Nutrition and Disorders of Gut–Brain Interaction
Abstract
1. Introduction
2. Materials and Methods
3. Results
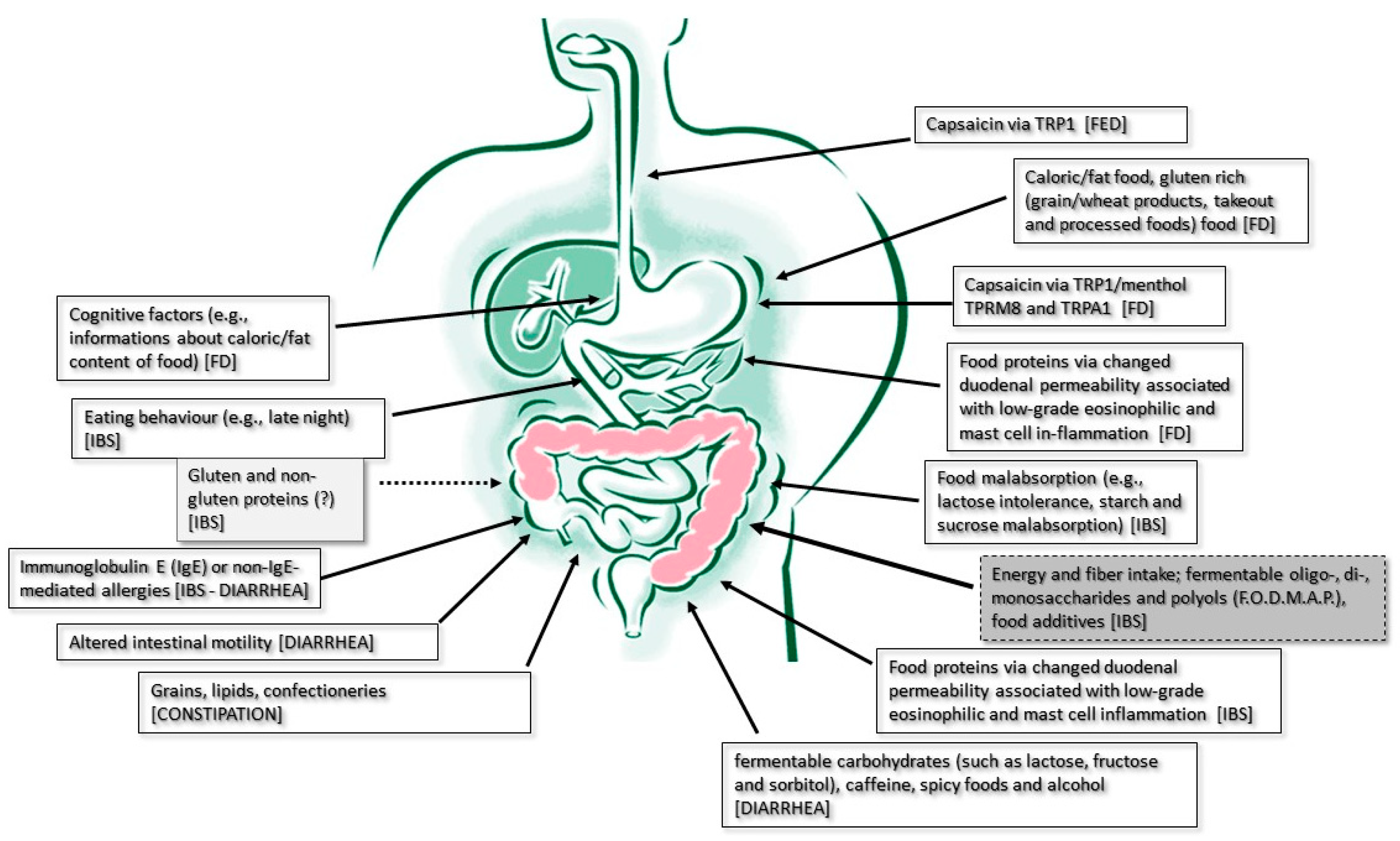
3.1. The Role of Food Intake as a Symptom Trigger and Therapeutic Target of Functional Esophageal Disorders (FEDs)
3.2. The Role of Food Intake as a Symptom Trigger and Therapeutic Target of Functional Dyspepsia
3.2.1. Food as a Trigger
3.2.2. Food Volume as a Trigger
3.2.3. Chemosensing as a Trigger
3.2.4. Caloric Content as a Trigger
3.2.5. Food Allergens as a Trigger
3.3. The Role of Food Intake as Symptoms’ Trigger in Lower GI Tract Functional Disorders
3.3.1. The Role of Food Intake as a Symptom Trigger and Therapeutic Target of Carbohydrate Malabsorption Syndromes
3.3.2. The Role of Food Intake as a Symptom Trigger and Therapeutic Target of Irritable Bowel Syndrome
3.3.3. The Role of Food Intake as a Symptom Trigger and Therapeutic Target of Diarrhea
3.3.4. The Role of Food Intake as a Symptom Trigger and Therapeutic Target of Constipation
3.3.5. The Role of Food and Gut Microbiota Interaction in DGBIs
3.3.6. The Role of Cognitive Factors in the Interaction between Food and DGBIs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simons, J.; Shajee, U.; Palsson, O.; Simren, M.; Sperber, A.D.; Törnblom, H.; Whitehead, W.; Aziz, I. Disorders of gut-brain interaction: Highly prevalent and burdensome yet under-taught within medical education. United Eur. Gastroenterol. J. 2022, 10, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, C.H.; Materna, A.; Wermelinger, C.; Schuler, J. Fructose and lactose intolerance and malabsorption testing: The relationship with symptoms in functional gastrointestinal disorders. Aliment. Pharmacol. Ther. 2013, 37, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Malagelada, J.R. The Brain-Gut Team. Dig. Dis. 2020, 38, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.E. The low FODMAP diet in clinical practice: Where are we and what are the long-term considerations? Proc. Nutr. Soc. 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.G.; Varney, J.E.; Ajamian, M.; Gibson, P.R. Gluten-free and low-FODMAP sourdoughs for patients with coeliac disease and irritable bowel syndrome: A clinical perspective. Int. J. Food Microbiol. 2019, 290, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Haller, E.; Scarlata, K. Diet Interventions for Irritable Bowel Syndrome: Separating the Wheat from the Chafe. Gastroenterol. Clin. N. Am. 2021, 50, 565–579. [Google Scholar] [CrossRef]
- Di Rosa, C.; Altomare, A.; Terrigno, V.; Carbone, F.; Tack, J.; Cicala, M.; Guarino, M.P.L. Constipation-Predominant Irritable Bowel Syndrome (IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 2023, 15, 1647. [Google Scholar] [CrossRef]
- Shankar, S.; Durairaj, E. Diet and Management of Diarrhea. Indian J. Pediatr. 2023. [Google Scholar] [CrossRef]
- Rettura, F.; Lambiase, C.; Grosso, A.; Rossi, A.; Tedeschi, R.; Ceccarelli, L.; Bellini, M. Role of Low-FODMAP diet in functional dyspepsia: “Why”, “When”, and “to Whom”. Best Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101831. [Google Scholar] [CrossRef]
- Ahonen, I.; Laurikka, P.; Koskimaa, S.; Huhtala, H.; Lindfors, K.; Kaukinen, K.; Kurppa, K.; Kivelä, L. Prevalence of vomiting and nausea and associated factors after chronic and acute gluten exposure in celiac disease. BMC Gastroenterol. 2023, 23, 301. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Q.; Fass, R.; Gyawali, C.P.; Miwa, H.; Pandolfino, J.E.; Zerbib, F. Functional Esophageal Disorders. Gastroenterology 2016, 130, 1459–1465. [Google Scholar]
- Gonlachanvit, S. Are rice and spicy diet good for functional gastrointestinal disorders? J. Neurogastroenterol. Motil. 2010, 16, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Milke, P.; Diaz, A.; Valdovinos, M.A.; Moran, S. Gastroesophageal reflux in healthy subjects induced by two different species of chilli (Capsicum annum). Dig. Dis. 2006, 24, 184–188. [Google Scholar] [CrossRef]
- Aziz, I.; Palsson, O.S.; Törnblom, H.; Sperber, A.D.; Whitehead, W.E.; Simrén, M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: A cross-sectional population-based study. Lancet Gastroenterol. Hepatol. 2018, 3, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Bisschops, R.; Karamanolis, G.; Arts, J.; Caenepeel, P.; Verbeke, K.; Janssens, J.; Tack, J. Relationship between symptoms and ingestion of a meal in functional dyspepsia. Gut 2008, 57, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Pilichiewicz, A.N.; Horowitz, M.; Holtmann, G.J.; Talley, N.J.; Feinle-Bisset, C. Relationship between symptoms and dietary patterns in patients with functional dyspepsia. Clin. Gastroenterol. Hepatol. 2009, 7, 317–322. [Google Scholar] [CrossRef]
- Duncanson, K.R.; Talley, N.J.; Walker, M.M.; Burrows, T.L. Food and functional dyspepsia: A systematic review. J. Hum. Nutr. Diet. 2018, 31, 390–407. [Google Scholar] [CrossRef]
- Pesce, M.; Cargiolli, M.; Cassarano, S.; Polese, B.; De Conno, B.; Aurino, L.; Mancino, N.; Sarnelli, G. Diet and functional dyspepsia: Clinical correlates and therapeutic perspectives. World J. Gastroenterol. 2020, 26, 456–465. [Google Scholar] [CrossRef]
- Tabibian, S.R.; Hajhashemy, Z.; Shaabani, P.; Saneei, P.; Keshteli, A.H.; Esmaillzadeh, A.; Adibi, P. The relationship between fruit and vegetable intake with functional dyspepsia in adults. Neurogastroenterol. Motil. 2021, 33, e14129. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef]
- Farré, R.; Vanheel, H.; Vanuytsel, T.; Masaoka, T.; Törnblom, H.; Simrén, M.; Van Oudenhove, L.; Tack, J.F. In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology 2013, 145, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Piessevaux, H.; Coulie, B.; Caenepeel, P.; Janssens, J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 1998, 115, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Vanheel, H.; Carbone, F.; Valvekens, L.; Simren, M.; Tornblom, H.; Vanuytsel, T.; Van Oudenhove, L.; Tack, J. Pathophysiological Abnormalities in Functional Dyspepsia Subgroups According to the Rome III Criteria. Am. J. Gastroenterol. 2017, 112, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. TRPV1 and the gut: From a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur. J. Pharmacol. 2004, 500, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Führer, M.; Vogelsang, H.; Hammer, J. A placebo-controlled trial of an oral capsaicin load in patients with functional dyspepsia. Neurogastroenterol. Motil. 2011, 23, 918-e397. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, Y.; Wong, R.K.; Ho, K.Y.; Wilder-Smith, C.H. Visceral and somatic sensory function in functional dyspepsia. Neurogastroenterol. Motil. 2013, 25, 246–253.e165. [Google Scholar] [CrossRef]
- Lacy, B.E.; Chey, W.D.; Epstein, M.S.; Shah, S.M.; Corsino, P.; Zeitzoff, L.R.; Cash, B.D. A novel duodenal-release formulation of caraway oil and L-menthol is a safe, effective and well tolerated therapy for functional dyspepsia. BMC Gastroenterol. 2022, 22, 105. [Google Scholar] [CrossRef]
- Chey, W.D.; Lacy, B.E.; Cash, B.D.; Epstein, M.; Corsino, P.E.; Shah, S.M. A Novel, Duodenal-Release Formulation of a Combination of Caraway Oil and L-Menthol for the Treatment of Functional Dyspepsia: A Randomized Controlled Trial. Clin. Transl. Gastroenterol. 2019, 10, e00021. [Google Scholar] [CrossRef]
- Boeckxstaens, G.E.; Hirsch, D.P.; van den Elzen, B.D.; Heisterkamp, S.H.; Tytgat, G.N. Impaired drinking capacity in patients with functional dyspepsia: Relationship with proximal stomach function. Gastroenterology 2001, 121, 1054–1063. [Google Scholar] [CrossRef]
- Kindt, S.; Coulie, B.; Wajs, E.; Janssens, J.; Tack, J. Reproducibility and symptomatic predictors of a slow nutrient drinking test in health and in functional dyspepsia. Neurogastroenterol. Motil. 2008, 20, 320–329. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Camilleri, M.; Burton, D.D.; Thieke, S.L.; Feuerhak, K.J.; Basu, A.; Zinsmeister, A.R. Increased nutrient sensitivity and plasma concentrations of enteral hormones during duodenal nutrient infusion in functional dyspepsia. Am. J. Gastroenterol. 2014, 109, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Feinle-Bisset, C.; Meier, B.; Fried, M.; Beglinger, C. Role of cognitive factors in symptom induction following high and low fat meals in patients with functional dyspepsia. Gut 2003, 52, 1414–1418, Erratum in Gut 2004, 53, 316. [Google Scholar] [CrossRef] [PubMed]
- Vanheel, H.; Vicario, M.; Vanuytsel, T.; Van Oudenhove, L.; Martinez, C.; Keita, Å.V.; Pardon, N.; Santos, J.; Söderholm, J.D.; Tack, J.; et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014, 63, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Fairlie, T.; Brown, G.; Jones, M.P.; Eslick, G.D.; Duncanson, K.; Thapar, N.; Keely, S.; Koloski, N.; Shahi, M.; et al. Duodenal Eosinophils and Mast Cells in Functional Dyspepsia: A Systematic Review and Meta-Analysis of Case-Control Studies. Clin. Gastroenterol. Hepatol. 2022, 20, 2229–2242.e29. [Google Scholar] [CrossRef] [PubMed]
- Hari, S.; Burns, G.L.; Hoedt, E.C.; Keely, S.; Talley, N.J. Eosinophils, Hypoxia-Inducible Factors, and Barrier Dysfunction in Functional Dyspepsia. Front. Allergy 2022, 3, 851482. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Dickman, R.; Drug, V.; Mulak, A.; Serra, J.; Enck, P.; Tack, J.; ESNM FD Consensus Group; Accarino, A.; Barbara, G.; et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on functional dyspepsia. Neurogastroenterol. Motil. 2021, 33, e14238. [Google Scholar] [CrossRef] [PubMed]
- Goyal, O.; Nohria, S.; Batta, S.; Dhaliwal, A.; Goyal, P.; Sood, A. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet versus traditional dietary advice for functional dyspepsia: A randomized controlled trial. J. Gastroenterol. Hepatol. 2022, 37, 301–309. [Google Scholar] [CrossRef]
- Van den Houte, K.; Carbone, F.; Toth, J.; Mariën, Z.; Schol, J.; Colomier, E.; Van den Bergh, J.; Vanderstappen, J.; Pauwels, N.; Matthys, C.; et al. Symptoms and duodenal mucosal integrity are improved by a dietary intervention in functional dyspepsia. Gastroenterology 2021, 160, S466. [Google Scholar] [CrossRef]
- Du, L.; Shen, J.; Kim, J.J.; He, H.; Chen, B.; Dai, N. Impact of gluten consumption in patients with functional dyspepsia: A case-control study. J. Gastroenterol. Hepatol. 2018, 33, 128–133. [Google Scholar] [CrossRef]
- Potter, M.D.E.; Walker, M.M.; Jones, M.P.; Koloski, N.A.; Keely, S.; Talley, N.J. Wheat Intolerance and Chronic Gastrointestinal Symptoms in an Australian Population-based Study: Association Between Wheat Sensitivity, Celiac Disease and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2018, 113, 1036–1044. [Google Scholar] [CrossRef]
- Montoro-Huguet, M.A.; Belloc, B.; Domínguez-Cajal, M. Small and Large Intestine (I): Malab-sorption of Nutrients. Nutrients 2021, 13, 1254. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef] [PubMed]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Suarez, F.L. A Comparison of Symptoms after the Consumption of Milk or Lac-tose-Hydrolyzed Milk by People with Self-Reported Severe Lactose Intolerance. N. Engl. J. Med. 1995, 333, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Naim, H.Y.; Heine, M.; Zimmer, K.-P. Congenital Sucrase-Isomaltase Deficiency: Heterogene-ity of Inheritance, Trafficking, and Function of an Intestinal Enzyme Complex. J. Pediatr. Gastroenterol. Nutr. 2012, 55, S13–S30. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Etxebarria, K.; Zheng, T.; Bonfiglio, F.; Bujanda, L.; Dlugosz, A.; Lindberg, G.; Schmidt, P.T.; Karling, P.; Ohlsson, B.; Simren, M.; et al. Increased Prevalence of Rare Sucrase-isomaltase Pathogenic Variants in Irritable Bowel Syndrome Patients. Clin. Gastroenterol. Hepatol. 2018, 16, 1673–1676. [Google Scholar] [CrossRef]
- Nilholm, C.; Larsson, E.; Roth, B.; Gustafsson, R.; Ohlsson, B. Irregular Dietary Habits with a High Intake of Cereals and Sweets Are Associated with More Severe Gastrointestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Zamfir-Taranu, A.; Löscher, B.-S.; Husein, D.M.; Hoter, A.; Garcia-Etxebarria, K.; Etxeberria, U.; Gayoso, L.; Mayr, G.; Nilholm, C.; Gustafsson, R.J.; et al. Sucrase-isomaltase genotype and response to a starch-reduced and su-crose-reduced diet in IBS-D patients. Gut 2023. [Google Scholar] [CrossRef]
- Zheng, T.; Eswaran, S.; Photenhauer, A.L.; Merchant, J.L.; Chey, W.D.; D’Amato, M. Reduced efficacy of low FODMAPs diet in patients with IBS-D carrying sucrase-isomaltase (SI) hypomorphic variants. Gut 2020, 69, 397–398. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar]
- Van den Houte, K.; Carbone, F.; Pannemans, J.; Corsetti, M.; Fischler, B.; Piessevaux, H.; Tack, J. Prevalence and impact of self-reported irritable bowel symptoms in the general population. United Eur. Gastroenterol. J. 2019, 7, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Posserud, I.; Strid, H.; Störsrud, S.; Törnblom, H.; Svensson, U.; Tack, J.; Van Oudenhove, L.; Simrén, M. Symptom pattern following a meal challenge test in patients with irritable bowel syndrome and healthy controls. United Eur. Gastroenterol. J. 2013, 1, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Colomier, E.; Van Oudenhove, L.; Tack, J.; Böhn, L.; Bennet, S.; Nybacka, S.; Störsrud, S.; Öhman, L.; Törnblom, H.; Simrén, M. Predictors of Symptom-Specific Treatment Response to Dietary Interventions in Irritable Bowel Syndrome. Nutrients 2022, 14, 397. [Google Scholar] [CrossRef] [PubMed]
- Clevers, E.; Törnblom, H.; Simrén, M.; Tack, J.; Van Oudenhove, L. Relations between food intake, psychological distress, and gastrointestinal symptoms: A diary study. United Eur. Gastroenterol. J. 2019, 7, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.K.; Mitchell, S.B.; Barrett, J.S.; Shepherd, S.J.; Irving, P.M.; Biesiekierski, J.R.; Smith, S.; Gibson, P.R.; Muir, J.G. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2010, 25, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.; Wilkinson-Smith, V.; Hoad, C.; Costigan, C.; Cox, E.; Lam, C.; Marciani, L.; Gowland, P.; Spiller, R.C. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am. J. Gastroenterol. 2014, 109, 110–119. [Google Scholar] [CrossRef]
- Whelan, K.; Martin, L.D.; Staudacher, H.M.; Lomer, M.C.E. The low FODMAP diet in the management of irritable bowel syndrome: An evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J. Hum. Nutr. Diet. 2018, 31, 239–255. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399.e2–1407.e2. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Gasbarrini, A.; Mele, M.C. Food Additives, Gut Microbiota, and Irritable Bowel Syndrome: A Hidden Track. Int. J. Environ. Res. Public. Health 2020, 17, 8816. [Google Scholar] [CrossRef] [PubMed]
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.M.; von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; Dierks, C.; et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903–911.e3. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef] [PubMed]
- Balsiger, L. Confocal Laser Endomicroscopy Shows High Rate of Acute Mucosal Reactions Following Food Administration in Irritable Bowel Syndrome. Available online: https://eposters.ddw.org/ddw/2023/ddw-2023/380552/lukas.balsiger.confocal.laser.endomicrosopy.shows.high.rate.of.acute.mucosal.html?f=listing%3D1%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D2%2Ace_id%3D2482%2Aot_id%3D27750%2Amarker%3D4159 (accessed on 15 September 2023).
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef]
- Savarino, E.; Zingone, F.; Barberio, B.; Marasco, G.; Akyuz, F.; Akpinar, H.; Barboi, O.; Bodini, G.; Bor, S.; Chiarioni, G.; et al. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 2022, 10, 556–584. [Google Scholar] [CrossRef]
- O’Brien, L.; Wall, C.L.; Wilkinson, T.J.; Gearry, R.B. What Are the Pearls and Pitfalls of the Dietary Management for Chronic Diarrhoea? Nutrients 2021, 13, 1393. [Google Scholar] [CrossRef]
- Schiller, L.R. Nutrition management of chronic diarrhea and malabsorption. Nutr. Clin. Pract. 2006, 21, 34–39. [Google Scholar] [CrossRef]
- Ford, C.K. Nutrition Considerations in Patients with Functional Diarrhea. Curr. Gastroenterol. Rep. 2023, 25, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.B.; Bailey, A.P.; Keshishian, A.C.; Silvernale, C.J.; Staller, K.; Eddy, K.T.; Thomas, J.J.; Kuo, B. Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Adult Neurogastroenterology Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 1995–2002.e1. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Ringel, Y. Treatment of functional diarrhea. Curr. Treat. Options Gastroenterol. 2006, 9, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Bliss, D.Z.; Jung, H.J.; Savik, K.; Lowry, A.; LeMoine, M.; Jensen, L.; Werner, C.; Schaffer, K. Supplementation with dietary fiber improves fecal incontinence. Nurs. Res. 2001, 50, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Bliss, D.Z.; Savik, K.; Jung, H.J.G.; Whitebird, R.; Lowry, A.; Sheng, X. Dietary Fiber Supplementation for Fecal Incontinence: A Randomized Clinical Trial. Res. Nurs. Health 2014, 37, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Menees, S.B.; Jackson, K.; Baker, J.R.; Fenner, D.E.; Eswaran, S.; Nojkov, B.; Saad, R.; Lee, A.A.; Chey, W.D. A Randomized Pilot Study to Compare the Effectiveness of a Low FODMAP Diet vs Psyllium in Patients with Fecal Incontinence and Loose Stools. Clin. Transl. Gastroenterol. 2022, 13, e00454. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.; Pohl, D.; Azpiroz, F.; Chiarioni, G.; Ducrotté, P.; Gourcerol, G.; Hungin, A.P.S.; Layer, P.; Mendive, J.M.; Pfeifer, J.; et al. European society of neurogastroenterology and motility guidelines on functional constipation in adults. Neurogastroenterol. Motil. 2020, 32, e13762. [Google Scholar] [CrossRef]
- Rollet, M.; Bohn, T.; Vahid, F.; On Behalf of the Oriscav Working Group. Association between Dietary Factors and Constipation in Adults Living in Luxembourg and Taking Part in the ORISCAV-LUX 2 Survey. Nutrients 2021, 14, 122. [Google Scholar] [CrossRef]
- Murakami, K.; Sasakii, S.; Okubo, H.; Takahashi, Y.; Hoso, Y.; Itabashi, M.; Freshmen in Dietetic Courses Study II Group. Food intake and functional constipation: A cross-sectional study of 3,835 Japanese women aged 18-20 years. J. Nutr. Sci. Vitaminol. 2007, 53, 30–36. [Google Scholar] [CrossRef][Green Version]
- Yurtdaş Depboylu, G.; Acar Tek, N.; Akbulut, G.; Günel, Z.; Kamanlı, B. Functional Constipation in Elderly and Related Determinant Risk Factors: Malnutrition and Dietary Intake. J. Am. Nutr. Assoc. 2023, 42, 541–547. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S.; Okubo, H.; Takahashi, Y.; Hosoi, Y.; Itabashi, M.; Freshmen in Dietetic Courses Study II Group. Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women. Eur. J. Clin. Nutr. 2007, 61, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, S.; Hisar, F. The efficacy of education programme for preventing constipation in women. Int. J. Nurs. Pract. 2014, 20, 275–282. [Google Scholar] [CrossRef] [PubMed]
- van der Schoot, A.; Drysdale, C.; Whelan, K.; Dimidi, E. The Effect of Fiber Supplementation on Chronic Constipation in Adults: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2022, 116, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, A.; Donahoe, R.; Valestin, J.; Brown, K.; Rao, S.S. Randomised clinical trial: Dried plums (prunes) vs. psyllium for constipation. Aliment. Pharmacol. Ther. 2011, 33, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Nagata, N.; Nishiura, K.; Miura, N.; Kawai, T.; Yamamoto, H. Prune Juice Containing Sorbitol, Pectin, and Polyphenol Ameliorates Subjective Complaints and Hard Feces While Normalizing Stool in Chronic Constipation: A Randomized Placebo-Controlled Trial. Am. J. Gastroenterol. 2022, 117, 1714–1717. [Google Scholar] [CrossRef]
- Vandenberghe, J.; Dupont, P.; Van Oudenhove, L.; Bormans, G.; Demyttenaere, K.; Fischler, B.; Geeraerts, B.; Janssens, J.; Tack, J. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology 2007, 132, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef]
- Futagami, S.; Itoh, T.; Sakamoto, C. Systematic review with meta-analysis: Post-infectious functional dyspepsia. Aliment. Pharmacol. Ther. 2015, 41, 177–188. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Gillilland, M., 3rd; Wu, X.; Leelasinjaroen, P.; Zhang, G.; Zhou, H.; Ye, B.; Lu, Y.; Owyang, C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J. Clin. Investig. 2018, 128, 267–280. [Google Scholar] [CrossRef]
- De Palma, G.; Shimbori, C.; Reed, D.E.; Yu, Y.; Rabbia, V.; Lu, J.; Jimenez-Vargas, N.; Sessenwein, J.; Lopez-Lopez, C.; Pigrau, M.; et al. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci. Transl. Med. 2022, 14, eabj1895. [Google Scholar] [CrossRef]
- Singh, P.; Grabauskas, G.; Zhou, S.Y.; Gao, J.; Zhang, Y.; Owyang, C. High FODMAP diet causes barrier loss via lipopolysaccharide-mediated mast cell activation. JCI Insight 2021, 6, e146529. [Google Scholar] [CrossRef]
- Van Oudenhove, L.; Vandenberghe, J.; Dupont, P.; Geeraerts, B.; Vos, R.; Dirix, S.; Van Laere, K.; Bormans, G.; Vanderghinste, D.; Demyttenaere, K.; et al. Regional brain activity in functional dyspepsia: A H(2)(15)O-PET study on the role of gastric sensitivity and abuse history. Gastroenterology 2010, 139, 36–47. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; de Araujo, I.E.; Rolls, E.T. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage 2004, 21, 781–788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpellini, E.; Balsiger, L.M.; Broeders, B.; Houte, K.V.D.; Routhiaux, K.; Raymenants, K.; Carbone, F.; Tack, J. Nutrition and Disorders of Gut–Brain Interaction. Nutrients 2024, 16, 176. https://doi.org/10.3390/nu16010176
Scarpellini E, Balsiger LM, Broeders B, Houte KVD, Routhiaux K, Raymenants K, Carbone F, Tack J. Nutrition and Disorders of Gut–Brain Interaction. Nutrients. 2024; 16(1):176. https://doi.org/10.3390/nu16010176
Chicago/Turabian StyleScarpellini, Emidio, Lukas Michaja Balsiger, Bert Broeders, Karen Van Den Houte, Karen Routhiaux, Karlien Raymenants, Florencia Carbone, and Jan Tack. 2024. "Nutrition and Disorders of Gut–Brain Interaction" Nutrients 16, no. 1: 176. https://doi.org/10.3390/nu16010176
APA StyleScarpellini, E., Balsiger, L. M., Broeders, B., Houte, K. V. D., Routhiaux, K., Raymenants, K., Carbone, F., & Tack, J. (2024). Nutrition and Disorders of Gut–Brain Interaction. Nutrients, 16(1), 176. https://doi.org/10.3390/nu16010176






