Food Intolerances, Food Allergies and IBS: Lights and Shadows
Abstract
1. Introduction
2. IBS: Pathogenesis
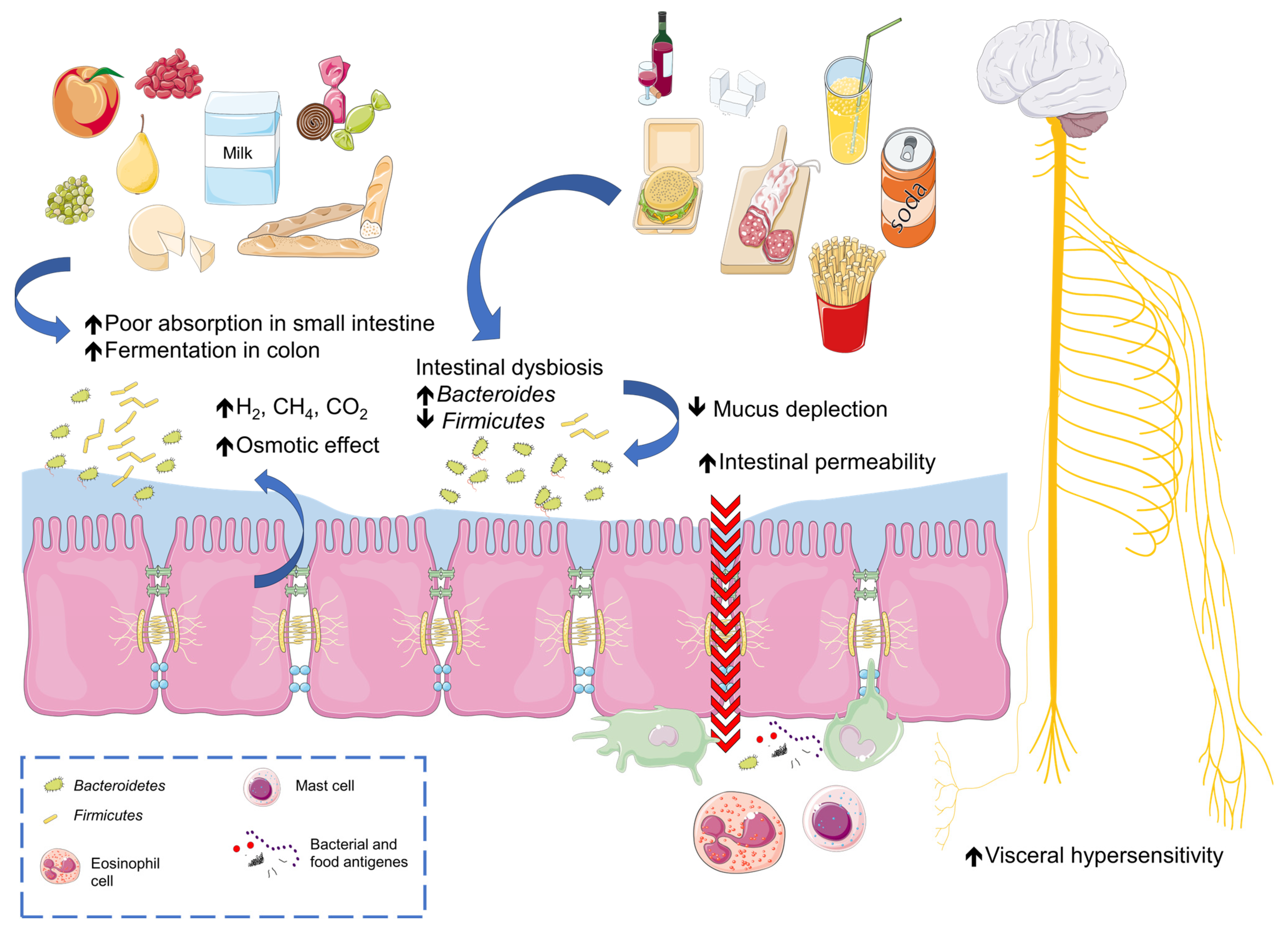
3. Adverse Food Reactions
4. Which Foods and Food Patterns Can Cause Intolerance?
4.1. Fermentable Oligo-, Di-, and Monosaccharides and Polyols (FODMAPs)
4.2. Fructose Malabsorption
4.3. Non-Celiac Gluten Sensitivity
4.4. Histamine
4.5. Food Additives
4.6. Other Foods Identified as Triggers in IBS Patients
5. Which Dietary Patterns Are Useful in Food Intolerance in IBS Patients?
5.1. Low-FODMAP Diet
5.2. Gluten-Free Diet
5.3. Lactose-Free Diet
5.4. Mediterranean Diet
5.5. Other Dietary Patterns for the Management of Food Intolerance in IBS Patients
6. Future Prospective and Unmet Needs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable Bowel Syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef]
- Lacy, B.E.; Patel, N.K. Clinical Medicine Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Trindade, I.A.; Melchior, C.; Törnblom, H.; Simrén, M. Quality of Life in Irritable Bowel Syndrome: Exploring Mediating Factors through Structural Equation Modelling. J. Psychosom. Res. 2022, 159, 110809. [Google Scholar] [CrossRef] [PubMed]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived Food Intolerance in Subjects with Irritable Bowel Syndrome—Etiology, Prevalence and Consequences. Eur. J. Clin. Nutr. 2005, 60, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Harer, K.N.; Eswaran, S.L. Irritable Bowel Syndrome: Food as a Friend or Foe? Gastroenterol. Clin. N. Am. 2021, 50, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, P.; D’alcamo, A.; Seidita, A.; Carroccio, A. Food Allergy in Irritable Bowel Syndrome: The Case of Non-Celiac Wheat Sensitivity. World J. Gastroenterol. 2015, 21, 7089–7109. [Google Scholar] [CrossRef]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Bégin, P.; Nadeau, K.C. Diagnosis of Food Allergy. Pediatr. Ann. 2013, 42, 102–109. [Google Scholar] [CrossRef]
- Van Den Houte, K.; Bercik, P.; Simren, M.; Tack, J.; Vanner, S. Mechanisms Underlying Food-Triggered Symptoms in Disorders of Gut-Brain Interactions. Am. J. Gastroenterol. 2022, 117, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R. Impact of Diet on Symptoms of the Irritable Bowel Syndrome. Nutrients 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.A.; Fraher, M.H.; Quigley, E.M.M. Irritable Bowel Syndrome: The Role of Food in Pathogenesis and Management. Gastroenterol. Hepatol. 2014, 10, 164. [Google Scholar]
- Martínez, C.; González-Castro, A.; Vicario, M.; Santos, J. Cellular and Molecular Basis of Intestinal Barrier Dysfunction in the Irritable Bowel Syndrome. Gut Liver 2012, 6, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zigich, S.; Heuberger, R. The Relationship of Food Intolerance and Irritable Bowel Syndrome in Adults. Gastroenterol. Nurs. 2013, 36, 275–282. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Irritable Bowel Syndrome: Treatment Based on Pathophysiology and Biomarkers. Gut 2023, 72, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.A. The Role of Genetics in IBS. Gastroenterol. Clin. N. Am. 2011, 40, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- O’Mahony, L.; Mccarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and Bifidobacterium in Irritable Bowel Syndrome: Symptom Responses and Relationship to Cytokine Profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- Park, J.; Choi, T.J.; Kang, K.S.; Choi, S.H. The Interrelationships between Intestinal Permeability and Phlegm Syndrome and Therapeutic Potential of Some Medicinal Herbs. Biomolecules 2021, 11, 284. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Öhman, L.; Simrén, M. Gut Microbiota as Potential Orchestrators of Irritable Bowel Syndrome. Gut Liver 2015, 9, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.W.; Rezaie, A.; Pimentel, M. Bile Acid and Gut Microbiota in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2022, 28, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, M.; Dong, W.; Liu, T.; Song, X.; Gu, Y.; Wang, S.; Liu, Y.; Abla, Z.; Qiao, X.; et al. Gut Dysbiosis and Abnormal Bile Acid Metabolism in Colitis-Associated Cancer. Gastroenterol. Res. Pract. 2021, 2021, 6645970. [Google Scholar] [CrossRef] [PubMed]
- Browning, K.N.; Travagli, R.A. Central Nervous System Control of Gastrointestinal Motility and Secretion and Modulation of Gastrointestinal Functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar] [CrossRef] [PubMed]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, V.; Rossi, R.E.; D’Arrigo, A.M.; Priori, A.; Gambini, O.; Demartini, B. Functional Neuroimaging in Irritable Bowel Syndrome: A Systematic Review Highlights Common Brain Alterations With Functional Movement Disorders. J. Neurogastroenterol. Motil. 2022, 28, 185. [Google Scholar] [CrossRef]
- Saha, L. Irritable Bowel Syndrome: Pathogenesis, Diagnosis, Treatment, and Evidence-Based Medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Jiang, A.-J.; Wang, X.-Y.; Wang, H.; Guan, Y.-Y.; Li, F.; Shen, G.-M. Uncovering the Pathophysiology of Irritable Bowel Syndrome by Exploring the Gut-Brain Axis: A Narrative Review. Ann. Transl. Med. 2021, 9, 1187. [Google Scholar] [CrossRef]
- Carco, C.; Young, W.; Gearry, R.B.; Talley, N.J.; McNabb, W.C.; Roy, N.C. Increasing Evidence That Irritable Bowel Syndrome and Functional Gastrointestinal Disorders Have a Microbial Pathogenesis. Front. Cell Infect. Microbiol. 2020, 10, 468. [Google Scholar] [CrossRef]
- Turnbull, J.L.; Adams, H.N.; Gorard, D.A. Review Article: The Diagnosis and Management of Food Allergy and Food Intolerances. Aliment Pharmacol. Ther. 2015, 41, 3–25. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Poowutikul, P.; Pansare, M.; Kamat, D. Food Allergy: A Review. Pediatr. Ann. 2020, 49, e50–e58. [Google Scholar] [CrossRef] [PubMed]
- Connors, L.; O’Keefe, A.; Rosenfield, L.; Kim, H. Non-IgE-Mediated Food Hypersensitivity. Allergy Asthma Clin. Immunol. 2018, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Chey, W.D.; Talley, N.J.; Malhotra, A.; Spiegel, B.M.R.; Moayyedi, P. Yield of Diagnostic Tests for Celiac Disease in Individuals With Symptoms Suggestive of Irritable Bowel Syndrome: Systematic Review and Meta-Analysis. Arch. Intern. Med. 2009, 169, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Di Nardo, G.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian Guidelines for the Management of Irritable Bowel Syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig. Liver Dis. 2023, 55, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Zhang, A.J.; DeKoven, J.G.; Maibach, H.I.; Belsito, D.V.; Sasseville, D.; Fowler, J.F.; Fransway, A.F.; Mathias, T.; Pratt, M.D.; et al. Epidemiology of Nickel Sensitivity: Retrospective Cross-Sectional Analysis of North American Contact Dermatitis Group Data 1994–2014. J. Am. Acad. Dermatol. 2019, 80, 701–713. [Google Scholar] [CrossRef]
- Saito, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Molecular Mechanisms of Nickel Allergy. Int. J. Mol. Sci 2016, 17, 202. [Google Scholar] [CrossRef]
- Rizzi, A.; Nucera, E.; Laterza, L.; Gaetani, E.; Valenza, V.; Corbo, G.M.; Inchingolo, R.; Buonomo, A.; Schiavino, D.; Gasbarrini, A. Irritable Bowel Syndrome and Nickel Allergy: What Is the Role of the Low Nickel Diet? J. Neurogastroenterol. Motil. 2017, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Coşgun, S.; Polat, U.; Kaya, M.; Sezikli, M.; Coşgun, S.; Polat, U.; Kaya, M.; Sezikli, M. Nickel Sensitivity in Patients With Irritable Bowel Syndrome. Cureus 2023, 15, e45196. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Cui, H.; Peng, X.; Pan, K.; Fang, J.; Zuo, Z.; Deng, J.; Wang, X.; Huang, J. Toxicological Effects of Dietary Nickel Chloride on Intestinal Microbiota. Ecotoxicol. Environ. Saf. 2014, 109, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pizzutelli, S. Systemic Nickel Hypersensitivity and Diet: Myth or Reality? Eur. Ann. Allergy Clin. Immunol. 2011, 43, 5–18. [Google Scholar]
- Sharma, A.D. Low Nickel Diet in Dermatology. Indian J. Dermatol. 2013, 58, 240. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, A.; Hagen, R.; Mitchell, M.; Ghareeb, E.; Fang, W.; Correa, R.; Zinn, Z.; Gayam, S. The Effect of a Low-Nickel Diet and Nickel Sensitization on Gastroesophageal Reflux Disease: A Pilot Study. Indian J. Gastroenterol. 2021, 40, 137–143. [Google Scholar] [CrossRef]
- Lombardi, F.; Fiasca, F.; Minelli, M.; Maio, D.; Mattei, A.; Vergallo, I.; Cifone, M.G.; Cinque, B.; Minelli, M. The Effects of Low-Nickel Diet Combined with Oral Administration of Selected Probiotics on Patients with Systemic Nickel Allergy Syndrome (SNAS) and Gut Dysbiosis. Nutrients 2020, 12, 1040. [Google Scholar] [CrossRef]
- Tuck, C.J.; Biesiekierski, J.R.; Schmid-Grendelmeier, P.; Pohl, D. Food Intolerances. Nutrients 2019, 11, 1684. [Google Scholar] [CrossRef]
- DI Costanzo, M.; Berni Canani, R. Lactose Intolerance: Common Misunderstandings. Ann. Nutr. Metab 2018, 73 (Suppl. S4), 30–37. [Google Scholar] [CrossRef]
- Usai-Satta, P.; Lai, M.; Oppia, F. Lactose Malabsorption and Presumed Related Disorders: A Review of Current Evidence. Nutrients 2022, 14, 584. [Google Scholar] [CrossRef]
- Lomer, M.C.E.; Parkes, G.C.; Sanderson, J.D. Review Article: Lactose Intolerance in Clinical Practice—Myths and Realities. Aliment. Pharmacol. Ther. 2008, 27, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020. [Google Scholar] [CrossRef] [PubMed]
- Montalto, M.; Curigliano, V.; Santoro, L.; Vastola, M.; Cammarota, G.; Manna, R.; Gasbarrini, A.; Gasbarrini, G. Management and Treatment of Lactose Malabsorption. World J. Gastroenterol. WJG 2006, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Zingone, F.; Iovino, P.; Bucci, C.; Ciacci, C. Coeliac Disease: No Difference in Milk and Dairy Products Consumption in Comparison with Controls. BMJ Nutr. Prev. Health 2019, 2, e000022. [Google Scholar] [CrossRef]
- Wanes, D.; Husein, D.M.; Naim, H.Y. Congenital Lactase Deficiency: Mutations, Functional and Biochemical Implications, and Future Perspectives. Nutrients 2019, 11, 461. [Google Scholar] [CrossRef]
- Cancarevic, I.; Rehman, M.; Iskander, B.; Lalani, S.; Malik, B.H. Is There a Correlation Between Irritable Bowel Syndrome and Lactose Intolerance? Cureus 2020, 12, 6170. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, C.; Van de Maele, K.; Hauser, B.; Vandenplas, Y. Hydrogen and Methane Breath Test in the Diagnosis of Lactose Intolerance. Nutrients 2021, 13, 3261. [Google Scholar] [CrossRef]
- Catanzaro, R.; Sciuto, M.; Marotta, F. Lactose Intolerance: An Update on Its Pathogenesis, Diagnosis, and Treatment. Nutr. Res. 2021, 89, 23–34. [Google Scholar] [CrossRef]
- Furnari, M.; Bonfanti, D.; Parodi, A.; Franzè, J.; Savarino, E.; Bruzzone, L.; Moscatelli, A.; Di Mario, F.; Dulbecco, P.; Savarino, V. A Comparison between Lactose Breath Test and Quick Test on Duodenal Biopsies for Diagnosing Lactase Deficiency in Patients with Self-Reported Lactose Intolerance. J. Clin. Gastroenterol. 2013, 47, 148–152. [Google Scholar] [CrossRef]
- Algera, J.; Colomier, E.; Simrén, M. The Dietary Management of Patients with Irritable Bowel Syndrome: A Narrative Review of the Existing and Emerging Evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef]
- Jansson-Knodell, C.L.; White, M.; Lockett, C.; Xu, H.; Shin, A. Associations of Food Intolerance with Irritable Bowel Syndrome, Psychological Symptoms, and Quality of Life. Clin. Gastroenterol. Hepatol. 2022, 20, 2121. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Personal View: Food for Thought--Western Lifestyle and Susceptibility to Crohn’s Disease. The FODMAP Hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Salem, A.; Nanavati, J.; Mullin, G.E. The Role of Diet in the Treatment of Irritable Bowel Syndrome: A Systematic Review. Gastroenterol. Clin. N. Am. 2018, 47, 107–137. [Google Scholar] [CrossRef] [PubMed]
- Sultan, N.; Varney, J.E.; Halmos, E.P.; Biesiekierski, J.R.; Yao, C.K.; Muir, J.G.; Gibson, P.R.; Tuck, C.J. How to Implement the 3-Phase FODMAP Diet Into Gastroenterological Practice. J. Neurogastroenterol. Motil. 2022, 28, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Serra, J.; Fernandez-Bañares, F.; Mearin, F. The Low-FODMAP Diet for Irritable Bowel Syndrome: Lights and Shadows. Gastroenterol. Hepatol. 2016, 39, 55–65. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Spiller, R. How Do FODMAPs Work? J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 36–39. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, A.; Chey, W.D. A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv. Nutr. 2017, 8, 587–596. [Google Scholar] [CrossRef]
- Aguilera-Lizarraga, J.; Florens, M.V.; Viola, M.F.; Jain, P.; Decraecker, L.; Appeltans, I.; Cuende-Estevez, M.; Fabre, N.; Van Beek, K.; Perna, E.; et al. Local Immune Response to Food Antigens Drives Meal-Induced Abdominal Pain. Nature 2021, 590, 151. [Google Scholar] [CrossRef]
- Bashashati, M.; Moossavi, S.; Cremon, C.; Barbaro, M.R.; Moraveji, S.; Talmon, G.; Rezaei, N.; Hughes, P.A.; Bian, Z.X.; Choi, C.H.; et al. Colonic Immune Cells in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Neurogastroenterol. Motil. 2018, 30, e13192. [Google Scholar] [CrossRef]
- Hussein, H.; Boeckxstaens, G.E. Immune-Mediated Food Reactions in Irritable Bowel Syndrome. Curr. Opin. Pharmacol. 2022, 66, 102285. [Google Scholar] [CrossRef]
- Fedewa, A.; Rao, S.S.C. Dietary Fructose Intolerance, Fructan Intolerance and FODMAPs. Curr. Gastroenterol. Rep. 2014, 16, 370. [Google Scholar] [CrossRef]
- Giussani, M.; Lieti, G.; Orlando, A.; Parati, G.; Genovesi, S. Fructose Intake, Hypertension and Cardiometabolic Risk Factors in Children and Adolescents: From Pathophysiology to Clinical Aspects. A Narrative Review. Front. Med. 2022, 9, 792949. [Google Scholar] [CrossRef]
- Herman, M.A.; Birnbaum, M.J. Molecular Aspects of Fructose Metabolism and Metabolic Disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef]
- Benardout, M.; Le Gresley, A.; Elshaer, A.; Wren, S.P. Fructose Malabsorption: Causes, Diagnosis and Treatment. Br. J. Nutr. 2022, 127, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Ikechi, R.; Fischer, B.D.; Desipio, J.; Phadtare, S. Irritable Bowel Syndrome: Clinical Manifestations, Dietary Influences, and Management. Healthcare 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Melchior, C.; Douard, V.; Coëffier, M.; Gourcerol, G. Fructose and Irritable Bowel Syndrome. Nutr. Res. Rev. 2020, 33, 235–243. [Google Scholar] [CrossRef]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef]
- Jung, K.W.; Seo, M.; Cho, Y.H.; Park, Y.O.; Yoon, S.Y.; Lee, J.; Yang, D.H.; Yoon, I.J.; Seo, S.Y.; Lee, H.J.; et al. Prevalence of Fructose Malabsorption in Patients With Irritable Bowel Syndrome after Excluding Small Intestinal Bacterial Overgrowth. J. Neurogastroenterol. Motil. 2018, 24, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966. [Google Scholar] [CrossRef]
- Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Figueroa-Salcido, O.G.; Ontiveros, N. Non-Celiac Gluten Sensitivity: An Update. Medicina 2021, 57, 526. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of Gluten Related Disorders: Celiac Disease, Wheat Allergy and Non-Celiac Gluten Sensitivity. World J. Gastroenterol. WJG 2015, 21, 7110. [Google Scholar] [CrossRef] [PubMed]
- Rej, A.; Aziz, I.; Sanders, D.S. Coeliac Disease and Noncoeliac Wheat or Gluten Sensitivity. J. Intern. Med. 2020, 288, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, U.N.; Pervaiz, A.; Khan, Z.B.; Sultana, T. Diagnostic Dilemma, Possible Non-Celiac Gluten Sensitivity: Consideration in Approach and Management. Cureus 2022, 14, 25302. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R.; Bagnato, C.; Belcari, C.; Bellantoni, A.; Caio, G.; Calella, F.; et al. An Italian Prospective Multicenter Survey on Patients Suspected of Having Non-Celiac Gluten Sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Rotondi Aufiero, V.; Fasano, A.; Mazzarella, G. Non-Celiac Gluten Sensitivity: How Its Gut Immune Activation and Potential Dietary Management Differ from Celiac Disease. Mol. Nutr. Food Res. 2018, 62, 1700854. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, P.; Seidita, A.; D’Alcamo, A.; Carroccio, A. Non-Celiac Gluten Sensitivity: Literature Review. J. Am. Coll Nutr. 2014, 33, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Zopf, Y. Gluten and FODMAPS-Sense of a Restriction/When Is Restriction Necessary? Nutrients 2019, 11, 1957. [Google Scholar] [CrossRef]
- Makharia, A.; Catassi, C.; Makharia, G.K. The Overlap between Irritable Bowel Syndrome and Non-Celiac Gluten Sensitivity: A Clinical Dilemma. Nutrients 2015, 7, 10417. [Google Scholar] [CrossRef]
- Ahmed, A.; Dixit, K.; Singh, A.; Agarwal, A.; Mehtab, W.; Prasad, S.; Rajput, M.S.; Chauhan, A.; Agarwal, A.; Mehta, S.; et al. Sieving out Non-Celiac Gluten Sensitivity amongst Patients with Irritable Bowel Syndrome. Dig. Liver Dis. 2023; in press. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten Causes Gastrointestinal Symptoms in Subjects without Celiac Disease: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Gastroenterol. 2011, 106, 508–514. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No Effects of Gluten in Patients with Self-Reported Non-Celiac Gluten Sensitivity after Dietary Reduction of Fermentable, Poorly Absorbed, Short-Chain Carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Durak-Dados, A.; Michalski, M.; Osek, J. Histamine and Other Biogenic Amines in Food. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Schnedl, W.J.; Enko, D. Histamine Intolerance Originates in the Gut. Nutrients 2021, 13, 1262. [Google Scholar] [CrossRef]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Soler-Singla, L.; Vidal-Carou, M.C. Diamine Oxidase (DAO) Supplement Reduces Headache in Episodic Migraine Patients with DAO Deficiency: A Randomized Double-Blind Trial. Clin. Nutr. 2019, 38, 152–158. [Google Scholar] [CrossRef]
- Smolinska, S.; Winiarska, E.; Globinska, A.; Jutel, M. Histamine: A Mediator of Intestinal Disorders—A Review. Metabolites 2022, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.O.; Nechaev, V.M.; Popova, I.R.; Deeva, T.A.; Kopylov, A.T.; Malsagova, K.A.; Kaysheva, A.L.; Ivashkin, V.T. Food Intolerance: The Role of Histamine. Nutrients 2021, 13, 3207. [Google Scholar] [CrossRef]
- Schink, M.; Konturek, P.C.; Tietz, E.; Dieterich, W.; Pinzer, T.C.; Wirtz, S.; Neurath, M.F.; Zopf, Y. Microbial Patterns in Patients with Histamine Intolerance. J. Physiol. Pharmacol. 2018, 69, 579–593. [Google Scholar] [CrossRef]
- Vanuytsel, T.; Bercik, P.; Boeckxstaens, G. Understanding Neuroimmune Interactions in Disorders of Gut–Brain Interaction: From Functional to Immune-Mediated Disorders. Gut 2023, 72, 787. [Google Scholar] [CrossRef]
- Partridge, D.; Lloyd, K.A.; Rhodes, J.M.; Walker, A.W.; Johnstone, A.M.; Campbell, B.J. Food Additives: Assessing the Impact of Exposure to Permitted Emulsifiers on Bowel and Metabolic Health—Introducing the FADiets Study. Nutr. Bull 2019, 44, 329. [Google Scholar] [CrossRef]
- Laudisi, F.; Stolfi, C.; Monteleone, G. Impact of Food Additives on Gut Homeostasis. Nutrients 2019, 11, 2334. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Gasbarrini, A.; Mele, M.C. Food Additives, Gut Microbiota, and Irritable Bowel Syndrome: A Hidden Track. Int. J. Environ. Res. Public Health 2020, 17, 8816. [Google Scholar] [CrossRef]
- Chi, L.; Bian, X.; Gao, B.; Tu, P.; Lai, Y.; Ru, H.; Lu, K. Effects of the Artificial Sweetener Neotame on the Gut Microbiome and Fecal Metabolites in Mice. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhan, S.; Tian, Z.; Li, N.; Li, T.; Wu, D.; Zeng, Z.; Zhuang, X. Food Additives Associated with Gut Microbiota Alterations in Inflammatory Bowel Disease: Friends or Enemies? Nutrients 2022, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct Impact of Commonly Used Dietary Emulsifiers on Human Gut Microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohns. Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Cozma-Petrut, A.; Loghin, F.; Miere, D.; Dumitrascu, D.L. Diet in Irritable Bowel Syndrome: What to Recommend, Not What to Forbid to Patients! World J. Gastroenterol. 2017, 23, 3771. [Google Scholar] [CrossRef]
- Capili, B.; Anastasi, J.K.; Chang, M. Addressing the Role of Food in Irritable Bowel Syndrome Symptom Management. J. Nurse Pract. 2016, 12, 324–329. [Google Scholar] [CrossRef]
- Nehlig, A. Effects of Coffee on the Gastro-Intestinal Tract: A Narrative Review and Literature Update. Nutrients 2022, 14, 399. [Google Scholar] [CrossRef]
- Iriondo-Dehond, A.; Uranga, J.A.; Del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis. Nutrients 2021, 13, 88. [Google Scholar] [CrossRef]
- Koochakpoor, G.; Salari-Moghaddam, A.; Keshteli, A.H.; Esmaillzadeh, A.; Adibi, P. Association of Coffee and Caffeine Intake With Irritable Bowel Syndrome in Adults. Front. Nutr. 2021, 8, 632469. [Google Scholar] [CrossRef] [PubMed]
- Gonlachanvit, S.; Mahayosnond, A.; Kullavanijaya, P. Effects of Chili on Postprandial Gastrointestinal Symptoms in Diarrhoea Predominant Irritable Bowel Syndrome: Evidence for Capsaicin-Sensitive Visceral Nociception Hypersensitivity. Neurogastroenterol. Motil. 2009, 21, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xu, X.; Zhang, T.; Wu, X.; Fan, D.; Hu, Y.; Ding, J.; Yang, X.; Lou, J.; Du, Q.; et al. Beneficial Effects of Dietary Capsaicin in Gastrointestinal Health and Disease. Exp. Cell Res. 2022, 417, 113227. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-Reported Food-Related Gastrointestinal Symptoms in IBS Are Common and Associated with More Severe Symptoms and Reduced Quality of Life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.; Salvioli, B.; Azpiroz, F.; Malagelada, J.R. Lipid-Induced Intestinal Gas Retention in Irritable Bowel Syndrome. Gastroenterology 2002, 123, 700–706. [Google Scholar] [CrossRef]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association Systematic Review and Evidence-Based Practice Guidelines for the Dietary Management of Irritable Bowel Syndrome in Adults (2016 Update). J. Hum. Nutr. Diet 2016, 29, 549–575. [Google Scholar] [CrossRef]
- Michalak, A.; Mosińska, P.; Fichna, J. Polyunsaturated Fatty Acids and Their Derivatives: Therapeutic Value for Inflammatory, Functional Gastrointestinal Disorders, and Colorectal Cancer. Front. Pharmacol. 2016, 7, 459. [Google Scholar] [CrossRef]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary Fiber in Irritable Bowel Syndrome (Review). Int. J. Mol. Med. 2017, 40, 607. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Bonetto, S.; Fagoonee, S.; Battaglia, E.; Grassini, M.; Saracco, G.M.; Pellicano, R. Recent Advances in the Treatment of Irritable Bowel Syndrome. Pol. Arch. Intern. Med. 2021, 131, 709–715. [Google Scholar] [CrossRef]
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol. Res. 2017, 38, 163. [Google Scholar]
- Qamar, N.; Castano, D.; Patt, C.; Chu, T.; Cottrell, J.; Chang, S.L. Meta-Analysis of Alcohol Induced Gut Dysbiosis and the Resulting Behavioral Impact. Behav. Brain Res. 2019, 376, 112196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Ge, C.; Feng, G.; Li, Y.; Luo, D.; Dong, J.; Li, H.; Wang, H.; Cui, M.; Fan, S. Gut Microbiota Modulates Alcohol Withdrawal-Induced Anxiety in Mice. Toxicol. Lett. 2018, 287, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Martinho-Grueber, M.; Kapoglou, I.; Bravo, F.; Sarraj, R.; Benz, E.; Restellini, S.; Biedermann, L.; Rogler, G.; Vavricka, S.R.; Schoepfer, A.; et al. Alcohol and Cannabis Consumption in Patients with Inflammatory Bowel Disease: Prevalence, Pattern of Consumption and Impact on the Disease. Eur. J. Gastroenterol. Hepatol. 2023, 35, 21–30. [Google Scholar] [CrossRef]
- Chen, G.; Shi, F.; Yin, W.; Guo, Y.; Liu, A.; Shuai, J.; Sun, J. Gut Microbiota Dysbiosis: The Potential Mechanisms by Which Alcohol Disrupts Gut and Brain Functions. Front. Microbiol. 2022, 13, 916765. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef]
- Rej, A.; Avery, A.; Aziz, I.; Black, C.J.; Bowyer, R.K.; Buckle, R.L.; Seamark, L.; Shaw, C.C.; Thompson, J.; Trott, N.; et al. Diet and Irritable Bowel Syndrome: An Update from a UK Consensus Meeting. BMC Med. 2022, 20, 287. [Google Scholar] [CrossRef]
- Paduano, D.; Cingolani, A.; Tanda, E.; Usai, P. Effect of Three Diets (Low-FODMAP, Gluten-Free and Balanced) on Irritable Bowel Syndrome Symptoms and Health-Related Quality of Life. Nutrients 2019, 11, 1566. [Google Scholar] [CrossRef]
- Domżał-Magrowska, D.; Kowalski, M.K.; Małecka-Wojciesko, E. The Incidence of Adult Type Hypolactasia in Patients with Irritable Bowel Syndrome. Prz. Gastroenterol. 2023, 18, 110. [Google Scholar] [CrossRef]
- Krieger-Grübel, C.; Hutter, S.; Hiestand, M.; Brenner, I.; Güsewell, S.; Borovicka, J. Treatment Efficacy of a Low FODMAP Diet Compared to a Low Lactose Diet in IBS Patients: A Randomized, Cross-over Designed Study. Clin. Nutr. ESPEN 2020, 40, 83–89. [Google Scholar] [CrossRef]
- Raoul, P.; Cintoni, M.; Palombaro, M.; Basso, L.; Rinninella, E.; Gasbarrini, A.; Mele, M.C. Food Additives, a Key Environmental Factor in the Development of IBD through Gut Dysbiosis. Microorganisms 2022, 10, 167. [Google Scholar] [CrossRef]
- Burtness, B.; Powell, M.; Catalano, P.; Berlin, J.; Liles, D.K.; Chapman, A.E.; Mitchell, E.; Benson, A.B. Randomized Phase II Trial of Irinotecan/Docetaxel or Irinotecan/Docetaxel Plus Cetuximab for Metastatic Pancreatic Cancer: An Eastern Cooperative Oncology Group Study. Am. J. Clin. Oncol. Cancer Clin. Trials 2016, 39, 340–345. [Google Scholar] [CrossRef]
- van Lanen, A.S.; de Bree, A.; Greyling, A. Efficacy of a Low-FODMAP Diet in Adult Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2021, 60, 3505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Camilleri, M.; Vanner, S.; Tuck, C. Review Article: Biological Mechanisms for Symptom Causation by Individual FODMAP Subgroups—The Case for a More Personalised Approach to Dietary Restriction. Aliment. Pharmacol. Ther. 2019, 50, 517–529. [Google Scholar] [CrossRef]
- Barrett, J.S.; Barrett, J. How to Institute the Low-FODMAP Diet. J. Gastroenterol. Hepatol. 2017, 32, 8–10. [Google Scholar] [CrossRef]
- Halmos, E.P.; Gibson, P.R. Controversies and Reality of the FODMAP Diet for Patients with Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2019, 34, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Tuck, C.; Gibson, P.R.; Chey, W.D. The Role of Food in the Treatment of Bowel Disorders: Focus on Irritable Bowel Syndrome and Functional Constipation. Am. J. Gastroenterol. 2022, 117, 947. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Choi, S.W. Dietary Modulation of Gut Microbiota for the Relief of Irritable Bowel Syndrome. Nutr. Res. Pract. 2021, 15, 411. [Google Scholar] [CrossRef]
- Lomer, M.C.E. The Low FODMAP Diet in Clinical Practice: Where Are We and What Are the Long-Term Considerations? Proc. Nutr. Soc. 2023, 7, 1–11. [Google Scholar] [CrossRef]
- Rej, A.; Sanders, D.S. Gluten-Free Diet and Its ‘Cousins’ in Irritable Bowel Syndrome. Nutrients 2018, 10, 1727. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, E.; Alijanzadeh, D.; Sadeghi, A.; Khoshdel, S.; Hekmatdoost, A.; Kord-Varkaneh, H.; Abdehagh, M. Gluten Restriction in Irritable Bowel Syndrome, Yes or No?: A GRADE-Assessed Systematic Review and Meta-Analysis. Front. Nutr. 2023, 10, 1273629. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Cremon, C.; Stanghellini, V.; Barbara, G. Recent Advances in Understanding Non-Celiac Gluten Sensitivity. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef]
- Nirmala Prasadi, V.P.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Fry, L.; Madden, A.M.; Fallaize, R. An Investigation into the Nutritional Composition and Cost of Gluten-Free versus Regular Food Products in the UK. J. Hum. Nutr. Diet. 2018, 31, 108–120. [Google Scholar] [CrossRef]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Casaburi, G.; Precone, V.; Pagliuca, C.; Colicchio, R.; Sarnataro, D.; Discepolo, V.; Kim, S.M.; Russo, I.; Del Vecchio Blanco, G.; et al. Metagenomics Reveals Dysbiosis and a Potentially Pathogenic N. Flavescens Strain in Duodenum of Adult Celiac Patients. Am. J. Gastroenterol. 2016, 111, 879. [Google Scholar] [CrossRef]
- Hansen, L.B.S.; Roager, H.M.; Søndertoft, N.B.; Gøbel, R.J.; Kristensen, M.; Vallès-Colomer, M.; Vieira-Silva, S.; Ibrügger, S.; Lind, M.V.; Mærkedahl, R.B.; et al. A Low-Gluten Diet Induces Changes in the Intestinal Microbiome of Healthy Danish Adults. Nat. Commun. 2018, 9, 4630. [Google Scholar] [CrossRef]
- Parker, T.J.; Woolner, J.T.; Prevost, A.T.; Tuffnell, Q.; Shorthouse, M.; Hunter, J.O. Irritable Bowel Syndrome: Is the Search for Lactose Intolerance Justified? Eur. J. Gastroenterol. Hepatol. 2001, 13, 219–225. [Google Scholar] [CrossRef]
- Marabotto, E.; Ferone, D.; Sheijani, A.D.; Vera, L.; Ziola, S.; Savarino, E.; Bodini, G.; Furnari, M.; Zentilin, P.; Savarino, V.; et al. Prevalence of Lactose Intolerance in Patients with Hashimoto Thyroiditis and Impact on LT4 Replacement Dose. Nutrients 2022, 14, 17. [Google Scholar] [CrossRef]
- Facioni, M.S.; Raspini, B.; Pivari, F.; Dogliotti, E.; Cena, H. Nutritional Management of Lactose Intolerance: The Importance of Diet and Food Labelling. J. Transl. Med. 2020, 18, 260. [Google Scholar] [CrossRef] [PubMed]
- Firrman, J.; Liu, L.S.; Mahalak, K.; Hu, W.; Bittinger, K.; Moustafa, A.; Jones, S.M.; Narrowe, A.; Tomasula, P. An in Vitro Analysis of How Lactose Modifies the Gut Microbiota Structure and Function of Adults in a Donor-Independent Manner. Front. Nutr. 2023, 9, 1040744. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and Potential Health Benefits of the Mediterranean Diet: Views from Experts around the World. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef]
- Chen, E.Y.; Mahurkar-Joshi, S.; Liu, C.; Jaffe, N.; Labus, J.S.; Dong, T.S.; Gupta, A.; Patel, S.; Mayer, E.A.; Chang, L. The Association Between a Mediterranean Diet and Symptoms of Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2023, 22, 164–172.e6. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Mahoney, S.; Canale, K.; Opie, R.S.; Loughman, A.; So, D.; Beswick, L.; Hair, C.; Jacka, F.N. Clinical Trial: A Mediterranean Diet Is Feasible and Improves Gastrointestinal and Psychological Symptoms in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2023, 1–12. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. “The Mediterranean Diet, Its Components, and Cardiovascular Disease”. Am. J. Med. 2015, 128, 229. [Google Scholar] [CrossRef] [PubMed]
- Werlang, M.E.; Palmer, W.C.; Lacy, B.E. Irritable Bowel Syndrome and Dietary Interventions. Gastroenterol.Hepatol. 2019, 15, 16. [Google Scholar]
- Hsieh, M.S.; Hsu, W.H.; Wang, J.W.; Wang, Y.K.; Hu, H.M.; Chang, W.K.; Chen, C.Y.; Wu, D.C.; Kuo, F.C.; Su, W.W. Nutritional and Dietary Strategy in the Clinical Care of Inflammatory Bowel Disease. J. Formos. Med. Assoc. 2020, 119, 1742–1749. [Google Scholar] [CrossRef]
- Singh, P.; Lembo, A. Emerging Role of the Gut Microbiome in Irritable Bowel Syndrome. Gastroenterol. Clin. N. Am. 2021, 50, 523–545. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, Q.; Song, L.; Duan, L. Alterations in Fecal Short-Chain Fatty Acids in Patients with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e14513. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in Silico Analysis. Front. Neurosci. 2019, 13, 493713. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Singh, R.; Ghoshal, U.C. Enterochromaffin Cells–Gut Microbiota Crosstalk: Underpinning the Symptoms, Pathogenesis, and Pharmacotherapy in Disorders of Gut-Brain Interaction. J. Neurogastroenterol. Motil. 2022, 28, 357. [Google Scholar] [CrossRef]
- Singh, P.; Grabauskas, G.; Zhou, S.Y.; Gao, J.; Zhang, Y.; Owyang, C. High FODMAP Diet Causes Barrier Loss via Lipopolysaccharide-Mediated Mast Cell Activation. JCI Insight 2021, 6, e146529. [Google Scholar] [CrossRef] [PubMed]
- Hillestad, E.M.R.; van der Meeren, A.; Nagaraja, B.H.; Bjørsvik, B.R.; Haleem, N.; Benitez-Paez, A.; Sanz, Y.; Hausken, T.; Lied, G.A.; Lundervold, A.; et al. Gut Bless You: The Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. World J. Gastroenterol. 2022, 28, 412. [Google Scholar] [CrossRef] [PubMed]
- Mahurkar-Joshi, S.; Chang, L. Epigenetic Mechanisms in Irritable Bowel Syndrome. Front. Psychiatry 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Henström, M.; D’Amato, M. Genetics of Irritable Bowel Syndrome. Mol. Cell Pediatr. 2016, 3, 7. [Google Scholar] [CrossRef]
- Atkinson, W.; Sheldon, T.A.; Shaath, N.; Whorwell, P.J. Food Elimination Based on IgG Antibodies in Irritable Bowel Syndrome: A Randomised Controlled Trial. Gut 2004, 53, 1459. [Google Scholar] [CrossRef]
- Vita, A.A.; Zwickey, H.; Bradley, R. Associations between Food-Specific IgG Antibodies and Intestinal Permeability Biomarkers. Front. Nutr. 2022, 9, 962093. [Google Scholar] [CrossRef]
- Stapel, S.O.; Asero, R.; Ballmer-Weber, B.K.; Knol, E.F.; Strobel, S.; Vieths, S.; Kleine-Tebbe, J. Testing for IgG4 against Foods Is Not Recommended as a Diagnostic Tool: EAACI Task Force Report. Allergy 2008, 63, 793–796. [Google Scholar] [CrossRef]
- Bock, S.A. AAAAI Support of the EAACI Position Paper on IgG4. J. Allergy Clin. Immunol. 2010, 125, 1410. [Google Scholar] [CrossRef]
- Kasoju, N.; Remya, N.S.; Sasi, R.; Sujesh, S.; Soman, B.; Kesavadas, C.; Muraleedharan, C.V.; Harikrishna Varma, P.R.; Behari, S. Digital Health: Trends, Opportunities and Challenges in Medical Devices, Pharma and Bio-Technology. CSI Trans. ICT 2023, 11, 11–30. [Google Scholar] [CrossRef]
- Kelly, J.T.; Reidlinger, D.P.; Hoffmann, T.C.; Campbell, K.L. Telehealth Methods to Deliver Dietary Interventions in Adults with Chronic Disease: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2016, 104, 1693–1702. [Google Scholar] [CrossRef]
| FOODS | MECHANISM OF ACTION | ASSOCIATION WITH IBS | SPECIFIC DIETARY APPROACH |
|---|---|---|---|
| FODMAPs [126] |
| High prevalence in IBS → 33% of patients with functional gastrointestinal disorders, including IBS Symptoms associated: Triggers bloating, abdominal discomfort, and altered bowel habits | Low-FODMAP diet to identify triggers:
|
| Fructose [77] |
| Common in IBS patients → The prevalence of fructose intolerance in patients with IBS is about 22% Symptoms associated: Causes bloating, abdominal pain, flatulence, and diarrhea | Fructose-restricted diet (FRD):
|
| Non-Celiac Gluten Sensitivity [127,128] |
| Prevalence of NCGS in IBS → between 23 and 49% Symptoms associated: Intestinal and extra-intestinal symptoms (i.e., altered bowel habit, abdominal pain, bloating, headache, fatigue, and joint pain) | Gluten-free diet:
|
| Lactose [129,130] |
| Misdiagnosed as IBS → 60.7% of the patients with IBS and 43.5% in the control group Symptoms associated: Triggers bloating and diarrhea after ingestion | Lactose-free diet:
|
| Food Additives [131] |
| Potential trigger of IBS onset → Between 1 and 2% of general population, with no direct data on IBS Symptoms associated: Effects on gut health are ongoing | Avoidance of artificial sweeteners, emulsifiers, and colorants:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasta, A.; Formisano, E.; Calabrese, F.; Plaz Torres, M.C.; Bodini, G.; Marabotto, E.; Pisciotta, L.; Giannini, E.G.; Furnari, M. Food Intolerances, Food Allergies and IBS: Lights and Shadows. Nutrients 2024, 16, 265. https://doi.org/10.3390/nu16020265
Pasta A, Formisano E, Calabrese F, Plaz Torres MC, Bodini G, Marabotto E, Pisciotta L, Giannini EG, Furnari M. Food Intolerances, Food Allergies and IBS: Lights and Shadows. Nutrients. 2024; 16(2):265. https://doi.org/10.3390/nu16020265
Chicago/Turabian StylePasta, Andrea, Elena Formisano, Francesco Calabrese, Maria Corina Plaz Torres, Giorgia Bodini, Elisa Marabotto, Livia Pisciotta, Edoardo Giovanni Giannini, and Manuele Furnari. 2024. "Food Intolerances, Food Allergies and IBS: Lights and Shadows" Nutrients 16, no. 2: 265. https://doi.org/10.3390/nu16020265
APA StylePasta, A., Formisano, E., Calabrese, F., Plaz Torres, M. C., Bodini, G., Marabotto, E., Pisciotta, L., Giannini, E. G., & Furnari, M. (2024). Food Intolerances, Food Allergies and IBS: Lights and Shadows. Nutrients, 16(2), 265. https://doi.org/10.3390/nu16020265








