7) | 39.2 | (9.0) | <0.0001 | |
| Long-term Recall | 26.4 | (12.8) | 33.2 | (12.3) | <0.0001 | |
| Short-term Recall c | 7.9 | (4.6) | 6.1 | (4.2) | <0.0001 | |
| Heaton | 1477 | |||||
| Immediate Recall | 9.8 | (3.8) | 9.1 | (3.4) | 0.0002 | |
| Delayed Recall | 7.3 | (4.5) | 6.4 | (3.9) | <0.0001 | |
| Copying | 15.1 | (2.2) | 15.1 | (2.0) | 0.9801 | |
| Mini-Mental State Exam | 1492 | 26.8 | (2.5) | 27.3 | (1.9) | <0.0001 |
| Serial 7’s | 1476 | 4.3 | (1.1) | 3.9 | (1.3) | <0.0001 |
| “World” Backward | 1500 | 4.7 | (0.9) | 4.8 | (0.6) | <0.0001 |
| Blessed Items | 1501 | 5.9 | (1.6) | 6.1 | (1.3) | 0.0428 |
| Trails B c | 1473 | 132.2 | (64.5) | 144.2 | (67.5) | 0.0007 |
| Category Fluency | 1504 | 18.3 | (5.4) | 17.1 | (4.8) | <0.0001 |
| Egg Consumption 1972–1974 | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0/Week | 1/Week | 2/Week | 3/Week | 4/Week | 5–6/Week | ≥7/Week | p-Value a | |
| Men | N = 34 | N = 68 | N = 103 | N = 91 | N = 107 | N = 103 | N = 111 | |
| Age, mean (sd) | 60.8 (8.4) | 60.2 (8.7) | 60.8 (8.4) | 60.2 (8.7) | 57.9 (7.9) | 59.0 (8.8) | 59.1 (8.0) | 0.1315 |
| BMI, mean (sd) | 26.1 (2.6) | 25.5 (2.8) | 25.8 (2.9) | 25.5 (2.3) | 25.8 (2.7) | 25.9 (2.8) | 25.8 (3.3) | 0.8877 |
| Cholesterol, mean (sd) | 218.1 (33.9) | 213.6 (35.2) | 214.6 (32.7) | 212.5 (36.2) | 206.2 (33.1) | 212.5 (34.7) | 203.0 (28.6) | 0.0668 |
| Glucose, mean (sd) | 111.9 (27.4) | 113.6 (21.6) | 109.0 (21.9) | 106.3 (16.8) | 104.5 (15.7) | 105.6 (15.7) | 109.1 (21.0) | 0.0465 |
| Triglycerides b, mdn (IQR) | 94.0 (60.0) | 119.5 (75.0) | 112.5 (88.0) | 123.0 (73.5) | 114.0 (75.0) | 107.0 (72.0) | 96.0 (74.0) | 0.3940 |
| College, n (%) | 24 (75.0) | 45 (67.2) | 81 (81.0) | 76 (84.4) | 82 (78.1) | 75 (72.8) | 93 (83.8) | 0.0792 |
| Smoking, n (%) | 3 (8.8) | 14 (20.6) | 11 (10.7) | 18.(19.8) | 25 (23.4) | 19 (18.4) | 25 (22.5) | 0.1526 |
| Chol-lowering meds n (%) | 5 (14.7) | 3 (4.4) | 5 (4.8) | 2 (2.2) | 1 (0.9) | 1 (1.0) | 1 (0.9) | --- c |
| Diabetes, n (%) | 1 (2.9) | 2 (2.9) | 3 (2.9) | 3 (3.3) | 4 (3.7) | 3 (2.9) | 7 (6.4) | --- c |
| Hx heart attack, n (%) | 2 (5.9) | 4 (5.9) | 8 (7.8) | 1 (1.1) | 2 (1.9) | 7 (6/8) | 3 (2.7) | --- c |
| Hx hypertension n (%) | 7 (20.6) | 9 (14.3) | 16 (15.5) | 27 (30.0) | 10 (17.8) | 23 (22.3) | 18 (16.4) | 0.1294 |
| 0/Week | 1/Week | 2/Week | 3/Week | 4/Week | 5–6/Week | ≥7/Week | p-Value a | |
| Women | N = 89 | N = 105 | N = 161 | N = 161 | N = 146 | N = 119 | N = 117 | |
| Age, mean (sd) | 59.3 (7.5) | 60.8 (7.9) | 60.0 (7.5) | 59.3 (7.8) | 58.5 (7.7) | 57.7 (8.3) | 57.1 (7.8) | 0.0036 |
| BMI, mean (sd) | 23.5 (3.6) | 23.4 (3.3) | 23.5 (2.9) | 23.3 (3.2) | 23.7 (3.1) | 23.7 (3.1) | 23/0 (2.7) | 0.8877 |
| Cholesterol, mean (sd) | 225.4 (42.7) | 226.1 (39.3) | 220.5 (34.1) | 223.5 (38.1) | 220.2 (35.4) | 218.5 (39.8) | 214.6 (35.3) | 0.2457 |
| Glucose, mean (sd) | 102.6 (13.8) | 105.6 (15.6) | 100.9 (15.4) | 103.0 (14.3) | 102.5 (17.7) | 101.0 (15.3) | 107.2(29.8) | 0.0643 |
| Triglycerides b, mdn (IQR) | 113.0 (68.0) | 107.0 (55.0) | 103.0 (62.0) | 101.0 (67.0) | 99.0 (52.0) | 96.0 (58.0) | 95.0 (57.0) | 0.1198 |
| College, n (%) | 50 (56.8) | 64 (60.9) | 93 (57.8) | 107 (66.9) | 87 (59.6) | 79 (66.4) | 84 (73.0) | 0.0930 |
| Smoking, n (%) | 21 (23.6) | 21 (20.0) | 35 (21.7) | 22 (20.5) | 35 (24.0) | 31 (26.0) | 45 (38.5) | 0.0155 |
| Chol-lowering meds, n (%) | 10 (11.2) | 4 (3.8) | 3 (1.9) | 1 (0.6) | 2 (1.4) | 4 (3.3) | 1 (0.9) | --- c |
| Diabetes, n (%) | 2 (2.2) | 2 (1.9) | 1 (0.6) | 2 (1.2) | 4 (2.8) | 2 (1.7) | 1 (0.9) | --- c |
| Hx heart attack, n (%) | 4 (4.5) | 4 (3.8) | 1 (0.6) | 1 (0.6) | 4 (2.8) | 2 (1.7) | 1 (0.9) | --- c |
| Hx hypertension, n (%) | 22 (25.0) | 24 (22.9) | 25 (15.6) | 38 (23.7) | 30 (20.5) | 29 (24.6) | 23 (19.7) | 0.4819 |
| Egg Consumption 1972–1974 | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0/Week | 1/Week | 2/Week | 3/Week | 4/Week | 5–6/Week | ≥7/Week | ||
| Men | N = 34 | N = 68 | N = 103 | N = 91 | N = 107 | N = 103 | N = 111 | p-Value a |
| Buschke | ||||||||
| Total Recall | 33.2 | 33.8 | 31.8 | 33.6 | 35.9 | 34.8 | 35.5 | 0.0527 |
| Long-Term Recall | 25.6 | 26.0 | 22.5 | 26.0 | 28.0 | 28.5 | 28.5 | 0.0316 |
| Short-Term Recall b | 7.6 | 7.8 | 9.2 | 7.5 | 7.9 | 7.0 | 7.0 | 0.0391 |
| Heaton | ||||||||
| Immediate Recall | 10.1 | 9.7 | 9.2 | 9.2 | 10.5 | 10.3 | 10.3 | 0.1471 |
| Delayed Recall | 7.5 | 7.1 | 6.6 | 6.4 | 8.3 | 7.5 | 7.5 | 0.0720 |
| Copying | 15.8 | 14.9 * | 14.7 * | 14.6 * | 15.4 | 15.3 | 15.3 | 0.0311 |
| MMSE | 26.7 | 26.8 | 26.3 | 26.0 | 27.4 | 26.9 | 26.9 | 0.0018 |
| Serial 7’s | 4.3 | 4.2 | 4.3 | 4.1 | 4.5 | 4.4 | 4.4 | 0.0763 |
| “World” Backward | 4.8 | 4.9 | 4.5 | 4.5 | 4.8 | 4.6 | 4.6 | 0.0577 |
| Blessed Items | 6.3 | 6.1 | 5.6 * | 5.3 * | 6.1 | 6.0 | 6.0 | 0.0004 |
| Trails B b | 124.1 | 119.3 | 147.7 | 136.5 | 118.3 | 138.6 | 138.6 | 0.0194 |
| Category Fluency | 18.9 | 18.5 | 17.1 | 17.7 | 18.9 | 18.4 | 18.4 | 0.1843 |
| 0/Week | 1/Week | 2/Week | 3/Week | 4/Week | 5–6/Week | ≥7/Week | ||
| Women | N = 89 | N = 105 | N = 161 | N = 161 | N = 146 | N = 119 | N = 117 | p-Value a |
| Buschke | ||||||||
| Total Recall | 37.8 | 38.0 | 39.3 | 39.2 | 38.1 | 40.1 | 41.7 * | 0.0115 |
| Long-Term Recall | 31.3 | 31.5 | 33.7 | 33.1 | 31.7 | 33.9 | 36.5 * | 0.0206 |
| Short-Term Recall b | 6.5 | 6.7 | 5.7 | 6.1 | 6.5 | 6.2 | 5.3 * | 0.1136 |
| Heaton | ||||||||
| Immediate Recall | 9.0 | 8.7 | 8.8 | 8.8 | 9.3 | 9.1 | 10.2 * | 0.0220 |
| Delayed Recall | 7.0 | 5.3 * | 6.2 | 5.9 * | 6.5 | 6.7 | 7.3 | 0.0021 |
| Copying | 15.4 | 14.8 | 14.9 | 15.0 | 15.1 | 15.0 | 15.3 | 0.2615 |
| MMSE | 27.1 | 27.1 | 27.3 | 27.2 | 27.2 | 27.6 | 17.4 | 0.3649 |
| Serial 7’s | 3.7 | 4.0 | 4.0 | 3.8 | 3.8 | 4.0 | 4.2 * | 0.0913 |
| “World” Backward | 4.9 | 4.8 | 4.9 | 4.8 | 4.8 | 4.9 | 4.9 | 0.9195 |
| Blessed Items | 5.9 | 6.1 | 6.1 | 6.0 | 6.0 | 6.2 | 6.1 | 0.7219 |
| Trails B b | 155.6 | 155.5 | 138.9 | 148.4 | 138.0 | 145.2 | 133.1 * | 0.0784 |
| Category Fluency | 16.6 | 16.7 | 17.1 | 16.7 | 17.0 | 17.7 | 18.3 * | 0.1587 |
| Men | Women | |||||
|---|---|---|---|---|---|---|
| B | p-Value | B | p-Value | |||
| Buschke | Total recall | Model 1 | 0.207 | 0.0475 | 0.133 | 0.2008 |
| Model 2 | 0.209 | 0.0447 | 0.130 | 0.2133 | ||
| Model 3 | 0.216 | 0.0415 | 0.114 | 0.2785 | ||
| Long-term recall | Model 1 | 0.318 | 0.0245 | 0.141 | 0.3223 | |
| Model 2 | 0.323 | 0.0224 | 0.139 | 0.3316 | ||
| Model 3 | 0.333 | 0.0204 | 0.113 | 0.4300 | ||
| Short-term recall a | Model 1 | −0.109 | 0.0578 | −0.018 | 0.7306 | |
| Model 2 | −0.111 | 0.0534 | −0.018 | 0.7731 | ||
| Model 3 | −0.115 | 0.0494 | −0.007 | 0.8864 | ||
| Heaton | Immediate recall | Model 1 | 0.066 | 0.1173 | 0.060 | 0.1359 |
| Model 2 | 0.068 | 0.1061 | 0.061 | 0.1315 | ||
| Model 3 | 0.065 | 0.1234 | 0.056 | 0.1677 | ||
| Delayed recall | Model 1 | 0.085 | 0.0890 | 0.048 | 0.2950 | |
| Model 2 | 0.087 | 0.0810 | 0.048 | 0.2887 | ||
| Model 3 | 0.073 | 0.1471 | 0.046 | 0.3153 | ||
| Copying | Model 1 | 0.027 | 0.3141 | −0.013 | 0.6011 | |
| Model 2 | 0.029 | 0.2808 | −0.010 | 0.6880 | ||
| Model 3 | 0.034 | 0.2067 | −0.013 | 0.6024 | ||
| Mini-Mental State Exam | Model 1 | 0.043 | 0.1344 | 0.006 | 0.7719 | |
| Model 2 | 0.044 | 0.1231 | 0.007 | 0.7444 | ||
| Model 3 | 0.050 | 0.0874 | 0.004 | 0.8418 | ||
| Serial 7’s | Model 1 | 0.017 | 0.2026 | 0.029 | 0.0816 | |
| Model 2 | 0.017 | 0.2094 | 0.031 | 0.0598 | ||
| Model 3 | 0.018 | 0.1946 | 0.028 | 0.0912 | ||
| “World” Backward | Model 1 | −0.003 | 0.7644 | −0.003 | 0.6652 | |
| Model 2 | −0.003 | 0.7740 | −0.003 | 0.6524 | ||
| Model 3 | −0.002 | 0.8254 | −0.002 | 0.7639 | ||
| Blessed Items | Model 1 | 0.020 | 0.2817 | −0.004 | 0.8061 | |
| Model 2 | 0.020 | 0.2895 | −0.004 | 0.8146 | ||
| Model 3 | 0.025 | 0.1978 | −0.005 | 0.7431 | ||
| Trails B a | Model 1 | 1.418 | 0.0505 | 0.002 | 0.9976 | |
| Model 2 | 1.389 | 0.0554 | −0.078 | 0.9193 | ||
| Model 3 | 1.187 | 0.1061 | 0.219 | 0.7771 | ||
| Category Fluency | Model 1 | 0.033 | 0.5865 | 0.096 | 0.0819 | |
| Model 2 | 0.034 | 0.5798 | 0.101 | 0.0690 | ||
| Model 3 | 0.050 | 0.4193 | 0.100 | 0.0743 | ||
| Men | Women | |||
|---|---|---|---|---|
| B | p-Value | B | p-Value | |
| Buschke | ||||
| Total recall | 0.226 | 0.0866 | 0.041 | 0.7419 |
| Long-term recall | 0.284 | 0.1246 | 0.022 | 0.8997 |
| Short-term recall a | −0.064 | 0.3729 | 0.001 | 0.9915 |
| Heaton | ||||
| Immediate recall | 0.073 | 0.1905 | 0.077 | 0.1346 |
| Delayed recall | 0.115 | 0.0883 | 0.075 | 0.2009 |
| Copying | 0.061 | 0.0352 | −0.036 | 0.1989 |
| Mini-Mental State Exam | 0.058 | 0.0154 | −0.0002 | 0.9266 |
| Serial 7’s | 0.008 | 0.6067 | 0.033 | 0.1138 |
| “World” backward | 0.003 | 0.7930 | −0.006 | 0.4947 |
| Blessed items | 0.025 | 0.1463 | −0.012 | 0.5040 |
| Trails B a | 1.749 | 0.0110 | 0.311 | 0.7196 |
| Category Fluency | 0.030 | 0.7021 | 0.145 | 0.0464 |
| Men | Women | |||
|---|---|---|---|---|
| Cognitive Function Test | OR | (95% CI) | OR | (95% CI) |
| Mini-Mental Status Exam ≤ 24 | 0.978 | (0.893–1.072) | 0.936 | (0.817–1.072) |
| Trails B ≥ 132 | 1.002 | (0.941–1.066) | 1.012 | (0.956–1.071) |
| Category Fluency ≤ 12 | 0.959 | (0.879–1.046) | 0.967 | (0.895–1.044) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kritz-Silverstein, D.; Bettencourt, R. The Longitudinal Association of Egg Consumption with Cognitive Function in Older Men and Women: The Rancho Bernardo Study. Nutrients 2024, 16, 53. https://doi.org/10.3390/nu16010053
Kritz-Silverstein D, Bettencourt R. The Longitudinal Association of Egg Consumption with Cognitive Function in Older Men and Women: The Rancho Bernardo Study. Nutrients. 2024; 16(1):53. https://doi.org/10.3390/nu16010053
Chicago/Turabian StyleKritz-Silverstein, Donna, and Ricki Bettencourt. 2024. "The Longitudinal Association of Egg Consumption with Cognitive Function in Older Men and Women: The Rancho Bernardo Study" Nutrients 16, no. 1: 53. https://doi.org/10.3390/nu16010053
APA StyleKritz-Silverstein, D., & Bettencourt, R. (2024). The Longitudinal Association of Egg Consumption with Cognitive Function in Older Men and Women: The Rancho Bernardo Study. Nutrients, 16(1), 53. https://doi.org/10.3390/nu16010053






