Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials
Abstract
1. Introduction
2. Methodology
3. Results
3.1. Flavonoids
3.1.1. Flavonoids and Breast Cancer
3.1.2. Flavonoids and Prostate Cancer
3.1.3. Flavonoids and Gastrointestinal Cancer
3.1.4. Flavonoids and Lung Cancer
3.2. Phytosterols
3.2.1. Phytosterols in Breast and Gynecological Cancers
3.2.2. Phytosterols in Prostate Neoplasms
3.2.3. Phytosterols in Colorectal Cancer
3.2.4. Phytosterols in Lung Cancer
3.2.5. Phytosterols in Stomach Cancer
3.2.6. Phytosterols in Pancreatic Cancer
3.3. Phenolic Acid
3.3.1. Phenolic Acid in Prostate Cancer
3.3.2. Phenolic Acid in Breast Cancer
3.3.3. Phenolic Acid in Lung Cancer
3.3.4. Phenolic Acid in Skin Cancer
3.3.5. Phenolic Acid in Esophageal Cancer
3.4. Stilbenes
3.4.1. Stilbenes and Colorectal Cancer
3.4.2. Stilbenes and Neuroendocrine Cancer
3.4.3. Stilbenes and Breast Cancer
3.4.4. Stilbenes and Prostate Cancer
3.4.5. Stilbenes and Multiple Myeloma
3.5. Carotenoids
3.5.1. Carotenoids and Prostate Cancer
3.5.2. Carotenoids and Breast Cancer
3.5.3. Carotenoids and Lung Cancer
3.5.4. Carotenoids and Gastrointestinal Cancers
3.5.5. Carotenoids and Colorectal Cancer
3.5.6. Carotenoids and Head and Neck Cancer
3.5.7. Carotenoids and Skin Cancer
3.5.8. Carotenoids and Gynecological Cancers
3.5.9. Carotenoids and Other Types of Cancers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, I.T. Phytochemicals and Cancer. Proc. Nutr. Soc. 2007, 66, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.B.L. Phytochemicals: Health Protective Effects. Can. J. Diet. Pract. Res. 1999, 60, 78. [Google Scholar]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and Its Phytochemicals: Hidden Health Benefits & Modulation of Signaling Cascade by Phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and Human Health. J. Sci. Food. Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of Action of Flavonoids as Anti-Inflammatory Agents: A Review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic Potential of Flavonoids and Their Mechanism of Action against Microbial and Viral Infections—A Review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the Gastrointestinal Tract: Local and Systemic Effects. Mol. Aspects Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Leitzmann, C. Characteristics and Health Benefits of Phytochemicals. Complement. Med. Res. 2016, 23, 69–74. [Google Scholar] [CrossRef]
- Liu, R.H. Health Benefits of Fruit and Vegetables Are from Additive and Synergistic Combinations of Phytochemicals. Am. J. Clin. Nutr. 2003, 78, S517–S520. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B. Health Benefits of Phytochemicals from Selected Canadian Crops. Trends Food Sci. Technol. 1999, 10, 193–198. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Sporn, M.B.; Suh, N. Chemoprevention of Cancer. Carcinogenesis 2000, 21, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.S.; Kim, E.S.; Hong, W.K. Chemoprevention of Cancer. CA Cancer J. Clin. 2004, 54, 150–180. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J. Cancer Chemoprevention with Dietary Phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Hadjzadeh, M.A.R.; Afshari, J.T.; Ghorbani, A.P. 144 The Effects of Aqueous Extract of Garlic (Allium sativum I.) On Laryngeal Cancer Cells (Hep-2) and 1929 Cells in Vitro. Oral Oncol. Suppl. 2005, 1, 190. [Google Scholar] [CrossRef]
- Chiu, C.-T.; Hsuan, S.-W.; Lin, H.-H.; Hsu, C.-C.; Chou, F.-P.; Chen, J.-H. Hibiscus sabdariffa Leaf Polyphenolic Extract Induces Human Melanoma Cell Death, Apoptosis, and Autophagy. J. Food Sci. 2015, 80, H649–H658. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chan, K.-C.; Sheu, J.-Y.; Hsuan, S.-W.; Wang, C.-J.; Chen, J.-H. Hibiscus Sabdariffa Leaf Induces Apoptosis of Human Prostate Cancer Cells in Vitro and in Vivo. Food Chem. 2012, 132, 880–891. [Google Scholar] [CrossRef]
- Tseng, T.-H.; Kao, T.-W.; Chu, C.-Y.; Chou, F.-P.; Lin, W.-L.; Wang, C.-J. Induction of Apoptosis by Hibiscus Protocatechuic Acid in Human Leukemia Cells via Reduction of Retinoblastoma (RB) Phosphorylation and Bcl-2 Expression. Biochem. Pharmacol. 2000, 60, 307–315. [Google Scholar] [CrossRef]
- Lin, H.-H.; Huang, H.-P.; Huang, C.-C.; Chen, J.-H.; Wang, C.-J. Hibiscus Polyphenol-Rich Extract Induces Apoptosis in Human Gastric Carcinoma Cells via P53 Phosphorylation and P38 MAPK/FasL Cascade Pathway. Mol. Carcinog. 2005, 43, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Nandhakumar, E.; Sachdanandam, P. Phytochemical Analysis and Anticancer Capacity of Shemamruthaa, a Herbal Formulation against DMBA- Induced Mammary Carcinoma in Rats. Asian Pac. J. Trop. Med. 2013, 6, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Mortazavian, S.M.; Ghorbani, A. Antiproliferative Effect of Viola Tricolor on Neuroblastoma Cells in Vitro. Aust. J. Herbalmed. 2012, 24, 93–96. [Google Scholar]
- Sadeghnia, H.R.; Ghorbani Hesari, T.; Mortazavian, S.M.; Mousavi, S.H.; Tayarani-Najaran, Z.; Ghorbani, A. Viola tricolor Induces Apoptosis in Cancer Cells and Exhibits Antiangiogenic Activity on Chicken Chorioallantoic Membrane. Biomed. Res. Int. 2014, 2014, 625792. [Google Scholar] [CrossRef] [PubMed]
- Berrington, D.; Lall, N. Anticancer Activity of Certain Herbs and Spices on the Cervical Epithelial Carcinoma (HeLa) Cell Line. Evid.-Based Complement. Altern. Med. 2012, 2012, 564927. [Google Scholar] [CrossRef]
- Chaudhry, G.-S.; Jan, R.; Mohamad, H.; Tengku Muhammad, T. Vitex Rotundifolia Fractions Induce Apoptosis in Human Breast Cancer Cell Line, MCF-7, via Extrinsic and Intrinsic Pathways. Res. Pharm. Sci. 2019, 14, 273. [Google Scholar] [CrossRef]
- Kim, H.; Yi, J.-M.; Kim, N.S.; Lee, Y.J.; Kim, J.; Oh, D.-S.; Oh, S.-M.; Bang, O.-S.; Lee, J. Cytotoxic Compounds from the Fruits of Vitex Rotundifolia against Human Cancer Cell Lines. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 433–437. [Google Scholar] [CrossRef]
- Tuorkey, M.J. Cancer Therapy with Phytochemicals: Present and Future Perspectives. Biomed. Environ. Sci. 2015, 28, 808–819. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 175. [Google Scholar] [CrossRef]
- Shu, L.; Cheung, K.-L.; Khor, T.O.; Chen, C.; Kong, A.-N. Phytochemicals: Cancer Chemoprevention and Suppression of Tumor Onset and Metastasis. Cancer Metastasis Rev. 2010, 29, 483–502. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed]
- Mollakhalili Meybodi, N.; Mortazavian, A.M.; Bahadori Monfared, A.; Sohrabvandi, S.; Aghaei Meybodi, F. Phytochemicals in Cancer Prevention: A Review of the Evidence. Iran. J. Cancer Prev. 2017; in press. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-Cancer Potential of Flavonoids: Recent Trends and Future Perspectives. Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Duckstein, N.; Rimbach, G. A Literature Review of Flavonoids and Lifespan in Model Organisms. Proc. Nutr. Soc. 2017, 76, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, M.P.; McKay, D.L.; Blumberg, J.B. Flavonoid Basics: Chemistry, Sources, Mechanisms of Action, and Safety. J. Nutr. Gerontol. Geriatr. 2012, 31, 176–189. [Google Scholar] [CrossRef]
- Le Marchand, L. Cancer Preventive Effects of Flavonoids—A Review. Biomed. Pharmacother. 2002, 56, 296–301. [Google Scholar] [CrossRef]
- Eid, O.; Elkady, W.M.; Ezzat, S.; El Sayed, A.; Abd Elsattar, E. Comprehensive Overview: The Effect of Using Different Solvents for Barley Extraction with Its Anti-Inflammatory and Antioxidant Activity. Chem. Biodivers. 2023, 20, e202200935. [Google Scholar] [CrossRef]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef]
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids Regulate Inflammation and Oxidative Stress in Cancer. Molecules 2020, 25, 5628. [Google Scholar] [CrossRef]
- Martinez-Perez, C.; Ward, C.; Cook, G.; Mullen, P.; McPhail, D.; Harrison, D.J.; Langdon, S.P. Novel Flavonoids as Anti-Cancer Agents: Mechanisms of Action and Promise for Their Potential Application in Breast Cancer. Biochem. Soc. Trans. 2014, 42, 1017–1023. [Google Scholar] [CrossRef]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- Cayetano-Salazar, L.; Nava-Tapia, D.A.; Astudillo-Justo, K.D.; Arizmendi-Izazaga, A.; Sotelo-Leyva, C.; Herrera-Martinez, M.; Villegas-Comonfort, S.; Navarro-Tito, N. Flavonoids as Regulators of TIMPs Expression in Cancer: Consequences, Opportunities, and Challenges. Life Sci. 2022, 308, 120932. [Google Scholar] [CrossRef]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of Cancer Cell Apoptosis by Flavonoids Is Associated with Their Ability to Inhibit Fatty Acid Synthase Activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef]
- Hsieh, T.-C.; Lin, C.-Y.; Lin, H.-Y.; Wu, J.M. AKT/MTOR as Novel Targets of Polyphenol Piceatannol Possibly Contributing to Inhibition of Proliferation of Cultured Prostate Cancer Cells. ISRN Urol. 2012, 2012, 272697. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Gawande, S.; Kotwal, S. Cancer Phytotherapeutics: Role for Flavonoids at the Cellular Level. Phytother. Res. 2008, 22, 567–577. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, 4005008. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as Prospective Compounds for Anti-Cancer Therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, X.; Jiang, Y.; Su, Q.; Li, Q.; Li, Z. Autophagy: Mechanisms and Therapeutic Potential of Flavonoids in Cancer. Biomolecules 2021, 11, 135. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Cutler, G.J.; Nettleton, J.A.; Ross, J.A.; Harnack, L.J.; Jacobs, D.R.; Scrafford, C.G.; Barraj, L.M.; Mink, P.J.; Robien, K. Dietary Flavonoid Intake and Risk of Cancer in Postmenopausal Women: The Iowa Women’s Health Study. Int. J. Cancer 2008, 123, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.; Sanders, K.; Kolybaba, M.; Lopez, D. Case-Control Study of Phyto-Oestrogens and Breast Cancer. Lancet 1997, 350, 990–994. [Google Scholar] [CrossRef]
- Dai, Q.; Franke, A.A.; Jin, F.; Shu, X.O.; Zheng, W. Urinary Excretion of Phytoestrogens and Risk of Breast Cancer among Chinese Women in Shanghai. Cancer Epidemiol. Biomark. Prev. 2002, 11, 815–821. [Google Scholar]
- Fink, B.N.; Steck, S.E.; Wolff, M.S.; Britton, J.A.; Kabat, G.C.; Schroeder, J.C.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Dietary Flavonoid Intake and Breast Cancer Risk among Women on Long Island. Am. J. Epidemiol. 2006, 165, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Lagiou, P.; Samoli, E.; Lagiou, A.; Katsouyanni, K.; La Vecchia, C.; Dwyer, J.; Trichopoulos, D. Flavonoid Intake and Breast Cancer Risk: A Case–Control Study in Greece. Br. J. Cancer 2003, 89, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gapstur, S.M.; Gaudet, M.M.; Peterson, J.J.; Dwyer, J.T.; McCullough, M.L. Evidence for an Association of Dietary Flavonoid Intake with Breast Cancer Risk by Estrogen Receptor Status Is Limited. J. Nutr. 2014, 144, 1603–1611. [Google Scholar] [CrossRef]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between Soy Isoflavone Intake and Breast Cancer Risk for Pre- and Post-Menopausal Women: A Meta-Analysis of Epidemiological Studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Qin, L.-Q. Soy Isoflavones Consumption and Risk of Breast Cancer Incidence or Recurrence: A Meta-Analysis of Prospective Studies. Breast Cancer Res. Treat. 2011, 125, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z. Soy Food Intake and Breast Cancer Risk: A Meta-Analysis. Wei Sheng Yan Jiu 2012, 41, 670–676. [Google Scholar]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, Flavonoid Subclasses and Breast Cancer Risk: A Meta-Analysis of Epidemiologic Studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef]
- Chi, F.; Wu, R.; Zeng, Y.-C.; Xing, R.; Liu, Y.; Xu, Z.-G. Post-Diagnosis Soy Food Intake and Breast Cancer Survival: A Meta-Analysis of Cohort Studies. Asian Pac. J. Cancer Prev. 2013, 14, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Gomez, S.L.; Chang, J.S.; Wey, M.; Wang, R.T.; Hsing, A.W. Soy and Isoflavone Consumption in Relation to Prostate Cancer Risk in China. Cancer Epidemiol. Biomark. Prev. 2003, 12, 665–668. [Google Scholar]
- Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Otani, T.; Inoue, M.; Tsugane, S. Soy Product and Isoflavone Consumption in Relation to Prostate Cancer in Japanese Men. Cancer Epidemiol. Biomark. Prev. 2007, 16, 538–545. [Google Scholar] [CrossRef]
- Geybels, M.S.; Verhage, B.A.J.; Arts, I.C.W.; van Schooten, F.J.; Goldbohm, R.A.; van den Brandt, P.A. Dietary Flavonoid Intake, Black Tea Consumption, and Risk of Overall and Advanced Stage Prostate Cancer. Am. J. Epidemiol. 2013, 177, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Travis, R.C.; Spencer, E.A.; Allen, N.E.; Appleby, P.N.; Roddam, A.W.; Overvad, K.; Johnsen, N.F.; Olsen, A.; Kaaks, R.; Linseisen, J.; et al. Plasma Phyto-Oestrogens and Prostate Cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2009, 100, 1817–1823. [Google Scholar] [CrossRef]
- van Die, M.D.; Bone, K.M.; Williams, S.G.; Pirotta, M.V. Soy and Soy Isoflavones in Prostate Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BJU Int. 2014, 113, E119–E130. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Agudo, A.; Luján-Barroso, L.; Romieu, I.; Ferrari, P.; Knaze, V.; Bueno-de-Mesquita, H.B.; Leenders, M.; Travis, R.C.; Navarro, C.; et al. Dietary Flavonoid and Lignan Intake and Gastric Adenocarcinoma Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Am. J. Clin. Nutr. 2012, 96, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Lee, J.; Choi, I.; Kim, C.; Lee, J.; Kwon, O.; Kim, J. Dietary Flavonoids and Gastric Cancer Risk in a Korean Population. Nutrients 2014, 6, 4961–4973. [Google Scholar] [CrossRef]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Trivers, K.F.; Abrahamson, P.E.; Engel, L.S.; He, K.; Chow, W.-H.; Mayne, S.T.; Risch, H.A.; et al. Dietary Intake of Flavonoids and Oesophageal and Gastric Cancer: Incidence and Survival in the United States of America (USA). Br. J. Cancer 2015, 112, 1291–1300. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Not, C.; Guinó, E.; Luján-Barroso, L.; García, R.M.; Biondo, S.; Salazar, R.; Moreno, V. Association between Habitual Dietary Flavonoid and Lignan Intake and Colorectal Cancer in a Spanish Case–Control Study (the Bellvitge Colorectal Cancer Study). Cancer Causes Control 2013, 24, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Le Marchand, L.; Murphy, S.P.; Hankin, J.H.; Wilkens, L.R.; Kolonel, L.N. Intake of Flavonoids and Lung Cancer. JNCI J. Natl. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Morgenstern, H.; Greenland, S.; Tashkin, D.P.; Mao, J.T.; Cai, L.; Cozen, W.; Mack, T.M.; Lu, Q.-Y.; Zhang, Z.-F. Dietary Flavonoid Intake and Lung Cancer-A Population-Based Case-Control Study. Cancer 2008, 112, 2241–2248. [Google Scholar] [CrossRef]
- Garcia-Closas, R.; Agudo, A.; Gonzalez, C.A.; Riboli, E. Intake of Specific Carotenoids and Flavonoids and the Risk of Lung Cancer in Women in Barcelona, Spain. Nutr. Cancer 1998, 32, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Nurmi, T.; Tuomainen, T.-P.; Salonen, J.T.; Pukkala, E.; Voutilainen, S. Intake of Flavonoids and Risk of Cancer in Finnish Men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Int. J. Cancer 2008, 123, 660–663. [Google Scholar] [CrossRef]
- De Stefani, E.; Boffetta, P.; Deneo-Pellegrini, H.; Mendilaharsu, M.; Carzoglio, J.C.; Ronco, A.; Olivera, L. Dietary Antioxidants and Lung Cancer Risk: A Case-Control Study in Uruguay. Nutr. Cancer 1999, 34, 100–110. [Google Scholar] [CrossRef]
- Tang, N.-P.; Zhou, B.; Wang, B.; Yu, R.-B.; Ma, J. Flavonoids Intake and Risk of Lung Cancer: A Meta-Analysis. Jpn. J. Clin. Oncol. 2009, 39, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.G.; Awad, A.B. Phytosterols as Anticancer Compounds. Mol. Nutr. Food Res. 2007, 51, 161–170. [Google Scholar] [CrossRef]
- Shahzad, N.; Khan, W.; MD, S.; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.A.; Abdel-Wahab, A.F.; et al. Phytosterols as a Natural Anticancer Agent: Current Status and Future Perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef]
- Bradford, P.G.; Awad, A.B. Modulation of Signal Transduction in Cancer Cells by Phytosterols. BioFactors 2010, 36, 241–247. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Awad, A.B. Role of Phytosterols in Cancer Prevention and Treatment. J. AOAC Int. 2015, 98, 735–738. [Google Scholar] [CrossRef]
- Cantrill, R. Phytosterols, Phytostanols and Their Esters Chemical and Technical Assessment; ScienceOpen, Inc.: Burlington, MA, USA, 2008. [Google Scholar]
- Craig, W.J.; Mangels, A.R. Position of the American Dietetic Association: Vegetarian Diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef]
- Fraser, G.E. Associations between Diet and Cancer, Ischemic Heart Disease, and All-Cause Mortality in Non-Hispanic White California Seventh-Day Adventists. Am. J. Clin. Nutr. 1999, 70, S532–S538. [Google Scholar] [CrossRef]
- Ho, X.L.; Liu, J.J.H.; Loke, W.M. Plant Sterol-Enriched Soy Milk Consumption Modulates 5-Lipoxygenase, 12-Lipoxygenase, and Myeloperoxidase Activities in Healthy Adults—A Randomized-Controlled Trial. Free Radic. Res. 2016, 50, 1396–1407. [Google Scholar] [CrossRef]
- Guo, L.; Liu, S.; Zhang, S.; Chen, Q.; Zhang, M.; Quan, P.; Lu, J.; Sun, X. C-Reactive Protein and Risk of Breast Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, 10508. [Google Scholar] [CrossRef]
- Fakih, O.; Sanver, D.; Kane, D.; Thorne, J.L. Exploring the Biophysical Properties of Phytosterols in the Plasma Membrane for Novel Cancer Prevention Strategies. Biochimie 2018, 153, 150–161. [Google Scholar] [CrossRef]
- Awad, A.B.; Tagle Hernandez, A.Y.; Fink, C.S.; Mendel, S.L. Effect of Dietary Phytosterols on Cell Proliferation and Protein Kinase C Activity in Rat Colonic Mucosa. Nutr. Cancer 1997, 27, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Janezic, S.A.; Rao, A.V. Dose-Dependent Effects of Dietary Phytosterol on Epithelial Cell Proliferation of the Murine Colon. Food Chem. Toxicol. 1992, 30, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Deschner, E.E.; Cohen, B.I.; Raicht, R.F. The Kinetics of the Protective Effect of β-Sitosterol against MNU-Induced Colonic Neoplasia. J. Cancer Res. Clin. Oncol. 1982, 103, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Downie, A.C.; Fink, C.S.; Kim, U. Dietary Phytosterol Inhibits the Growth and Metastasis of MDA-MB-231 Human Breast Cancer Cells Grown in SCID Mice. Anticancer. Res. 2000, 20, 821–824. [Google Scholar]
- Ju, Y.H.; Helferich, W.G.; Clausen, L.M.; Allred, K.F.; Almada, A.L. β-Sitosterol, β-Sitosterol Glucoside, and a Mixture of β-Sitosterol and β-Sitosterol Glucoside Modulate the Growth of Estrogen-Responsive Breast Cancer Cells In Vitro and in Ovariectomized Athymic Mice. J. Nutr. 2004, 134, 1145–1151. [Google Scholar] [CrossRef]
- Ronco, A.; De Stefani, E.; Boffetta, P.; Deneo-Pellegrini, H.; Mendilaharsu, M.; Leborgne, F. Vegetables, Fruits, and Related Nutrients and Risk of Breast Cancer: A Case-Control Study in Uruguay. Nutr. Cancer 1999, 35, 111–119. [Google Scholar] [CrossRef]
- McCann, S.E. Diet in the Epidemiology of Endometrial Cancer in Western New York (United States). Cancer Causes Control 2000, 11, 965–974. [Google Scholar] [CrossRef]
- McCann, S.E.; Freudenheim, J.L.; Graham, S.; Marshall, J.R. Risk of Human Ovarian Cancer Is Related to Dietary Intake of Selected Nutrients, Phytochemicals and Food Groups. J. Nutr. 2003, 133, 1937–1942. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial. JAMA Intern. Med. 2015, 175, 1752. [Google Scholar] [CrossRef]
- Torres-Sanchez, L.; Galvan-Portillo, M.; Wolff, M.S.; Lopez-Carrillo, L. Dietary Consumption of Phytochemicals and Breast Cancer Risk in Mexican Women. Public. Health Nutr. 2009, 12, 825–831. [Google Scholar] [CrossRef]
- Plant Sterol Intervention for Cancer Prevention (PINC). Available online: https://clinicaltrials.gov/ct2/show/NCT04147767 (accessed on 1 April 2023).
- Normén, A.L.; Brants, H.A.; Voorrips, L.E.; Andersson, H.A.; van den Brandt, P.A.; Goldbohm, R.A. Plant Sterol Intakes and Colorectal Cancer Risk in the Netherlands Cohort Study on Diet and Cancer. Am. J. Clin. Nutr. 2001, 74, 141–148. [Google Scholar] [CrossRef]
- Huang, J.; Xu, M.; Fang, Y.-J.; Lu, M.-S.; Pan, Z.-Z.; Huang, W.-Q.; Chen, Y.-M.; Zhang, C.-X. Association between Phytosterol Intake and Colorectal Cancer Risk: A Case–Control Study. Br. J. Nutr. 2017, 117, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Mendilaharsu, M.; De Stefani, E.; Deneo-Pellegrini, H.; Carzoglio, J.; Ronco, A. Phytosterols and Risk of Lung Cancer: A Case-Control Study in Uruguay. Lung Cancer 1998, 21, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E.; Beeson, W.L.; Phillips, R.L. Diet and Lung Cancer in California Seventh-Day Adventists. Am. J. Epidemiol. 1991, 133, 683–693. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Boffetta, P.; Ronco, A.L.; Brennan, P.; Deneo-Pellegrini, H.; Carzoglio, J.C.; Mendilaharsu, M. Plant Sterols and Risk of Stomach Cancer: A Case-Control Study in Uruguay. Nutr. Cancer 2000, 37, 140–144. [Google Scholar] [CrossRef]
- Mills, P.K.; Beeson, W.L.; Abbey, D.E.; Fraser, G.E.; Phillips, R.L. Dietary Habits and Past Medical History as Related to Fatal Pancreas Cancer Risk among Adventists. Cancer 1988, 61, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic Analysis of the Content of 502 Polyphenols in 452 Foods and Beverages: An Application of the Phenol-Explorer Database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Tseng, T.-H.; Lee, Y.-J. Evaluation of Natural and Synthetic Compounds from East Asiatic Folk Medicinal Plants on the Mediation of Cancer. Anticancer Agents Med. Chem. 2006, 6, 347–365. [Google Scholar] [CrossRef]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Antônio, M.; Araújo, D.; Galberto, M.; da Costa, J. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant Activity and Mechanism of Rhizoma Cimicifugae. Chem. Cent J. 2012, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Doble, M. Combination of Phenylpropanoids with 5-Fluorouracil as Anti-Cancer Agents against Human Cervical Cancer (HeLa) Cell Line. Phytomedicine 2013, 20, 151–158. [Google Scholar] [CrossRef]
- Zheng, L.-F.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.-L. Prooxidant Activity of Hydroxycinnamic Acids on DNA Damage in the Presence of Cu(II) Ions: Mechanism and Structure–Activity Relationship. Food Chem. Toxicol. 2008, 46, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Acharya, S.R.; Acharya, N.S. Clerodendrum serratum (L.) Moon.—A Review on Traditional Uses, Phytochemistry and Pharmacological Activities. J. Ethnopharmacol. 2014, 154, 268–285. [Google Scholar] [CrossRef]
- Touaibia, M.; Jean-Francois, J.; Doiron, J. Caffeic Acid, A Versatile Pharmacophore: An Overview. Mini-Rev. Med. Chem. 2011, 11, 695–713. [Google Scholar] [CrossRef]
- Russo, G.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef] [PubMed]
- Romanos-Nanclares, A.; Sánchez-Quesada, C.; Gardeazábal, I.; Martínez-González, M.Á.; Gea, A.; Toledo, E. Phenolic Acid Subclasses, Individual Compounds, and Breast Cancer Risk in a Mediterranean Cohort: The SUN Project. J. Acad. Nutr. Diet. 2020, 120, 1002–1015.e5. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Chen, Z.; Han, R.; Lu, L.; Li, Z.; Lin, J.; Hu, L.; Huang, X.; Lin, L. Comprehensive TCM Treatments Combined with Chemotherapy for Advanced Non-Small Cell Lung Cancer. Medicine 2021, 100, e25690. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Qian, X.; Zhou, J.; Li, C.; Jiao, Y.; Li, Y.Y.; Zhao, L. Multitarget and Multipathway Regulation of Zhenqi Fuzheng Granule against Non-Small Cell Lung Cancer Based on Network Pharmacology and Molecular Docking. Evid.-Based Complement. Altern. Med. 2022, 2022, 5967078. [Google Scholar] [CrossRef]
- Murray, J.C.; Burch, J.A.; Streilein, R.D.; Iannacchione, M.A.; Hall, R.P.; Pinnell, S.R. A Topical Antioxidant Solution Containing Vitamins C and E Stabilized by Ferulic Acid Provides Protection for Human Skin against Damage Caused by Ultraviolet Irradiation. J. Am. Acad. Dermatol. 2008, 59, 418–425. [Google Scholar] [CrossRef]
- Gao, S. GASC1 Inhibitor Caffeic Acid for Squamous Esophageal Cell Cancer (ESCC) (GiCAEC). Available online: https://clinicaltrials.gov/ct2/show/NCT04648917 (accessed on 1 April 2023).
- Gao, S. The Efficacy and Safety of Caffeic Acid for Esophageal Cancer (CAEC). Available online: https://clinicaltrials.gov/ct2/show/NCT03070262 (accessed on 1 April 2023).
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef]
- Li, Y.-H. Role of Phytochemicals in Colorectal Cancer Prevention. World J. Gastroenterol. 2015, 21, 9262. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Koushki, M.; Dashatan, N.A.; Meshkani, R. Effect of Resveratrol Supplementation on Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Ther. 2018, 40, 1180–1192.e5. [Google Scholar] [CrossRef]
- Kawada, N.; Seki, S.; Inoue, M.; Kuroki, T. Effect of Antioxidants, Resveratrol, Quercetin, AndN-Acetylcysteine, on the Functions of Cultured Rat Hepatic Stellate Cells and Kupffer Cells. Hepatology 1998, 27, 1265–1274. [Google Scholar] [CrossRef]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative Stress in Environmental-Induced Carcinogenesis. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 36–44. [Google Scholar] [CrossRef]
- Sale, S.; Tunstall, R.G.; Ruparelia, K.C.; Potter, G.A.; Steward, W.P.; Gescher, A.J. Comparison of the Effects of the Chemopreventive Agent Resveratrol and Its Synthetic Analogtrans 3,4,5,4?-Tetramethoxystilbene (DMU-212) on Adenoma Development in the ApcMin+ Mouse and Cyclooxygenase-2 in Human-Derived Colon Cancer Cells. Int. J. Cancer 2005, 115, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Medina, I.; Ortega, A.; Carretero, J.; Baño, M.C.; Obrador, E.; Estrela, J.M. Inhibition of Cancer Growth by Resveratrol Is Related to Its Low Bioavailability. Free. Radic. Biol. Med. 2002, 33, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and Biological Activity of Wild Blueberry (Vaccinium Angustifolium) Polyphenols during Simulated in Vitro Gastrointestinal Digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of Phenolic Compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Takada, Y. Role of Resveratrol in Prevention and Therapy of Cancer: Preclinical and Clinical Studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Azzolini, M. Pharmacology of Natural Polyphenols: Prodrugs and Biochemistry of Resveratrol and Pterostilbene. Ph.D. Thesis, University of Padwa, Padua, Italy, 2015. Available online: https://www.research.unipd.it/handle/11577/3423930?1/azzolini_michele_tesi.pdf (accessed on 1 April 2023).
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural Stilbenes: An Overview. Nat. Prod. Rep. 2009, 26, 916. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, B.; Bordun, K.-A.; Anderson, H. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Kalantari, H.; Das, D.K. Physiological Effects of Resveratrol. BioFactors 2010, 36, 401–406. [Google Scholar] [CrossRef]
- Berta, G.N.; Salamone, P.; Sprio, A.E.; Di Scipio, F.; Marinos, L.M.; Sapino, S.; Carlotti, M.E.; Cavalli, R.; Di Carlo, F. Chemoprevention of 7,12-Dimethylbenz[a]Anthracene (DMBA)-Induced Oral Carcinogenesis in Hamster Cheek Pouch by Topical Application of Resveratrol Complexed with 2-Hydroxypropyl-β-Cyclodextrin. Oral. Oncol. 2010, 46, 42–48. [Google Scholar] [CrossRef]
- Woodall, C.E.; Li, Y.; Liu, Q.H.; Wo, J.; Martin, R.C.G. Chemoprevention of Metaplasia Initiation and Carcinogenic Progression to Esophageal Adenocarcinoma by Resveratrol Supplementation. Anticancer Drugs 2009, 20, 437–443. [Google Scholar] [CrossRef]
- Lee, M.-H.; Choi, B.Y.; Kundu, J.K.; Shin, Y.K.; Na, H.-K.; Surh, Y.-J. Resveratrol Suppresses Growth of Human Ovarian Cancer Cells in Culture and in a Murine Xenograft Model: Eukaryotic Elongation Factor 1A2 as a Potential Target. Cancer Res. 2009, 69, 7449–7458. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-S.; Tsai, M.-L.; Nagabhushanam, K.; Wang, Y.-J.; Wu, C.-H.; Ho, C.-T.; Pan, M.-H. Pterostilbene Is More Potent than Resveratrol in Preventing Azoxymethane (AOM)-Induced Colon Tumorigenesis via Activation of the NF-E2-Related Factor 2 (Nrf2)-Mediated Antioxidant Signaling Pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-B. Anticancer Activity of Resveratrol on Implanted Human Primary Gastric Carcinoma Cells in Nude Mice. World J. Gastroenterol. 2005, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Gu, M.; Takahata, T.; Frederick, B.; Agarwal, C.; Siriwardana, S.; Agarwal, R.; Sclafani, R.A. Resveratrol Selectively Induces DNA Damage, Independent of Smad4 Expression, in Its Efficacy against Human Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2011, 17, 5402–5411. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.-W.; Tsai, L.-L.; Yu, C.-H.; Chen, P.-N.; Chou, M.-Y.; Yu, C.-C. Impairment of Tumor-Initiating Stem-like Property and Reversal of Epithelial-Mesenchymal Transdifferentiation in Head and Neck Cancer by Resveratrol Treatment. Mol. Nutr. Food Res. 2012, 56, 1247–1258. [Google Scholar] [CrossRef]
- Chen, Y.; Tseng, S.-H.; Lai, H.-S.; Chen, W.-J. Resveratrol-Induced Cellular Apoptosis and Cell Cycle Arrest in Neuroblastoma Cells and Antitumor Effects on Neuroblastoma in Mice. Surgery 2004, 136, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, F.; Sala, G.; Bagnacani, A.; Bertini, S.; Carboni, I.; Placanica, G.; Prota, G.; Rapposelli, S.; Sacchi, N.; Macchia, M.; et al. Synthesis of a Resveratrol Analogue with High Ceramide-Mediated Proapoptotic Activity on Human Breast Cancer Cells. J. Med. Chem. 2005, 48, 6783–6786. [Google Scholar] [CrossRef]
- Kaur, A.; Tiwari, R.; Tiwari, G.; Ramachandran, V. Resveratrol: A Vital Therapeutic Agent with Multiple Health Benefits. Drug Res. 2022, 72, 5–17. [Google Scholar] [CrossRef]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol Metabolites Inhibit Human Metastatic Colon Cancer Cells Progression and Synergize with Chemotherapeutic Drugs to Induce Cell Death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Leleu, X.; Sacco, A.; Moreau, A.-S.; Hatjiharissi, E.; Jia, X.; Xu, L.; Ciccarelli, B.; Patterson, C.J.; Ngo, H.T.; et al. Resveratrol Exerts Antiproliferative Activity and Induces Apoptosis in Waldenstrm’s Macroglobulinemia. Clin. Cancer Res. 2008, 14, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, R.F.; Nguyen, A.; Martinez Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope; Holcombe, R.F. Results of a Phase I Pilot Clinical Trial Examining the Effect of Plant-Derived Resveratrol and Grape Powder on Wnt Pathway Target Gene Expression in Colonic Mucosa and Colon Cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar] [CrossRef]
- Emily, R.; Winslow, M. A Biological Study of Resveratrol’s Effects on Notch-1 Signaling in Subjects with Low Grade Gastrointestinal Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT01476592 (accessed on 1 April 2023).
- Gayathri Nagaraj, M. Effect of Resveratrol on Serum IGF2 among African American Women. Available online: https://clinicaltrials.gov/ct2/show/NCT04266353 (accessed on 1 April 2023).
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S.; et al. A Phase I Study of Muscadine Grape Skin Extract in Men with Biochemically Recurrent Prostate Cancer: Safety, Tolerability, and Dose Determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef]
- Paller, C.J.; Zhou, X.C.; Heath, E.I.; Taplin, M.-E.; Mayer, T.; Stein, M.N.; Bubley, G.J.; Pili, R.; Hudson, T.; Kakarla, R.; et al. Muscadine Grape Skin Extract (MPX) in Men with Biochemically Recurrent Prostate Cancer: A Randomized, Multicenter, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 2018, 24, 306–315. [Google Scholar] [CrossRef]
- van Die, M.D.; Williams, S.G.; Emery, J.; Bone, K.M.; Taylor, J.M.G.; Lusk, E.; Pirotta, M.V. A Placebo-Controlled Double-Blinded Randomized Pilot Study of Combination Phytotherapy in Biochemically Recurrent Prostate Cancer. Prostate 2017, 77, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A Phase 2 Study of SRT501 (Resveratrol) with Bortezomib for Patients with Relapsed and or Refractory Multiple Myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Niranjana, R.; Gayathri, R.; Nimish Mol, S.; Sugawara, T.; Hirata, T.; Miyashita, K.; Ganesan, P. Carotenoids Modulate the Hallmarks of Cancer Cells. J. Funct. Foods 2015, 18, 968–985. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Health Protective Effects of Carotenoids and Their Interactions with Other Biological Antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of Microparticles of Carotenoids from Natural and Synthetic Sources for Applications in Food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The Role of Carotenoids in the Prevention of Human Pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and Protective Effects of Natural Carotenoids. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Khachik, F.; Spangler, C.J.; Smith, J.C.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, Quantification, and Relative Concentrations of Carotenoids and Their Metabolites in Human Milk and Serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Vitamin A Review. J. Nutr. 2016, 146, S1816–S1848. [Google Scholar] [CrossRef]
- Yang, D.-J.; Lin, J.-T.; Chen, Y.-C.; Liu, S.-C.; Lu, F.-J.; Chang, T.-J.; Wang, M.; Lin, H.-W.; Chang, Y.-Y. Suppressive Effect of Carotenoid Extract of Dunaliella Salina Alga on Production of LPS-Stimulated pro-Inflammatory Mediators in RAW264.7 Cells via NF-ΚB and JNK Inactivation. J. Funct. Foods 2013, 5, 607–615. [Google Scholar] [CrossRef]
- Amin, A.; Hamza, A.A.; Bajbouj, K.; Ashraf, S.S.; Daoud, S. Saffron: A Potential Candidate for a Novel Anticancer Drug against Hepatocellular Carcinoma. Hepatology 2011, 54, 857–867. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of Effect of Long-Term Supplementation with Beta Carotene on the Incidence of Malignant Neoplasms and Cardiovascular Disease. New Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef]
- Lee, I.-M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Hennekens, C.H. β-Carotene Supplementation and Incidence of Cancer and Cardiovascular Disease: The Women’s Health Study. JNCI J. Natl. Cancer Inst. 1999, 91, 2102–2106. [Google Scholar] [CrossRef] [PubMed]
- Connett, J.E.; Kuller, L.H.; Kjelsberg, M.O.; Polk, B.F.; Collins, G.; Rider, A.; Hulley, S.B. Relationship between Carotenoids and Cancer. The Multiple Risk Factor Intervention Trial (MRFIT) Study. Cancer 1989, 64, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L.; Pettinger, M.; Neuhouser, M.L.; Tinker, L.F.; Huang, Y.; Zheng, C.; Manson, J.E.; Mossavar-Rahmani, Y.; Anderson, G.L.; Lampe, J.W. Application of Blood Concentration Biomarkers in Nutritional Epidemiology: Example of Carotenoid and Tocopherol Intake in Relation to Chronic Disease Risk. Am. J. Clin. Nutr. 2019, 109, 1189–1196. [Google Scholar] [CrossRef]
- Rowles, J.L.; Erdman, J.W. Carotenoids and Their Role in Cancer Prevention. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef]
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased Dietary and Circulating Lycopene Are Associated with Reduced Prostate Cancer Risk: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of Carotene and Lycopene on the Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0137427. [Google Scholar] [CrossRef]
- Pu, Y.-S. Molecular Effects of a Multi-Carotenoids (MCS) New Agent on Prostate Cancer Chemoprevention. Available online: https://clinicaltrials.gov/ct2/show/NCT02426216 (accessed on 1 April 2023).
- Aune, D.; Chan, D.S.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Norat, T. Dietary Compared with Blood Concentrations of Carotenoids and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2012, 96, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gapstur, S.M.; Gaudet, M.M.; Furtado, J.D.; Campos, H.; McCullough, M.L. Plasma Carotenoids and Breast Cancer Risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Causes Control 2015, 26, 1233–1244. [Google Scholar] [CrossRef]
- He, J.; Gu, Y.; Zhang, S. Vitamin A and Breast Cancer Survival: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2018, 18, e1389–e1400. [Google Scholar] [CrossRef]
- Rock, C.L.; Natarajan, L.; Pu, M.; Thomson, C.A.; Flatt, S.W.; Caan, B.J.; Gold, E.B.; Al-Delaimy, W.K.; Newman, V.A.; Hajek, R.A.; et al. Longitudinal Biological Exposure to Carotenoids Is Associated with Breast Cancer–Free Survival in the Women’s Healthy Eating and Living Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 486–494. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.; Adams-Campbell, L.L.; Caan, B.J.; Chlebowski, R.T.; Neuhouser, M.L.; Shikany, J.M.; Rohan, T.E. Longitudinal Study of Serum Carotenoid, Retinol, and Tocopherol Concentrations in Relation to Breast Cancer Risk among Postmenopausal Women. Am. J. Clin. Nutr. 2009, 90, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, U.; Starkey, L.J.; O’Loughlin, J.; Gray-Donald, K. Fruit and Vegetable Consumption Is Lower and Saturated Fat Intake Is Higher among Canadians Reporting Smoking. J. Nutr. 2001, 131, 1952–1958. [Google Scholar] [CrossRef]
- Vergnaud, A.-C.; Norat, T.; Romaguera, D.; Mouw, T.; May, A.M.; Romieu, I.; Freisling, H.; Slimani, N.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; et al. Fruit and Vegetable Consumption and Prospective Weight Change in Participants of the European Prospective Investigation into Cancer and Nutrition–Physical Activity, Nutrition, Alcohol, Cessation of Smoking, Eating Out of Home, and Obesity Study. Am. J. Clin. Nutr. 2012, 95, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Abar, L.; Vieira, A.R.; Aune, D.; Stevens, C.; Vingeliene, S.; Navarro Rosenblatt, D.A.; Chan, D.; Greenwood, D.C.; Norat, T. Blood Concentrations of Carotenoids and Retinol and Lung Cancer Risk: An Update of the WCRF-AICR Systematic Review of Published Prospective Studies. Cancer Med. 2016, 5, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, L.; Boyd, K.; Matanoski, G.; Tao, X.; Chen, L.; Lam, T.K.; Shiels, M.; Hammond, E.; Robinson, K.A.; Caulfield, L.E.; et al. Carotenoids and the Risk of Developing Lung Cancer: A Systematic Review. Am. J. Clin. Nutr. 2008, 88, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Risk Factors for Lung Cancer and for Intervention Effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. JNCI J. Natl. Cancer Inst. 1996, 88, 1550–1559. [Google Scholar] [CrossRef]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L.; Omenn, G.S.; Valanis, B.; Williams, J.H. The Beta-Carotene and Retinol Efficacy Trial: Incidence of Lung Cancer and Cardiovascular Disease Mortality During 6-Year Follow-up After Stopping -Carotene and Retinol Supplements. JNCI J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef]
- Gary, E.; Goodman, M. Carotene and Retinol Efficacy Trial (CARET). Available online: https://clinicaltrials.gov/ct2/show/NCT00712647 (accessed on 1 April 2023).
- The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The Effect of Vitamin E and Beta Carotene on the Incidence of Lung Cancer and Other Cancers in Male Smokers. New Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Kubo, A.; Corley, D.A. Meta-Analysis of Antioxidant Intake and the Risk of Esophageal and Gastric Cardia Adenocarcinoma. Am. J. Gastroenterol. 2007, 102, 2323–2330. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, W.; Shao, L.; Zhong, D.; Wu, Y.; Cai, J. Association between Intake of Antioxidants and Pancreatic Cancer Risk: A Meta-Analysis. Int. J. Food Sci. Nutr. 2016, 67, 744–753. [Google Scholar] [CrossRef]
- Yang, T.; Yang, X.; Wang, X.; Wang, Y.; Song, Z. The Role of Tomato Products and Lycopene in the Prevention of Gastric Cancer: A Meta-Analysis of Epidemiologic Studies. Med. Hypotheses 2013, 80, 383–388. [Google Scholar] [CrossRef]
- Huang, J.; Lu, M.-S.; Fang, Y.-J.; Xu, M.; Huang, W.-Q.; Pan, Z.-Z.; Chen, Y.-M.; Zhang, C.-X. Serum Carotenoids and Colorectal Cancer Risk: A Case-Control Study in Guangdong, China. Mol. Nutr. Food Res. 2017, 61, 1700267. [Google Scholar] [CrossRef] [PubMed]
- Bairati, I.; Meyer, F.; Gélinas, M.; Fortin, A.; Nabid, A.; Brochet, F.; Mercier, J.-P.; Têtu, B.; Harel, F.; Abdous, B.; et al. Randomized Trial of Antioxidant Vitamins to Prevent Acute Adverse Effects of Radiation Therapy in Head and Neck Cancer Patients. J. Clin. Oncol. 2005, 23, 5805–5813. [Google Scholar] [CrossRef] [PubMed]
- Bairati, I.; Meyer, F.; Gelinas, M.; Fortin, A.; Nabid, A.; Brochet, F.; Mercier, J.-P.; Tetu, B.; Harel, F.; Masse, B.; et al. A Randomized Trial of Antioxidant Vitamins to Prevent Second Primary Cancers in Head and Neck Cancer Patients. JNCI J. Natl. Cancer Inst. 2005, 97, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Bairati, I.; Jobin, É.; Gélinas, M.; Fortin, A.; Nabid, A.; Têtu, B. Acute Adverse Effects of Radiation Therapy and Local Recurrence in Relation to Dietary and Plasma Beta Carotene and Alpha Tocopherol in Head and Neck Cancer Patients. Nutr. Cancer 2007, 59, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bairati, I.; Meyer, F.; Jobin, E.; Gélinas, M.; Fortin, A.; Nabid, A.; Brochet, F.; Têtu, B. Antioxidant Vitamins Supplementation and Mortality: A Randomized Trial in Head and Neck Cancer Patients. Int. J. Cancer 2006, 119, 2221–2224. [Google Scholar] [CrossRef]
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid Intake from Natural Sources and Head and Neck Cancer: A Systematic Review and Meta-Analysis of Epidemiological Studies. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1003–1011. [Google Scholar] [CrossRef]
- Heinen, M.M.; Hughes, M.C.; Ibiebele, T.I.; Marks, G.C.; Green, A.C.; van der Pols, J.C. Intake of Antioxidant Nutrients and the Risk of Skin Cancer. Eur. J. Cancer 2007, 43, 2707–2716. [Google Scholar] [CrossRef]
- Robert Greenberg, E.; Baron, J.A.; Stevens, M.M.; Stukel, T.A.; Mandel, J.S.; Spencer, S.K.; Elias, P.M.; Nicholas, L.; Nierenberg, D.N.; Bayrd, G.; et al. The Skin Cancer Prevention Study: Design of a Clinical Trial of Beta-Carotene among Persons at High Risk for Nonmelanoma Skin Cancer. Control. Clin. Trials 1989, 10, 153–166. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Baron, J.A.; Stukel, T.A.; Stevens, M.M.; Mandel, J.S.; Spencer, S.K.; Elias, P.M.; Lowe, N.; Nierenberg, D.W.; Bayrd, G.; et al. A Clinical Trial of Beta Carotene to Prevent Basal-Cell and Squamous-Cell Cancers of the Skin. New Engl. J. Med. 1990, 323, 789–795. [Google Scholar] [CrossRef]
- Frieling, U.M.; Schaumberg, D.A.; Kupper, T.S.; Muntwyler, J.; Hennekens, C.H. A Randomized, 12-Year Primary-Prevention Trial of Beta Carotene Supplementation for Nonmelanoma Skin Cancer in the Physicians’ Health Study. Arch Dermatol. 2000, 136, 179–184. [Google Scholar] [CrossRef]
- Green, A.; Williams, G.; Nèale, R.; Hart, V.; Leslie, D.; Parsons, P.; Marks, G.C.; Gaffney, P.; Battistutta, D.; Frost, C.; et al. Daily Sunscreen Application and Betacarotene Supplementation in Prevention of Basal-Cell and Squamous-Cell Carcinomas of the Skin: A Randomised Controlled Trial. Lancet 1999, 354, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Correlation between Skin Carotenoid Levels and Previous History of Skin Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00836342 (accessed on 1 April 2023).
- Li, X.; Xu, J. Meta-Analysis of the Association between Dietary Lycopene Intake and Ovarian Cancer Risk in Postmenopausal Women. Sci. Rep. 2014, 4, 4885. [Google Scholar] [CrossRef] [PubMed]
- Potischman, N.; Brinton, L.A. Nutrition and Cervical Neoplasia. Cancer Causes Control 1996, 7, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Potischman, N. Nutritional Epidemiology of Cervical Neoplasia. J. Nutr. 1993, 123, 424–429. [Google Scholar] [CrossRef]
- Herrero, R.; Potischman, N.; Brinton, L.A.; Reeves, W.C.; Brenes, M.M.; Tenorio, F.; de Britton, R.C.; Gaitan, E. A Case-Control Study of Nutrient Status and Invasive Cervical Cancer. Am. J. Epidemiol. 1991, 134, 1335–1346. [Google Scholar] [CrossRef]
- Brock, K.E.; Berry, G.; Mock, P.A.; MacLennan, R.; Truswell, A.S.; Brinton, L.A. Nutrients in Diet and Plasma and Risk of In Situ Cervical Cancer. JNCI J. Natl. Cancer Inst. 1988, 80, 580–585. [Google Scholar] [CrossRef]
- Wylie-Rosett, J.A.; Romney, S.L.; Slagle, N.S.; Wassertheil-Smoller, S.; Miller, G.L.; Palan, P.R.; Lucido, D.J.; Duttagupta, C. Influence of Vitamin a on Cervical Dysplasia and Carcinoma in situ. Nutr. Cancer 1985, 6, 49–57. [Google Scholar] [CrossRef]
- Marshall, J.R.; Graham, S.; Byers, T.; Swanson, M.; Brasure, J. Diet and smoking in the epidemiology of cancer of the cervix. J. Natl. Cancer Inst. 1983, 70, 847–851. [Google Scholar]
- Singh, V.; Gaby, S. Premalignant Lesions: Role of Antioxidant Vitamins and β-Carotene in Risk Reduction and Prevention of Malignant Transformation. Am. J. Clin. Nutr. 1991, 53, 386S–390S. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Papenfuss, M.; Nour, M.; Canfield, L.M.; Schneider, A.; Hatch, K. Antioxidant nutrients: Associations with persistent human papillomavirus infection. Cancer Epidemiol. Biomark. Prev. 1997, 6, 917–923. [Google Scholar]
- Van Eenwyk, J.; Davis, F.G.; Bowen, P.E. Dietary and Serum Carotenoids and Cervical Intraepithelial Neoplasia. Int. J. Cancer 2007, 48, 34–38. [Google Scholar] [CrossRef]
- Batieha, A.M.; Armenian, H.K.; Norkus, E.P.; Morris, J.S.; Spate, V.E.; Comstock, G.W. Serum micronutrients and the subsequent risk of cervical cancer in a population-based nested case-control study. Cancer Epidemiol. Biomark. Prev. 1993, 2, 335–339. [Google Scholar]
- Meyskens, F.L.; Surwit, E.; Moon, T.E.; Childers, J.M.; Davis, J.R.; Dorr, R.T.; Johnson, C.S.; Alberts, D.S. Enhancement of Regression of Cervical Intraepithelial Neoplasia II (Moderate Dysplasia) With Topically Applied All- Trans -Retinoic Acid: A Randomized Trial. JNCI J. Natl. Cancer Inst. 1994, 86, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J.; Boone, C.W.; Crowell, J.A.; Nayfield, S.G.; Hawk, E.; Steele, V.E.; Lubet, R.A.; Sigman, C.C. Strategies for Phase II Cancer Chemoprevention Trials: Cervix, Endometrium, and Ovary. J. Cell Biochem. 1995, 59, 1–9. [Google Scholar] [CrossRef]
- de Vet, H.C.W.; Knipschild, P.G.; Willebrand, D.; Schouten, H.J.A.; Sturmans, F. The Effect of Beta-Carotene on the Regression and Progression of Cervical Dysplasia: A Clinical Experiment. J. Clin. Epidemiol. 1991, 44, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Romney, S.L.; Ho, G.Y.F.; Palan, P.R.; Basu, J.; Kadish, A.S.; Klein, S.; Mikhail, M.; Hagan, R.J.; Chang, C.J.; Burk, R.D. Effects of β-Carotene and Other Factors on Outcome of Cervical Dysplasia and Human Papillomavirus Infection. Gynecol. Oncol. 1997, 65, 483–492. [Google Scholar] [CrossRef]
- Manetta, A.; Schubbert, T.; Chapman, J.; Schell, M.J.; Peng, Y.M.; Liao, S.Y.; Meyskens, F.J., Jr. beta-Carotene treatment of cervical intraepithelial neoplasia: A phase II study. Cancer Epidemiol. Biomark. Prev. 1996, 5, 929–932. [Google Scholar]
- Meyskens, F.; Manetta, A. Prevention of Cervical Intraepithelial Neoplasia and Cervical Cancer. Am. J. Clin. Nutr. 1995, 62, 1417S–1419S. [Google Scholar] [CrossRef]
- Mackerras, D.; Baghurst, P.; Fairley, C.; Irwig, L.; Weisberg, E.; Simpson, J. Beta-Carotene and Cervical Dysplasia Trials in Australia. Ann. N. Y Acad. Sci. 1993, 691, 253–254. [Google Scholar] [CrossRef]
- Chen, F.; Hu, J.; Liu, P.; Li, J.; Wei, Z.; Liu, P. Carotenoid Intake and Risk of Non-Hodgkin Lymphoma: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Ann. Hematol. 2017, 96, 957–965. [Google Scholar] [CrossRef] [PubMed]
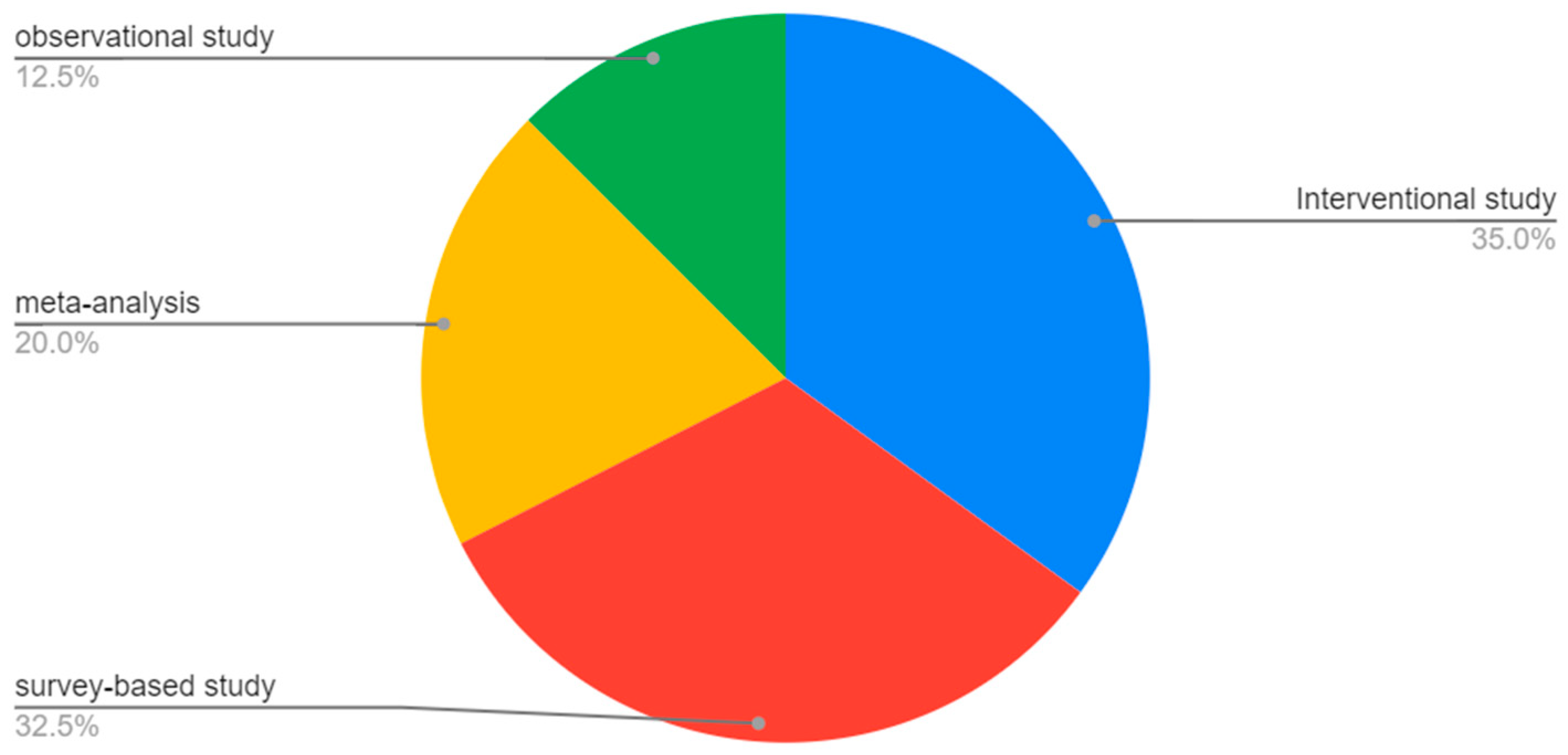




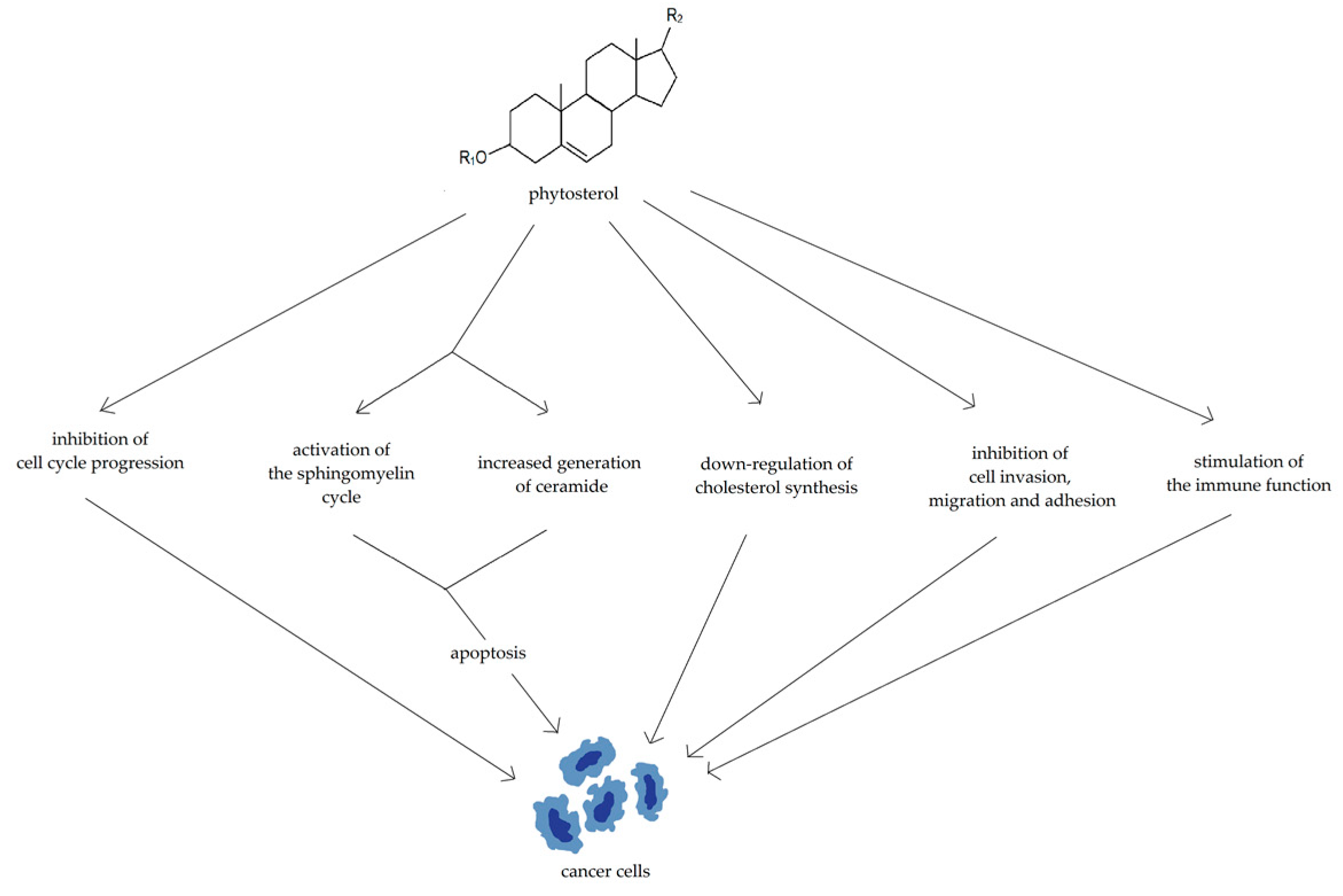
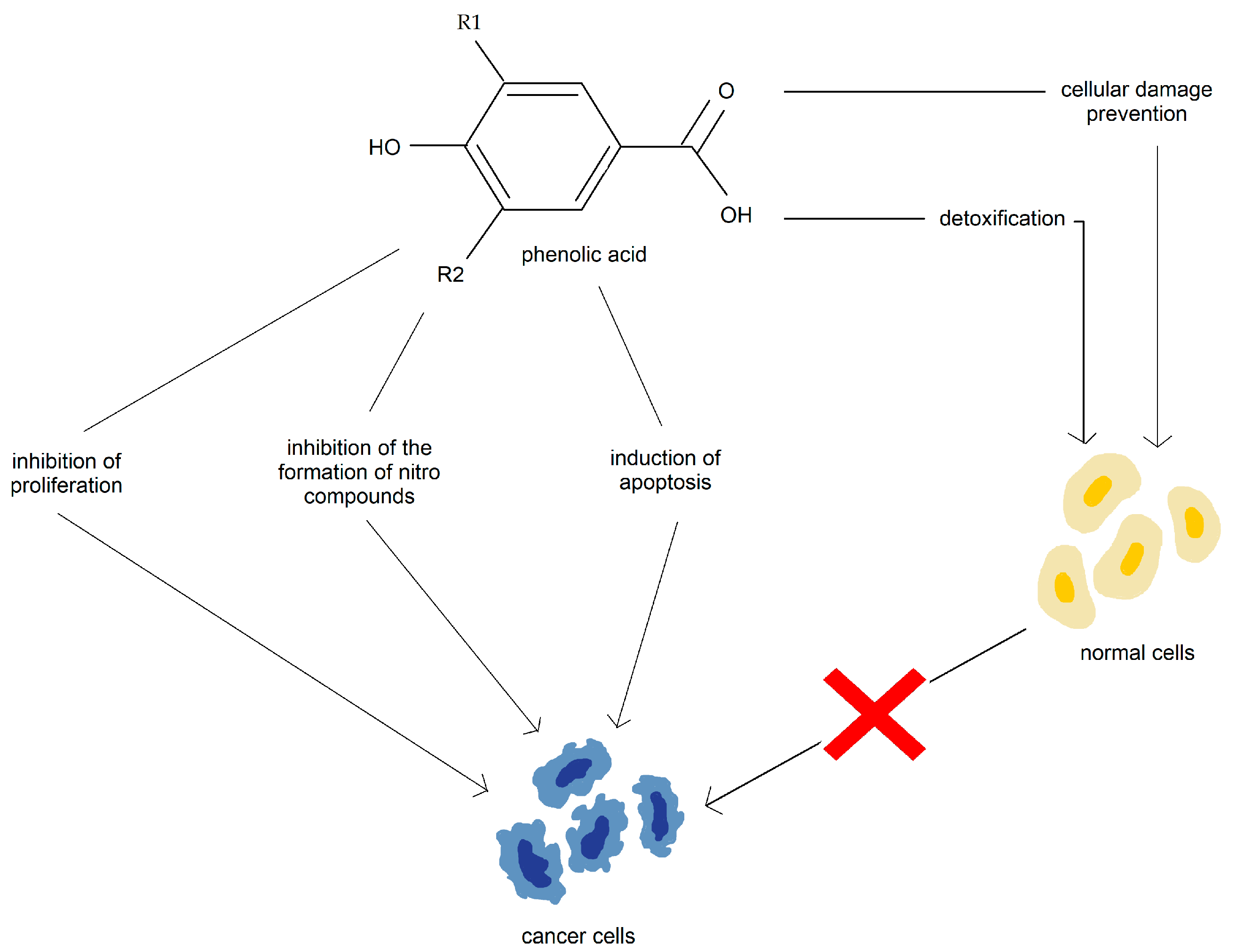


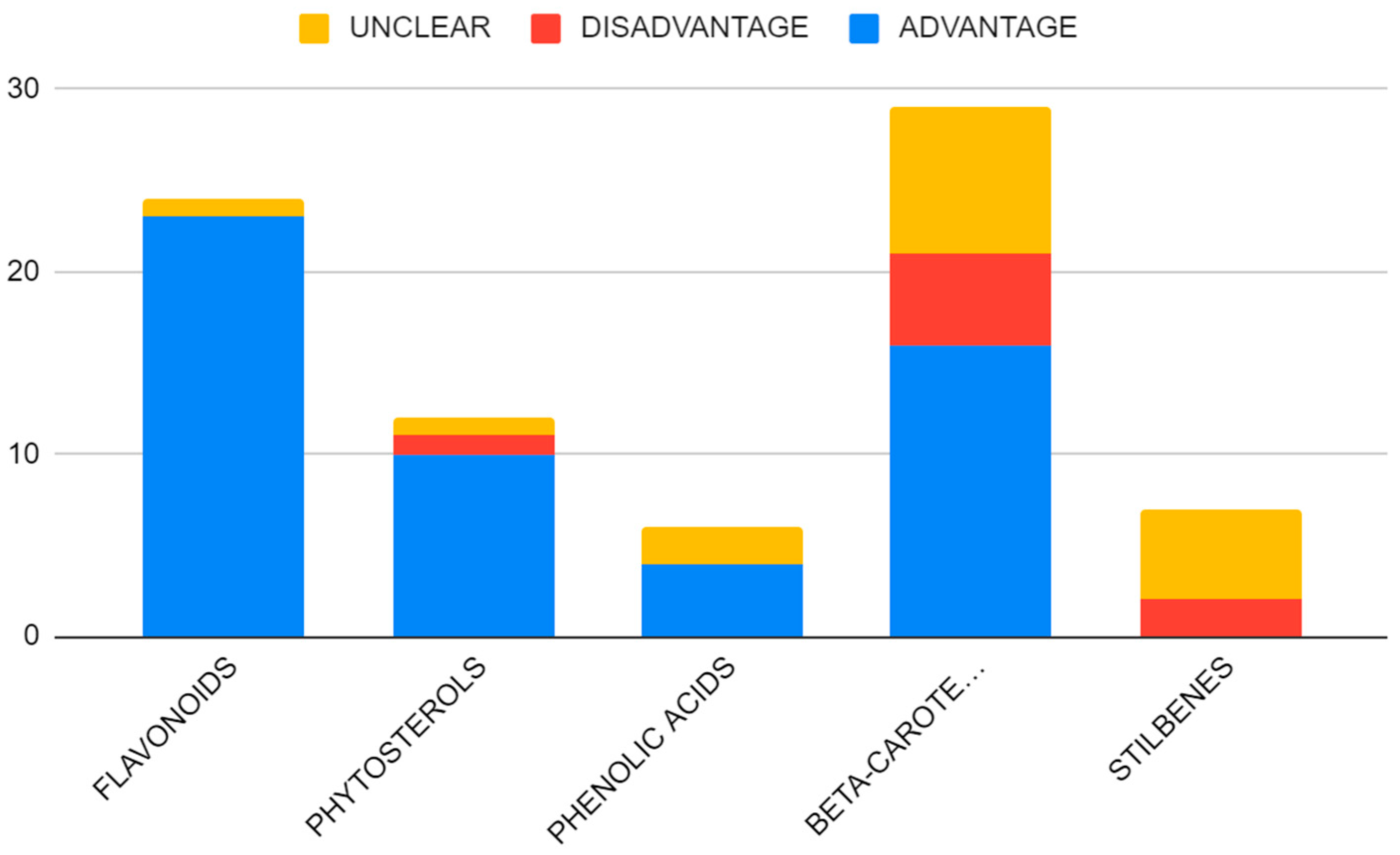
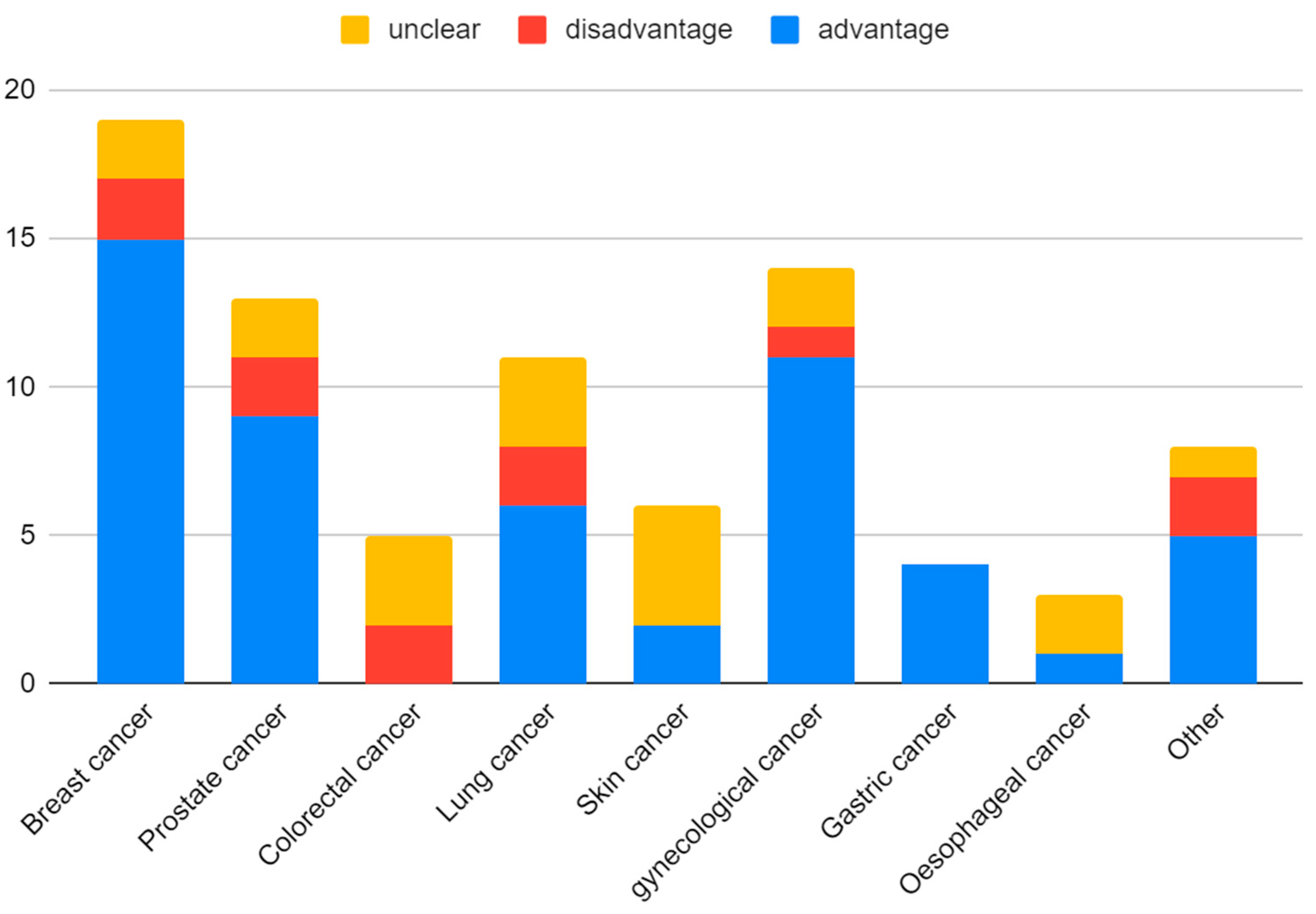
| Compound | Number of Studies |
|---|---|
| Flavonoids | 24 |
| Phytosterols | 9 |
| Phenolic acids | 6 |
| Beta-carotenoids | 27 |
| Stilbenes | 7 |
| Substance | Case Cohort | Control Cohort |
|---|---|---|
| Flavonoids | 556,799 | 245,864 |
| Phytosterols | 162,617 | 4378 |
| Phenolic acids | 11,327 | 222 |
| Beta-carotenoids | 3,778,821 | 27,735 |
| Stilbenes | 930 | 1440 |
| Trial Number | Years | Location | Condition (Only Significant for Article) | Number Recruited | Substance | Outcome Measure | Status |
|---|---|---|---|---|---|---|---|
| NCT00712647 | 05.1985–06.2005 | United States | Lung Cancer | 18,314 | Beta Carotene, Retinol | Prevention Of Lung Cancer | Completed |
| NCT00003094 | 04.2004–05.2014 | United States | Cervical Cancer | 0 | Preventative Dietary Intervention | Carotenoid Level; Progression Of Cervical Dysplasia | Withdrawn * |
| NCT00098969 | 12.2004–12.2012 | United States, United Kingdom | Unspecified Adult Solid Tumor, Protocol Specific | 40 | Resveratrol | Concentration Of Resveratrol and its Metabolites in the Plasma, Urine, and Feces; Determination of the Drug Safety in Participants | Completed |
| NCT00169845 | 09.2005–03.2018 | Canada | Head and Neck Neoplasms | 540 | Alpha-Tocopherol; Beta-Carotene | Prevention of Second Primary Cancers | Completed |
| NCT00256334 | 06.2005–04.2009 | United States | Colon Cancer | 11 | Resveratrol | Expression of a Panel of Wnt Target Genes | Completed |
| NCT00342992 | 06.2006–09.2020 | Finland | Cancer | 29,133 | Alpha-Tocopherol; Beta-Carotene | Annual Linkage with Finnish Cancer Registry | Completed |
| NCT00578396 | 12.2007–01.2021 | United States | Colon Cancer | 0 | Grapes | Localization of β-Catenin and Wnt Target Gene Expression in Intestinal Mucosa | Withdrawn ** |
| NCT00433576 | 02.2007–09.2014 | United States | Adenocarcinoma of the Colon Adenocarcinoma of the Rectum Colon Cancer (Stafe I–III) Rectal Cancer (Stage I–III) | 20 | Resveratrol | Pharmacodynamics of Resveratrol; Concentrations of Biomarkers | Completed |
| NCT00712647 | 07.2008–09.2012 | United States | Lung Cancer | 18,314 | Beta Carotene; Retinol | Lung Cancer Incidence [bi-annual ] | Completed |
| NCT00836342 | 02.2009–06.2012 | United States | Skin Cancer Basal Cell Carcinoma Squamous Cell Carcinoma | 81 | Skin Carotenoid | Skin Carotenoid Levels | Completed |
| NCT00920556 | 03.2009–11.2010 | Denmark, United Kingdom | Multiple Myeloma | 24 | SRT501, Bortezomib | Safety Assessments | Terminated |
| NCT01476592 | 11.2011–10.2013 | United States | Neuroendocrine Tumor | 7 | Resveratrol | Notch1 Activation in Tumor Biopsy Specimens | Completed |
| NCT01692340 | 09.2012–03.2019 | United States | Healthy | 12 | Lycopene, Phytoene, Phytofluene | Plasma Half-Life of Labeled Carotenoid; Maximal Plasma Carotenoid Concentration; Time of Maximal Carotenoid Concentration | Completed |
| NCT01538316 | 02.2012–05.2012 | Germany | Primary Prevention of Prostate Cancer | 60 | Quercetin; Genistein | Log2-Transformed PSA Measurements | Unknown |
| NCT02261844 | 10.2014–03.2017 | United States | Liver Cancer | 0 | Resveratrol | Improvement of the Metabolic Profile of Liver Cells | Withdrawn *** |
| NCT02426216 | 04.2015–10.2015 | Taiwan | Prostate Cancer | 300 | Multi-carotenoids | Cumulative Histologically Proven Prostate Cancer Incidence at 2 years | Unknown |
| NCT03433092 | 11. 2017–12.2017 | China | Gastrointestinal Tract Cancer | 40,641 | Serum Carotenoids | Serum Carotenoid Levels; Relative Risk (RR) or OR and Corresponding 95% CI | completed |
| NCT03070262 | 01.2017–12.2021 | China | Esophagus Cancer | 300 | Caffeic Acid | 1-Year Overall Survival (OS) | Unknown |
| NCT04648917 | 05. 2019–05.2022 | China | Esophagus Cancer, Stage III | 80 | Caffeic Acid | 1-Year Overall Survival (OS) | Unknown |
| NCT04266353 | 02.2020–05.2022 | United States | Chemoprevention | 50 | Resveratrol | Serum/plasma IGF-II/IGFBP-3 Concentrations | Suspended |
| NCT04147767 | 02.2020–12.2021 | United Kingdom | Breast Cancer | 50 | Dietary Supplement: Cholesterol Reducing Yogurt Drink | Serum/Plasma Oxysterol Concentrations | Recruiting |
| Substance | Additional Substances | Time Interval | Dosage | Outcome |
|---|---|---|---|---|
| Phytosterols | ||||
| Phytosterols | 20 weeks | 2 g/d free plant sterols | Results not available [97] | |
| 6 years | 1 L/week of extra virgin olive oil | 62% relatively lower risk of malignant breast cancer [95] | ||
| 6 years | 30 g/d of mixed nuts | Nonsignificant breast cancer risk reduction [95] | ||
| Phenolic acid | ||||
| Caffeic acid | 1 year | 100–200 mg/d, depending on body mass | Results not available [118] | |
| 1 year | 300 mg/d | Results not available [119] | ||
| Beta-carotene | ||||
| Beta-carotene | 8 years | 20 mg/d | Inverse association between dietary intake of alpha-tocopherol and beta carotene and the risk of lung cancer; higher mortality among recipients of beta carotene [187] | |
| 8 years | 20 mg + 50 mg/d of alpha-tocopherol | |||
| beta-cryptoxanthin, lutein, lycopene | 5 years | daily intake of 5 vegetable servings, 16 oz of vegetable juice or vegetable equivalents, 3 fruit servings, 30 g fiber, and 15–20% energy intake from fat | 21% relatively lower risk of malignant breast cancer [178] | |
| 9 months | 30 mg/day | Lack of beneficial effect on; CIN (cervical intraepithelial neoplasia) [217] | ||
| 12 months | 30 mg/day | 70% response rate of CIN at 6 months and 43% at 12 months [218] | ||
| 5 years | 30 mg/day + and a sun protection factor 15+ sunscreen | Slightly but not significantly lower incidence for basal-cell carcinoma (BCC); slightly but not significantly higher incidence of squamous-cell carcinoma (SCC) [201] | ||
| 15 mg + 300 mg vitamin C | ||||
| 30 mg/day | ||||
| vitamin C | 6 months | 15 mg + 500 mg vitamin C | Results not available [220] | |
| 12 months | 30 mg | |||
| alpha tocopherol | 3 years | 30 mg + 400 IU of alpha-tocopherol | Fewer severe acute adverse effects [139] | |
| 2 years | 30 mg + 25,000 IU retinil palmitate | Lack of chemopreventive benefit and excess lung cancer incidence and mortality [184,185,186] | ||
| 5 years | 50 mg/day | Lack of beneficial effect on the rate of occurrence of first new non-melanoma (NMSC) skin cancer [198] | ||
| 12 years | 50 mg on alternate days | Lack of beneficial effect on first NMSC, including BCC and SCC, prevention [200] | ||
| Stilbenes | ||||
| Resveratrol SRT501 | 3 months | 5 mg/day in 2 divided doses of 2.5 mg | Results not available [148] | |
| bortezomib | 3 years | 5 g/day | Unacceptable safety profile and minimal efficacy in patients with relapsed/refractory MM [153] | |
| 2 weeks | 20, 80, 160 mg/day + grape extract 125 mg/day + 8 oz glass of water | Inhibitory effect on Wnt signal throughput in colonic mucosa-derived cell line [146] | ||
| Curcumin, catechins, fresh borccoli sprouts | 12 weeks | curcumin 100 mg/d; resveratrol 30 mg/d; catechins 100 mg/d; fresh broccoli sprouts equivalent to 2000 mg/d | Non-significant increase in the log-slope of PSA in the active treatment group [151] | |
| 6 weeks | 150 mg/day | Results not available [149] | ||
| MPX (Muscadine Grape Skin Extract) | 1 year | 500 mg/day to 4000 mg/day | No severe related adverse events were reported [149] | |
| 1 year | 4000 mg/d of MPX, 500 mg/d of MPX (1.2 mg of ellagic acid, 9.2 μg of quercetin, and 4.4 μg of trans-resveratrol) | No significant difference in PSADT change [150] | ||
| 1 year | 1.2 mg of ellagic acid, 9.2 μg of quercetin, and 4.4 μg of trans-resveratrol | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. https://doi.org/10.3390/nu15081896
Rudzińska A, Juchaniuk P, Oberda J, Wiśniewska J, Wojdan W, Szklener K, Mańdziuk S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients. 2023; 15(8):1896. https://doi.org/10.3390/nu15081896
Chicago/Turabian StyleRudzińska, Anna, Pola Juchaniuk, Jakub Oberda, Jolanta Wiśniewska, Witold Wojdan, Katarzyna Szklener, and Sławomir Mańdziuk. 2023. "Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials" Nutrients 15, no. 8: 1896. https://doi.org/10.3390/nu15081896
APA StyleRudzińska, A., Juchaniuk, P., Oberda, J., Wiśniewska, J., Wojdan, W., Szklener, K., & Mańdziuk, S. (2023). Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients, 15(8), 1896. https://doi.org/10.3390/nu15081896





