Abstract
Hyperhomocysteinemia (HHcy) is an independent risk factor for cardiovascular and cerebrovascular diseases where the plasma homocysteine (Hcy) concentration exceeds 15 µmol/L. HHcy is affected by vitamins B12, B6, and folic acid (fol); however, its relationship with other nutrients is not fully understood. We investigated the nutritional and genetic factors associated with HHcy and the possible dose–response relationships or threshold effects in patients in Northeast China. Genetic polymorphisms and micronutrients were tested with polymerase chain reaction and mass spectrometry, respectively. This trial was registered under trial number ChiCTR1900025136. The HHcy group had significantly more males and higher body mass index (BMI), methylenetetrahydrofolate reductase (MTHFR 677TT) polymorphism proportion, and uric acid, Zn, Fe, P, and vitamin A levels than the control group. After adjusting for age, sex, BMI, vitamin B12, fol, and MTHFR C677T, the lowest Zn quartile reduced the odds ratio of HHcy compared with the highest Zn quartile. The dose–response curves for the association between plasma Zn and HHcy were S-shaped. High plasma Zn concentrations were significantly correlated with high HHcy odds ratios, and the curve leveled off or slightly decreased. Most importantly, HHcy risk decreased with decreasing plasma Zn concentration; the threshold was 83.89 µmol/L. Conclusively, individuals residing in Northeast China, especially those with the MTHFR 677TT polymorphism, must pay attention to their plasma Zn and Hcy levels.
1. Introduction
Homocysteine (Hcy) is a sulfur-containing amino acid and an intermediate metabolite of methionine and cysteine, mainly through remethylation and sulfuration [1]. In the physiological state, Hcy levels in the body are maintained at 5–15 µmol/L [2]. Many factors, such as heredity, medicine use, diseases, and living habits, may lead to increased Hcy levels (>15 µmol/L), resulting in hyperhomocysteinemia (HHcy) [3]. HHcy is considered a cytotoxic factor associated with various diseases such as coronary heart disease, stroke, Alzheimer’s disease, peripheral vascular disease, cancer, diabetes, and osteoporosis [4,5,6]. Furthermore, numerous epidemiological reports have established HHcy as an independent risk factor for cardiovascular disease, cerebrovascular disease, dementia-type disorders, and associated fractures [7,8,9].
During remethylation, Hcy can be methylated twice via two different pathways. First, methylenetetrahydrofolate reductase (MTHFR) reduces 5,10-methyltetrahydrofolate (MTHF) to form 5-MTHF [10]. Then 5-MTHF, assisted by the cofactor vitamin B12, adds a methyl group to Hcy, which again produces methionine. This requires the key enzyme methionine synthase reductase (MTRR) [11,12].
More than 40 polymorphism sites are present in MTHFR [13], of which C677T and A1298C are the most impactful [14]. Compared with the 677CC genotype, the 677TT genotype shows approximately 34% MTHFR activity, while the heterozygote 677CT shows approximately 65% MTHFR activity [15]. In MTRR, the most important polymorphism is A66G [16], which regulates Hcy levels through DNA hypomethylation [17]. Polymorphisms differ between distinct geographical areas and ethnic populations [18,19,20].
Folic acid (fol) is a water-soluble vitamin involved in nucleic acid synthesis, DNA methylation, repair, cell division, and embryonic development [21,22,23]. Hcy remethylation and trans-sulfur pathways require vitamins B12 and B6 as coenzymes, respectively. A two-month-long, randomized, double-blind study found that vitamin D supplementation decreased hypertension and reduced Hcy levels [24].
Minerals also greatly influence the occurrence of diseases; for example, Zn [25], Fe [26], and Mg [27] are related to atherosclerosis and vasospasm [28]. Further, minerals are involved in the complex physiological reactions in the body, serving as components of some metabolic enzymes or as auxiliary factors regulating enzyme activity. For example, Zn regulates the activity of more than 100 enzymes [28,29]. However, there are few studies that have elucidated whether minerals are relevant factors influencing Hcy metabolic enzymes. Sadako Matsui’s [30] cross-sectional study showed that Zn was negatively correlated to log Hcy in men, but not in women. Esfandiar Heidarian [31] conducted a randomized, double-blind, controlled, crossover study which showed that Zn supplementation reduced serum Hcy and increased vitamin B12 and fol concentrations in T2DM patients with microalbuminuria. However, Véronique Ducros [32] showed that Zn supplementation did not modify Hcy, vitamin B12, or RBC fol values in aging healthy people.
Therefore, in this study, we aimed to investigate the relationship between plasma Hcy levels and genetic variation in MTHFR and MTRR in patients from Northeast China. In addition, as several vitamins play key roles in the metabolism of Hcy, we studied the relationship between vitamin levels and HHcy. Moreover, we explored the nutritional and genetic factors associated with HHcy and the potential dose–response relationships and threshold effects.
2. Materials and Methods
2.1. Study Participants
From June 2019 to December 2020, according to the inclusion and exclusion criteria, 203 participants were enrolled at the clinical nutrition department of Shengjing Hospital, China Medical University (Shenyang, China). Specific study inclusion requirements were no serious organic disease, independent mobility, clear awareness, and voluntary participation. Exclusion criteria were severe organ lesions, immobility, and unconsciousness. No age or sex restrictions were laid. All participants were tested for HHcy through the enzyme cycle method and divided into the normal (plasma Hcy 5–15 µmol/L) and HHcy (plasma Hcy > 15 µmol/L) groups [14]. Then, 135 healthy individuals (control group) and 68 patients (HHcy group) were included in the study. This study fulfilled the principles of the Declaration of Helsinki and was approved by the ethics committee at Shengjing Hospital of China Medical University, China, and registered with a trial number ChiCTR1900025136. All participants received written informed consent to use their clinical data for research purposes.
2.2. Experimental Instruments and Reagents
Fasting venous samples (5 mL) were collected from all subjects in the morning, and plasma was centrifuged after anticoagulant treatment (3000 rpm for 15 min). A nucleic acid extraction reagent (EE201-01) was purchased from TransGen Biotech (Beijing, China) and a human MTHFR gene detection kit was purchased from Wuhan Youzhiyou Medical Technology (Wuhan, China). Polymerase chain reaction (PCR) amplification devices (7500-fast, ABI, Alameda, CA, USA) were used in this study. Hcy and other nutrients were completed in the clinical laboratory of Shengjing Hospital according to clinical testing standards. Plasma Hcy levels were determined through chemiluminescence using a commercial kit (Jiuqiang Biological Company, Beijing, China) and an automatic chemiluminassay analyzer (Abbott-i16200, Shanghai, China), following the detection methods reported by Yin [33,34]. Plasma Zn, Cu, Ca, P, Mg, and Pb levels were determined using an atomic absorption spectrometer (Jingbohui Biotechnology Company, Beijing, China), following the method by Komarova [35,36]. Plasma vitamin A, vitamin D, fol, and vitamin B12 levels were determined using an automatic electrochemiluminescence immune analyzer (Roche, Cobas e601E-E, Shanghai, China), following the method by Stokes [37,38,39] and its companion Detection special kit, in strict accordance with the Electrochemistry Luminescence method, instrument operating specifications, and detection kit instructions.
2.3. Sample Collection, DNA Preparation, and MTHFR Genotyping
The exfoliated cells were collected with disposable oral swabs and subjected to DNA extraction using a commercial DNA extraction kit, following the manufacturers’ instructions. The extracted DNA was stored at −20 °C. The PCR amplification conditions were 37 °C for 10 min, 95 °C for 5 min, 95 °C for 15 s (40 cycles), and 60 °C for 60 s. The fluorescence channel settings used were FAM, VIC, and ROX. According to the comparison of the two fluorescence signal intensities, the genotype was determined as wild-type, heterozygous, or homozygous (Figure 1).
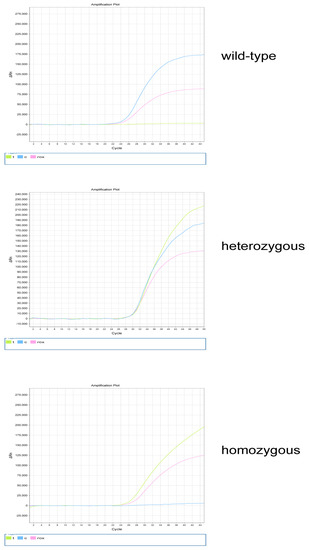
Figure 1.
MTHFR genotyping. The wild−type genotypes include MTHFR 677CC, MTHFR 1298AA, and MTRR 66AA; the heterozygous genotypes include MTHFR 677CT, MTHFR 1298AC, and MTRR 66AG; and the homozygous genotypes include MTHFR 677TT, MTHFR 1298CC, and MTRR 66GG; t represents the FAM fluorescence channel, c represents the VIC fluorescence channel, and ROX represents internal reference.
2.4. Statistical Analysis
The statistical software SPSS 20 (IBM Corp., Armonk, NY, USA) was used for data processing and statistical analyses. Results with p < 0.05 were considered significant. The chi-square or Fisher’s exact tests were used for categorical variables, and a one-way analysis of variance was used for continuous variables. Odds ratios (ORs) with 95% confidence intervals were estimated using logistic regression analysis. We also used restricted cubic splines (RCSs) to test for linearity and explore the shape of the dose–response effect between plasma Zn concentration and HHcy.
3. Results
3.1. Participant Characteristics
The characteristics of the 203 participants are listed in Table 1. According to the criteria of plasma Hcy concentration >15 µmol/L, 68 patients had HHcy (HHcy group). Additionally, 135 healthy individuals (control group) were included. The average plasma Hcy concentration was 35.89 and 9.26 µmol/L in the HHcy and control groups, respectively. The HHcy group had more male and overweight/obese participants and showed higher alkaline phosphatase (ALKP) and uric acid (UA) levels than the control group. The MTHFR C677T mutation distribution was significantly different between the groups (p < 0.001). The HHcy group had more proportion of participants with MTHFR 677TT type than the control group (51.50 vs. 22.20%). There was no significant difference in MTHFR 1298 and MTRR 66 mutations between the groups (p = 0.083 and p = 0.853, respectively).

Table 1.
Characteristics of the control and HHcy group participants.
3.2. Comparison of Hcy Concentration among Different Genotypes
Table 2 shows an overall comparison of Hcy concentrations for different genotypes and a comparison of Hcy concentrations in gender subgroups. The results of all Hardy–Weinberg tests were p > 0.05. Hcy levels were significantly higher in the MTHFR 677TT group than in the MTHFR 677CC or MTHFR 677CT groups. This difference was significant in the total and male groups (p < 0.001), but not statistically significant in the female group (p = 0.095). However, there were no significant differences in the Hcy levels in the wild-type, heterozygous, and homozygous MTHFR A1298C and MTRR A66G groups, indicating that Hcy levels were affected by the MTHFR C677T polymorphism, instead of MTHFR A1298C or MTRR A66G.

Table 2.
Comparison of Hcy concentrations among different genotypes in sex subgroups.
3.3. Nutrient Differences among Participants
Table 3 shows the nutrient differences between the control and HHcy groups. The HHcy group had significantly higher plasma Zn, Fe, and vitamin A levels than the control group. In addition, the HHcy group had significantly lower plasma fol, vitamin B12, and P levels than the control group. There were no significant differences in plasma vitamin D, Pb, Cu, Ca, and Mg levels between the two groups.

Table 3.
Nutrient differences between the control and HHcy groups.
3.4. Associations between Zn and HHcy ORs
Table 4 shows the associations between Zn and HHcy ORs. Plasma Zn levels were divided into four quartiles. Model 1 was an unadjusted model; Model 2 was adjusted for age, sex, and body mass index (BMI); and Model 3 was further adjusted for vitamin B12, fol, and MTHFR C677T. All three models indicated that Q1 had a significantly lower HHcy risk than Q4.

Table 4.
Associations between Zn and the HHcy odds ratio.
3.5. Dose–Response Association between Plasma Zn Level and HHcy Risk
The spline regression model showed that the HHcy OR increased significantly when plasma Zn concentration was >83.89 µmol/L. Correspondingly, when plasma Zn was <83.89 µmol/L, the HHcy OR decreased significantly (Figure 2).
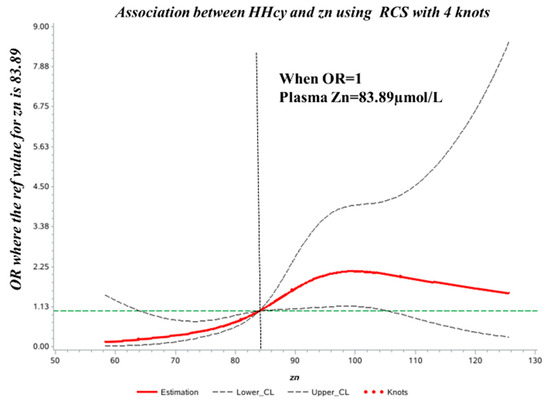
Figure 2.
Dose–response relationship between plasma Zn concentration and HHcy odds ratio (OR). The ORs (solid lines) and 95% confidence intervals (dashed lines) of plasma Zn concentration are indicated by straight lines based on restrictive cubic splines. Age, sex, vitamin B12, folic acid, and MTHFR C677T were adjusted.
4. Discussion
The incidence of hypertension and cardiovascular diseases in Shenyang is higher than that in other cities in China [40]. HHcy is an independent risk factor for cardiovascular and cerebrovascular diseases, but studies on its relationship with nutrients are scarce. Therefore, using clinical data from a large tertiary care hospital, we investigated the genetic and nutritional factors associated with HHcy.
We observed that more males were in the HHcy group than in the control group, as in the study by Yating Yang [41]. They enrolled 330 Han Chinese patients with schizophrenia (SZ) and 190 healthy controls and found that male sex and older age were independent risk factors for HHcy in patients with SZ. This sex-related difference may have been caused by genetic factors or hormone levels. Similarly, our study showed the HHcy group to be older than the control group, but not significantly.
In addition to sex and age, the HHcy group showed a higher BMI. Kittisak Thawnashom [42] included 149 Thai overweight/obese and 113 control participants to analyze the association between the MTHFR C677T polymorphism and plasma Hcy, fol, and vitamin B12 concentrations. They found that the overweight/obese group had higher Hcy levels than the control group. Furthermore, Gallistl [43] enrolled 84 children and adolescents to assess the association among plasma Mg concentration, the MTHFR C677T mutation, and metabolic risk factors for coronary heart disease (CHD). Their results also showed that after adjusting for age and sex, Hcy levels were significantly correlated with BMI.
The prevalence of MTHFR C677T mutation was significantly higher and that of MTHFR A1298C was lower in our study than in Boyi Yang’s [44] study. Additionally, there was little difference in the prevalence of MTRR A66G between the two studies. It should be noted that the Hcy levels in MTHFR 1298CC homozygous mutants were significantly lower than that in the wild-type 1298AA. Similar to the results from Oliveira’s study [45], the Hcy concentration in the MTHFR 1298 CC group decreased by 10% in males and by 5% in females, compared to that in the 1298AA group. In addition, we obtained the same result as Zappacosta [46]: the Hcy value was higher in the wild-type (1298 AA) group than in the homozygote mutation (1298 CC) group. In our study, although the MTHFR TT polymorphism significantly contributed to an increase in Hcy concentration, there were still many individuals with MTHFR TT in the group with normal Hcy, which should be reflected in the real value of gene detection and the interaction between gene and environment. Individuals with gene mutations should pay special attention to their daily diet and even take supplements to obtain healthy levels of vitamin B, especially at a young age. This way, they could avoid having high blood Hcy levels for several years without knowing, which can eventually lead to the occurrence of disease. Therefore, to reduce the incidence of HHcy-related diseases, nutritional and lifestyle improvements should be implemented in young children with genetic mutations.
In addition to the gene mutations mentioned above, micronutrients and vitamins play a basic role in regulating the metabolism of Hcy as enzyme cofactors. Our results showed that Zn, which is essential for the activity of many enzymes as an intracellular ion, was present in higher levels in the HHcy group than in the control group. Methionine synthase (MTR) and BHMT are Zn-dependent methyltransferases that participate in the remethylation of Hcy [47]. The high Zn content in the HHcy group in this study may be because the diet of the patients was mainly composed of meat containing high levels of purine and Zn. In their mother–infant pair-based study, Dilli Det [48] enrolled 108 newborns with CHD and 103 healthy newborns. They found that high levels of Hcy and Zn with low levels of vitamin D might be involved in the pathogenesis of CHD. Hector Vázquez-Lorente [49] recruited 51 healthy postmenopausal woman volunteers to take a 50 mg/day Zn supplement or placebo for eight weeks to assess the effect of Zn on plasma Hcy concentration. They confirmed that Zn supplementation enhanced plasma fol and Hcy levels.
There are relatively few studies on Zn and HHcy and examining the dose–response relationship or threshold effect between them. Regardless, the problem of excess Zn has been studied extensively in recent years. Abigail-Podany [50] used neonatal mice to test the effects of excess dietary Zn on intestinal function and host—microbe interactions during early life. The authors found that excess Zn in the diet causes oxidative stress, increases the number of cupped cells and mucus production, and is associated with increased intestinal permeability and systemic inflammation. Panpan-He [51] used data from 16,257 participants from the China Health and Nutrition Survey (CHNS). These individuals were free of diabetes, but during follow-up (median duration of 9.0 years), 1097 participants developed new-onset diabetes. The authors later analyzed the dietary Zn intake and the risk of diabetes onset and found a U-shaped relationship between dietary Zn and diabetes incidence in Chinese adults, with a breakpoint of approximately 9.1 mg/day. As dietary Zn is the main source of Zn in the body, the results of our study also support the idea that Zn intake is manageable and that excessive Zn intake can cause disease. Moreover, Wolfgang Maret [52] showed that high intakes of Zn can cause Cu deficiency and that the current assumed range between safe and unsafe Zn intake is relatively narrow.
This study has several strengths. First, the samples were randomly selected from a representative clinical hospital in Northeast China with a complete quality control system in the clinical laboratory, which could ensure the comparability and stability of the detection of various nutrients. Second, the interaction between genes and nutrients and HHcy is relatively little-studied in China, which is of great significance for disease prevention. Third, we comprehensively analyzed the relationship between plasma Zn concentration and HHcy and their dose-effect and threshold-effect relationships. In the logistic regression and RCS models, we considered several covariates, including gene polymorphisms, to obtain accurate and robust results.
Nevertheless, our study has several limitations. As we considered multiple factors in the study, a larger sample size is needed to validate our findings. In addition, we could not establish a generalized causal relationship between nutrients and HHcy risk among the Chinese population, justifying a more randomized controlled trial studying plasma Zn concentration and HHcy.
5. Conclusions
In summary, the HHcy group had more males and a higher MTHFR 677TT proportion, higher BMI, and higher UA, Zn, Fe, vitamin A, and P levels. We also found a positive association between plasma Zn levels and HHcy in patients in Northeast China. The risk of HHcy was reduced with decreasing plasma Zn concentration, and the threshold value was 83.89 µmol/L. Our results highlight that individuals residing in Northeast China, especially those with the MTHFR 677TT polymorphism, should monitor their plasma Zn and Hcy levels.
Author Contributions
Conceptualization, C.S. and D.D.; methodology, C.S.; software, Z.W.; validation, J.K. and C.S.; formal analysis, D.D.; investigation, C.S.; resources, C.S.; data curation, C.S.; writing—original draft, D.D.; writing—review and editing, J.K. and C.Z.; visualization, C.Z.; supervision, J.K.; project administration, C.S. and D.D.; funding acquisition, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (82073545, 81570811), the Strategic emerging disciplines program of China Medical University in 2023, grant number 3110230017, and the Clinical project support plan in Shengjing Hospital in 2019, grant number M0465.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Shengjing Hospital of China Medical University, China, which had been registered with a trial number ChiCTR1900025136.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank all participants that took part in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alam, S.F.; Kumar, S.; Ganguly, P. Measurement of homocysteine: A historical perspective. J. Clin. Biochem. Nutr. 2019, 65, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, X.; Kong, W. Hyperhomocysteinaemia and vascular injury: Advances in mechanisms and drug targets. Br. J. Pharmacol. 2018, 175, 1173–1189. [Google Scholar] [CrossRef] [PubMed]
- Fezeu, L.K.; Ducros, V.; Guéant, J.L.; Guilland, J.C.; Andreeva, V.A.; Hercberg, S.; Galan, P. MTHFR 677C → T genotype modulates the effect of a 5-year supplementation with B-vitamins on homocysteine concentration: The SU.FOL.OM3 randomized controlled trial. PLoS ONE 2018, 13, e0193352. [Google Scholar] [CrossRef] [PubMed]
- Zaric, B.L.; Obradovic, M.; Bajic, V.; Haidara, M.A.; Jovanovic, M.; Isenovic, E.R. Homocysteine and Hyperhomocysteinaemia. Curr. Med. Chem. 2019, 26, 2948–2961. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.C.; Gupta, E.D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: Epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 2015, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Panini, R.; Verlato, C.; Scarpetta, G.; Salvioli, G. Hyperhomocysteinemia and related factors in 600 hospitalized elderly subjects. Metab. Clin. Exp. 2001, 50, 1466–1471. [Google Scholar] [CrossRef]
- Maron, B.A.; Loscalzo, J. The treatment of hyperhomocysteinemia. Annu. Rev. Med. 2009, 60, 39–54. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Zhao, Q.F.; Li, J.Q.; Wang, J.; Yu, J.T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Sakamoto, W.; Isomura, H.; Fujie, K.; Deyama, Y.; Kato, A.; Nishihira, J.; Izumi, H. Homocysteine attenuates the expression of osteocalcin but enhances osteopontin in MC3T3-E1 preosteoblastic cells. Biochim. Biophys. Acta 2005, 1740, 12–16. [Google Scholar] [CrossRef]
- Price, B.R.; Wilcock, D.M.; Weekman, E.M. Hyperhomocysteinemia as a Risk Factor for Vascular Contributions to Cognitive Impairment and Dementia. Front. Aging Neurosci. 2018, 10, 350. [Google Scholar] [CrossRef]
- Födinger, M.; Buchmayer, H.; Sunder-Plassmann, G. Molecular genetics of homocysteine metabolism. Miner. Electrolyte Metab. 1999, 25, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.; den Heijer, M.; Kluijtmans, L.A.; van den Heuvel, L.P.; et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Moll, S.; Varga, E.A. Homocysteine and MTHFR Mutations. Circulation 2015, 132, e6–e9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Hou, J.; Wu, H.; Zhong, M. Analysis of genetic polymorphism of methylenetetrahydrofolate reductase in a large ethnic Hakka population in southern China. Medicine 2018, 97, e13332. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.S.; Shyu, H.Y.; Shieh, J.C.; Fu, Y.P.; Chin, T.Y.; Wang, H.W.; Cheng, C.W. Association of MTHFR, MTR, and MTRR polymorphisms with Parkinson’s disease among ethnic Chinese in Taiwan. Clin. Chim. Acta Int. J. Clin. Chem. 2011, 412, 332–338. [Google Scholar] [CrossRef]
- Lajin, B.; Alhaj Sakur, A.; Michati, R.; Alachkar, A. Association between MTHFR C677T and A1298C, and MTRR A66G polymorphisms and susceptibility to schizophrenia in a Syrian study cohort. Asian J. Psychiatry 2012, 5, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.F.; Karakus, N.; Ulusoy, A.N.; Özaslan, C.; Kara, N. Association of the MTHFR Gene C677T Polymorphism with Breast Cancer in a Turkish Population. Oncol. Res. Treat. 2016, 39, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Silva, A.; Figuera, L.E.; Soto-Quintana, O.M.; Puebla-Pérez, A.M.; Ramírez-Patiño, R.; Gutiérrez-Hurtado, I.; Carrillo-Moreno, D.I.; Zúñiga-González, G.M.; Dávalos-Rodríguez, I.P.; Gallegos-Arreola, M.P. Association of the C677T polymorphism in the methylenetetrahydrofolate reductase gene with breast cancer in a Mexican population. Genet. Mol. Res. GMR 2015, 14, 4015–4026. [Google Scholar] [CrossRef]
- Couto, F.D.; Adorno, E.V.; Menezes, J.F.; Moura Neto, J.P.; Rêgo, M.A.; Reis, M.G.; Gonçalves, M.S. C677T polymorphism of the MTHFR gene and variant hemoglobins: A study in newborns from Salvador, Bahia, Brazil. Cad. De Saude Publica 2004, 20, 529–533. [Google Scholar] [CrossRef]
- Ford, T.C.; Downey, L.A.; Simpson, T. The Effect of a High-Dose Vitamin B Multivitamin Supplement on the Relationship between Brain Metabolism and Blood Biomarkers of Oxidative Stress: A Randomized Control Trial. Nutrients 2018, 10, 1860. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica Fate Foreign Compd. Biol. Syst. 2014, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayyari, N.; Al-Zeidaneen, S.; Hailat, R.; Hamadneh, J. Vitamin D(3) prevents cardiovascular diseases by lowering serum total homocysteine concentrations in overweight reproductive women: A randomized, placebo-controlled clinical trial. Nutr. Res. 2018, 59, 65–71. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, X.; Li, T.; He, J.; Huang, L.; Ouyang, Z.; He, L.; Wei, T.; He, Q. Improved Sp1 and Betaine Homocysteine-S-Methyltransferase Expression and Homocysteine Clearance Are Involved in the Effects of Zinc on Oxidative Stress in High-Fat-Diet-Pretreated Mice. Biol. Trace Elem. Res. 2018, 184, 436–441. [Google Scholar] [CrossRef]
- Baggott, J.E.; Tamura, T. Homocysteine, iron and cardiovascular disease: A hypothesis. Nutrients 2015, 7, 1108–1118. [Google Scholar] [CrossRef]
- Józefczuk, J.; Kasprzycka, W.; Czarnecki, R.; Graczyk, A.; Józefczuk, P.; Magda, K.; Lampart, U. Homocysteine as a Diagnostic and Etiopathogenic Factor in Children with Autism Spectrum Disorder. J. Med. Food 2017, 20, 744–749. [Google Scholar] [CrossRef]
- Dragan, S.; Buleu, F.; Christodorescu, R.; Cobzariu, F.; Iurciuc, S.; Velimirovici, D.; Xiao, J.; Luca, C.T. Benefits of multiple micronutrient supplementation in heart failure: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 965–981. [Google Scholar] [CrossRef]
- Houston, M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J. Cardiol. 2014, 6, 38–66. [Google Scholar] [CrossRef]
- Matsui, S.; Hiraishi, C.; Sato, R.; Kojima, T.; Ando, K.; Fujimoto, K.; Yoshida, H. Associations of Homocysteine with B Vitamins and Zinc in Serum Levels of Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study. J. Nutr. Sci. Vitaminol. 2021, 67, 417–423. [Google Scholar] [CrossRef]
- Heidarian, E.; Amini, M.; Parham, M.; Aminorroaya, A. Effect of zinc supplementation on serum homocysteine in type 2 diabetic patients with microalbuminuria. Rev. Diabet. Stud. 2009, 6, 64–70. [Google Scholar] [CrossRef]
- Ducros, V.; Andriollo-Sanchez, M.; Arnaud, J.; Meunier, N.; Laporte, F.; Hininger-Favier, I.; Coudray, C.; Ferry, M.; Roussel, A.M. Zinc supplementation does not alter plasma homocysteine, vitamin B12 and red blood cell folate concentrations in French elderly subjects. J. Trace Elem. Med. Biol. 2009, 23, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhen, B.; Sun, J.; Ouyang, J.; Na, N. Detection of glutathione, cysteine, and homocysteine by online derivatization-based electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2022, 36, e9291. [Google Scholar] [CrossRef] [PubMed]
- Słowiński, D.; Świerczyńska, M.; Romański, J.; Podsiadły, R. HPLC Study of Product Formed in the Reaction of NBD-Derived Fluorescent Probe with Hydrogen Sulfide, Cysteine, N-acetylcysteine, and Glutathione. Molecules 2022, 27, 8305. [Google Scholar] [CrossRef] [PubMed]
- Komarova, T.; McKeating, D.; Perkins, A.V.; Tinggi, U. Trace Element Analysis in Whole Blood and Plasma for Reference Levels in a Selected Queensland Population, Australia. Int. J. Environ. Res. Public Health 2021, 18, 2652. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, M.; Deng, Y.; Qiu, J.; Zhang, X.; Tan, J. Associations of toxic and essential trace elements in serum, follicular fluid, and seminal plasma with In vitro fertilization outcomes. Ecotoxicol. Environ. Saf. 2020, 204, 110965. [Google Scholar] [CrossRef]
- Stokes, C.S.; Lammert, F.; Volmer, D.A. Analytical Methods for Quantification of Vitamin D and Implications for Research and Clinical Practice. Anticancer Res. 2018, 38, 1137–1144. [Google Scholar] [CrossRef]
- Lin, H.; Ding, L.; Zhang, B.; Huang, J. Detection of nitrite based on fluorescent carbon dots by the hydrothermal method with folic acid. R. Soc. Open Sci. 2018, 5, 172149. [Google Scholar] [CrossRef]
- Ma, F.; Zhou, X.; Li, Q.; Zhao, J.; Song, A.; An, P.; Du, Y.; Xu, W.; Huang, G. Effects of Folic Acid and Vitamin B12, Alone and in Combination on Cognitive Function and Inflammatory Factors in the Elderly with Mild Cognitive Impairment: A Single-blind Experimental Design. Curr. Alzheimer Res. 2019, 16, 622–632. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, W.; Sa, R.; Liu, F. Prevalence and Risk Factors of Hypertension, Diabetes, and Dyslipidemia among Adults in Northwest China. Int. J. Hypertens. 2021, 2021, 5528007. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Xiong, Z.; Yao, X.; Zhang, Y.; Ning, X.; Zhong, Y.; Liu, Z.; Zhang, Y.; Zhao, T.; et al. Prevalence and clinical demography of hyperhomocysteinemia in Han Chinese patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Thawnashom, K.; Tungtrongchitr, R.; Petmitr, S.; Pongpaew, P.; Phonrat, B.; Tungtrongchitr, A.; Schelp, F.P. Methylenetetrahydrofolate reductase (MTHFR) polymorphism (C677T) in relation to homocysteine concentration in overweight and obese Thais. Southeast Asian J. Trop. Med. Public Health 2005, 36, 459–466. [Google Scholar] [PubMed]
- Gallistl, S.; Sudi, K.; Mangge, H.; Erwa, W.; Borkenstein, M. Insulin is an independent correlate of plasma homocysteine levels in obese children and adolescents. Diabetes Care 2000, 23, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, Y.; Li, Y.; Fan, S.; Zhi, X.; Lu, X.; Wang, D.; Zheng, Q.; Wang, Y.; Wang, Y.; et al. Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in China: Findings from 15357 adults of Han nationality. PLoS ONE 2013, 8, e57917. [Google Scholar] [CrossRef]
- Oliveira, I.O.; Silva, L.P.; Borges, M.C.; Cruz, O.M.; Tessmann, J.W.; Motta, J.V.; Seixas, F.K.; Horta, B.L.; Gigante, D.P. Interactions between lifestyle and MTHFR polymorphisms on homocysteine concentrations in young adults belonging to the 1982 Pelotas Birth Cohort. Eur. J. Clin. Nutr. 2017, 71, 259–266. [Google Scholar] [CrossRef]
- Zappacosta, B.; Graziano, M.; Persichilli, S.; Di Castelnuovo, A.; Mastroiacovo, P.; Iacoviello, L. 5,10-Methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms: Genotype frequency and association with homocysteine and folate levels in middle-southern Italian adults. Cell Biochem. Funct. 2014, 32, 1–4. [Google Scholar] [CrossRef]
- Jing, M.; Rech, L.; Wu, Y.; Goltz, D.; Taylor, C.G.; House, J.D. Effects of zinc deficiency and zinc supplementation on homocysteine levels and related enzyme expression in rats. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2015, 30, 77–82. [Google Scholar] [CrossRef]
- Dilli, D.; Dogan, N.N.; Orun, U.A.; Koc, M.; Zenciroglu, A.; Karademir, S.; Akduman, H. Maternal and neonatal micronutrient levels in newborns with CHD. Cardiol. Young 2018, 28, 523–529. [Google Scholar] [CrossRef]
- Vázquez-Lorente, H.; Herrera-Quintana, L.; Molina-López, J.; Gamarra, Y.; Planells, E. Effect of zinc supplementation on circulating concentrations of homocysteine, vitamin B(12), and folate in a postmenopausal population. J. Trace Elem. Med. Biol. 2022, 71, 126942. [Google Scholar] [CrossRef]
- Maywald, M.; Wessels, I.; Rink, L. Zinc Signals and Immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef]
- He, P.; Li, H.; Liu, M.; Zhang, Z.; Zhang, Y.; Zhou, C.; Li, Q.; Liu, C.; Qin, X. U-shaped Association Between Dietary Zinc Intake and New-onset Diabetes: A Nationwide Cohort Study in China. J. Clin. Endocrinol. Metab. 2022, 107, e815–e824. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).