Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle–Brain Axis
Abstract
1. Introduction
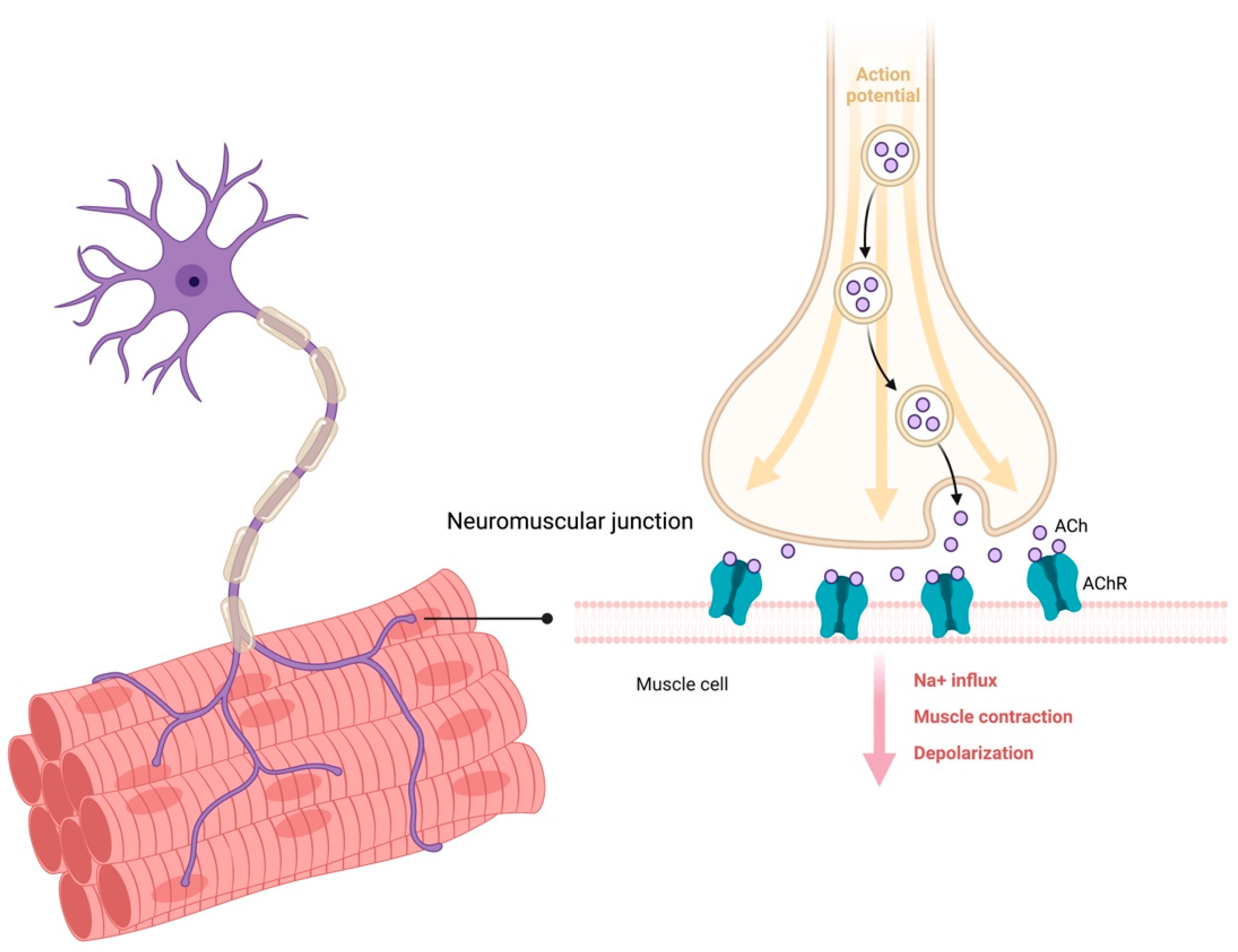
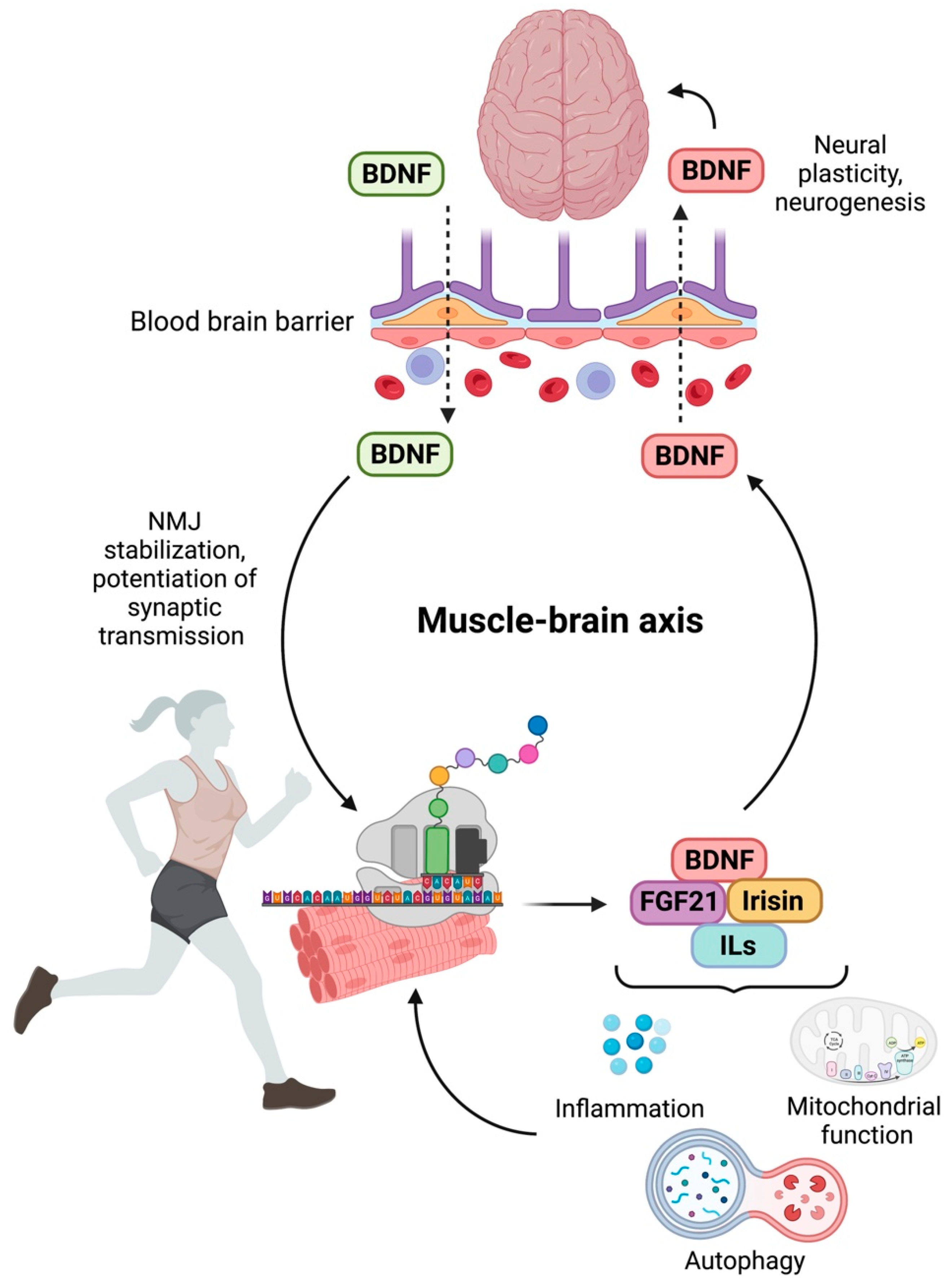
2. Molecular Mechanisms and Mediators of Muscle–Brain Crosstalk
3. Sarcopenia and Cognitive Decline in Older Adults: Myokines at the Interface of the Muscle–Brain Axis
4. Targeting the Muscle–Brain Axis: Current Strategies and Emerging Targets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short physical performance battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef]
- Suzuki, T.; Bean, J.F.; Fielding, R.A. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J. Am. Geriatr. Soc. 2001, 49, 1161–1167. [Google Scholar] [CrossRef]
- Bean, J.F.; Kiely, D.K.; Larose, S.; Goldstein, R.; Frontera, W.R.; Leveille, S.G. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? J. Am. Geriatr. Soc. 2010, 58, 2363–2368. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.G.; Bauer, J.J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Uchida, M.C.; Picca, A.; Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Marzetti, E. Evidence-based recommendations for resistance and power training to prevent frailty in community-dwellers. Aging Clin. Exp. Res. 2021, 33, 2069–2086. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Trichopoulou, A.; Panza, F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 70, 101395. [Google Scholar] [CrossRef]
- Cacciatore, S.; Calvani, R.; Marzetti, E.; Picca, A.; Coelho-Júnior, H.J.; Martone, A.M.; Massaro, C.; Tosato, M.; Landi, F. Low adherence to mediterranean diet is associated with probable sarcopenia in community-dwelling older adults: Results from the Longevity Check-Up (Lookup) 7+ Project. Nutrients 2023, 15, 1026. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International exercise recommendations in older adults (ICFSR): Expert consensus guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Martone, A.M.A.M.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Santoro, L.; Di Giorgio, A.; Nesci, A.; Sisto, A.; Santoliquido, A.; et al. Exercise and protein intake: A synergistic approach against sarcopenia. BioMed Res. Int. 2017, 2017, 2672435. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Tosato, M.; Landi, F.; Picca, A.; Marzetti, E. Protein intake and physical function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 81, 101731. [Google Scholar] [CrossRef]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef]
- Marzetti, E.; Guerra, F.; Calvani, R.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Primiano, A.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; et al. Circulating mitochondrial-derived vesicles, inflammatory biomarkers and amino acids in older adults with physical frailty and sarcopenia: A Preliminary BIOSPHERE multi-marker study using sequential and orthogonalized covariance selection–Linear discriminant analysis. Front. Cell Dev. Biol. 2020, 8, 564417. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Neves, J.; Sousa-Victor, P. Understanding muscle regenerative decline with aging: New approaches to bring back youthfulness to aged stem cells. FEBS J. 2020, 287, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Cohen-Mansfield, J.; Ferrucci, L.; Leveille, S.; Volpato, S.; De Rekeneire, N.; Guralnik, J.M. Incidence of loss of ability to walk 400 meters in a functionally limited older population. J. Am. Geriatr. Soc. 2004, 52, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Fanning, J.; Rejeski, W.J.; Chen, S.H.; Guralnik, J.; Pahor, M.; Miller, M.E. Relationships between profiles of physical activity and major mobility disability in the LIFE Study. J. Am. Geriatr. Soc. 2020, 68, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Pahor, M.; Guralnik, J.M.; Anton, S.D.; Ambrosius, W.T.; Blair, S.N.; Church, T.S.; Espeland, M.A.; Fielding, R.A.; Gill, T.M.; Glynn, N.W.; et al. Impact and lessons from the lifestyle interventions and independence for elders (LIFE) clinical trials of physical activity to prevent mobility disability. J. Am. Geriatr. Soc. 2020, 68, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Groessl, E.J.; Kaplan, R.M.; Rejeski, W.J.; Katula, J.A.; Glynn, N.W.; King, A.C.; Anton, S.D.; Walkup, M.; Lu, C.J.; Reid, K.; et al. Physical activity and performance impact long-term quality of life in older adults at risk for major mobility disability. Am. J. Prev. Med. 2019, 56, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Smith, L.; Cereda, E.; Maggi, S.; Barbagallo, M.; Dominguez, L.J.; Koyanagi, A. Multimorbidity increases the risk for sarcopenia onset: Longitudinal analyses from the English Longitudinal Study of Ageing. Exp. Gerontol. 2021, 156, 111624. [Google Scholar] [CrossRef]
- Lim, H.-S.; Park, Y.-H.; Suh, K.; Yoo, M.H.; Park, H.K.; Kim, H.J.; Lee, J.-H.; Byun, D.-W. Association between sarcopenia, sarcopenic obesity, and chronic disease in Korean elderly. J. Bone Metab. 2018, 25, 187–193. [Google Scholar] [CrossRef]
- Casati, M.; Costa, A.S.; Capitanio, D.; Ponzoni, L.; Ferri, E.; Agostini, S.; Lori, E. The biological foundations of sarcopenia: Established and promising markers. Front. Med. 2019, 6, 184. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The neuromuscular junction: Aging at the crossroad between nerves and muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Coelho-Junior, H.J.; Cesari, M.; Bossola, M.; et al. Identification of biomarkers for physical frailty and sarcopenia through a new multi-marker approach: Results from the BIOSPHERE study. GeroScience 2020, 43, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Corsetti, G.; Aquilani, R.; Romano, C.; Picca, A.; Calvani, R.; Dioguardi, F.S. Protein-amino acid metabolism disarrangements: The hidden enemy of chronic age-related conditions. Nutrients 2018, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Scisciola, L.; Fontanella, R.A.; Surina; Cataldo, V.; Paolisso, G.; Barbieri, M. Sarcopenia and cognitive function: Role of myokines in muscle brain cross-talk. Life 2021, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Baehr, L.M.; Hughes, D.C.; Waddell, D.S.; Bodine, S.C. SnapShot: Skeletal muscle atrophy. Cell 2022, 185, 1618–1618.e1. [Google Scholar] [CrossRef]
- Philp, A.; Hamilton, D.L.; Baar, K. Highlighted topic signals mediating skeletal muscle remodeling by activity signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J. Appl. Physiol. 2011, 110, 561–568. [Google Scholar] [CrossRef]
- Castro, R.; Taetzsch, T.; Vaughan, S.K.; Godbe, K.; Chappell, J.; Settlage, R.E.; Valdez, G. Specific labeling of synaptic schwann cells reveals unique cellular and molecular features. eLife 2020, 9, e56935. [Google Scholar] [CrossRef]
- Feng, Z.; Ko, C.P. Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-beta1. J. Neurosci. 2008, 28, 9599–9609. [Google Scholar] [CrossRef]
- Sugiura, Y.; Lin, W. Neuron-glia interactions: The roles of Schwann cells in neuromuscular synapse formation and function. Biosci. Rep. 2011, 31, 295–302. [Google Scholar] [CrossRef]
- Court, F.A.; Gillingwater, T.H.; Melrose, S.; Sherman, D.L.; Greenshields, K.N.; Morton, A.J.; Harris, J.B.; Willison, H.J.; Ribchester, R.R. Identity, developmental restriction and reactivity of extralaminar cells capping mammalian neuromuscular junctions. J. Cell Sci. 2008, 121, 3901–3911. [Google Scholar] [CrossRef]
- Hickling, J.K.; Fenton, C.M.; Howland, K.; Marsh, S.G.E.; Rothbard, J.B. Peptides recognized by class I restricted T cells also bind to MHC class II molecules. Int. Immunol. 1990, 2, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; De Vito, G.; Narici, M.; Boreham, C. Neuromuscular junction aging: A role for biomarkers and exercise. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Lepore, E.; Casola, I.; Dobrowolny, G.; Musarò, A. Neuromuscular junction as an entity of nerve-muscle communication. Cells 2019, 8, 906. [Google Scholar] [CrossRef] [PubMed]
- Siparsky, P.N.; Kirkendall, D.T.; Garrett, W.E. Muscle changes in aging: Understanding sarcopenia. Sports Health 2014, 6, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Vandervoort, A.A.; Symons, T.B. Functional and metabolic consequences of sarcopenia. Can. J. Appl. Physiol. 2001, 26, 90–101. [Google Scholar] [CrossRef]
- Clark, B.C. Neuromuscular changes with aging and sarcopenia. J. Frailty Aging 2019, 8, 7–9. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Baker, B.A. Efficacy of age-specific high-intensity stretch-shortening contractions in reversing dynapenia, sarcopenia, and loss of skeletal muscle quality. J. Funct. Morphol. Kinesiol. 2018, 3, 36. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M. Sarcopenia =/= dynapenia. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 829–834. [Google Scholar] [CrossRef]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Stewart Coats, A.J.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. European working group on sarcopenia in older people sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Yazar, T.; Olgun Yazar, H. Prevalance of sarcopenia according to decade. Clin. Nutr. ESPEN 2019, 29, 137–141. [Google Scholar] [CrossRef]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Pasco, J.A. Skeletal muscle health and cognitive function: A narrative review. Int. J. Mol. Sci. 2020, 22, 255. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, R.; Khan, M.M.; Witzemann, V. Motor endplate-anatomical, functional, and molecular concepts in the historical perspective. Cells 2019, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Mège, R.M.; Goudou, D.; Giaume, C.; Nicolet, M.; Rieger, F. Is intercellular communication via gap junctions required for myoblast fusion? Cell Adhes. Commun. 1994, 2, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol. Rev. 1996, 76, 371–423. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Lorenzi, M.; Martone, A.M.; Tosato, M.; Drey, M.; D’Angelo, E.; Capoluongo, E.; Russo, A.; Bernabei, R.; et al. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community-dwellers: Results from the ilSIRENTE study. Exp. Gerontol. 2016, 79, 31–36. [Google Scholar] [CrossRef]
- Shigemoto, K.; Kubo, S.; Mori, S.; Yamada, S.; Akiyoshi, T.; Miyazaki, T. Muscle weakness and neuromuscular junctions in aging and disease. Geriatr. Gerontol. Int. 2010, 10 (Suppl. 1), S137–S147. [Google Scholar] [CrossRef]
- Bütikofer, L.; Zurlinden, A.; Bolliger, M.F.; Kunz, B.; Sonderegger, P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 2011, 25, 4378–4393. [Google Scholar] [CrossRef]
- Ibebunjo, C.; Chick, J.M.; Kendall, T.; Eash, J.K.; Li, C.; Zhang, Y.; Vickers, C.; Wu, Z.; Clarke, B.A.; Shi, J.; et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol. Cell. Biol. 2013, 33, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Dalkin, W.; Taetzsch, T.; Valdez, G. The fibular nerve injury method: A reliable assay to identify and test factors that repair neuromuscular junctions. J. Vis. Exp. 2016, 114, 54186. [Google Scholar] [CrossRef]
- Vainshtein, A.; Sandri, M. Signaling pathways that control muscle mass. Int. J. Mol. Sci. 2020, 21, 4759. [Google Scholar] [CrossRef] [PubMed]
- Ham, D.J.; Börsch, A.; Lin, S.; Thürkauf, M.; Weihrauch, M.; Reinhard, J.R.; Delezie, J.; Battilana, F.; Wang, X.; Kaiser, M.S.; et al. The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia. Nat. Commun. 2020, 11, 4510. [Google Scholar] [CrossRef] [PubMed]
- Baraldo, M.; Geremia, A.; Pirazzini, M.; Nogara, L.; Solagna, F.; Türk, C.; Nolte, H.; Romanello, V.; Megighian, A.; Boncompagni, S.; et al. Skeletal muscle mTORC1 regulates neuromuscular junction stability. J. Cachexia Sarcopenia Muscle 2020, 11, 208–225. [Google Scholar] [CrossRef]
- Tang, H.; Inoki, K.; Brooks, S.V.; Okazawa, H.; Lee, M.; Wang, J.; Kim, M.; Kennedy, C.L.; Macpherson, P.C.D.; Ji, X.; et al. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell 2019, 18, e12943. [Google Scholar] [CrossRef]
- Cohen, S.; Zhai, B.; Gygi, S.P.; Goldberg, A.L. Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy. J. Cell Biol. 2012, 198, 575–589. [Google Scholar] [CrossRef]
- Mokhonova, E.I.; Avliyakulov, N.K.; Kramerova, I.; Kudryashova, E.; Haykinson, M.J.; Spencer, M.J. The E3 ubiquitin ligase TRIM32 regulates myoblast proliferation by controlling turnover of NDRG2. Hum. Mol. Genet. 2015, 24, 2873–2883. [Google Scholar] [CrossRef]
- Skoglund, E.; Grönholdt-Klein, M.; Rullman, E.; Thornell, L.E.; Strömberg, A.; Hedman, A.; Cederholm, T.; Ulfhake, B.; Gustafsson, T. Longitudinal muscle and myocellular changes in community-dwelling men over two decades of successful aging-The ULSAM cohort revisited. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 654–663. [Google Scholar] [CrossRef]
- Graziani, C.; Talocco, C.; De Sire, R.; Petito, V.; Lopetuso, L.R.; Gervasoni, J.; Persichilli, S.; Franceschi, F.; Ojetti, V.; Gasbarrini, A.; et al. Intestinal permeability in physiological and pathological conditions: Major determinants and assessment modalities. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 795–810. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M. Muscle wasting: The gut microbiota as a new therapeutic target? Int. J. Biochem. Cell. Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Lochlainn, M.N.; Bowyer, R.C.E.; Steves, C.J. Dietary protein and muscle in aging people: The potential role of the gut microbiome. Nutrients 2018, 10, 929. [Google Scholar] [CrossRef]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Sirago, G.; Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Marzetti, E. Mammalian target of rapamycin (MTOR) Signaling at the crossroad of muscle fiber fate in sarcopenia. Int. J. Mol. Sci. 2022, 23, 13823. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Picca, A.; Calvani, R.; Uchida, M.C.; Marzetti, E. If my muscle could talk: Myokines as a biomarker of frailty. Exp. Gerontol. 2019, 127, 110715. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Marini, F.; Cesari, M.; Tosato, M.; Anker, S.D.; von Haehling, S.; Miller, R.R.; Bernabei, R.; Landi, F.; Marzetti, E. SPRINTT consortium Biomarkers for physical frailty and sarcopenia: State of the science and future developments. J. Cachexia Sarcopenia Muscle 2015, 6, 278–286. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef]
- Ojima, K.; Oe, M.; Nakajima, I.; Shibata, M.; Muroya, S.; Chikuni, K.; Hattori, A.; Nishimura, T. The importance of subfragment 2 and C-terminus of myosin heavy chain for thick filament assembly in skeletal muscle cells. Anim. Sci. J. 2015, 86, 459–467. [Google Scholar] [CrossRef]
- Briana, D.D.; Malamitsi-Puchner, A. Developmental origins of adult health and disease: The metabolic role of BDNF from early life to adulthood. Metabolism 2018, 81, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Åström, M.B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef] [PubMed]
- Hempstead, B.L.; Martin-Zanca, D.; Kaplan, D.R.; Parada, L.F.; Chao, M.V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature 1991, 350, 678–683. [Google Scholar] [CrossRef]
- Moreira-Pais, A.; Ferreira, R.; Oliveira, P.A.; Duarte, J.A. A neuromuscular perspective of sarcopenia pathogenesis: Deciphering the signaling pathways involved. GeroScience 2022, 44, 1199–1213. [Google Scholar] [CrossRef]
- Colombo, E.; Bedogni, F.; Lorenzetti, I.; Landsberger, N.; Previtali, S.C.; Farina, C. Autocrine and immune cell-derived BDNF in human skeletal muscle: Implications for myogenesis and tissue regeneration. J. Pathol. 2013, 231, 190–198. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J. Biomed. Biotechnol. 2011, 2011, 201696. [Google Scholar] [CrossRef]
- Leßmann, V.; Brigadski, T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 2009, 65, 11–22. [Google Scholar] [CrossRef]
- Lu, B. Pro-region of neurotrophins: Role in synaptic modulation. Neuron 2003, 39, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Aby, K.; Antony, R.; Eichholz, M.; Srinivasan, R.; Li, Y. Enhanced pro-BDNF-p75NTR pathway activity in denervated skeletal muscle. Life Sci. 2021, 286, 120067. [Google Scholar] [CrossRef]
- Diniz, C.R.A.F.; Casarotto, P.C.; Resstel, L.; Joca, S.R.L. Beyond good and evil: A putative continuum-sorting hypothesis for the functional role of proBDNF/BDNF-propeptide/mBDNF in antidepressant treatment. Neurosci. Biobehav. Rev. 2018, 90, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Matti, U.; Nehring, R.B.; Binz, T.; Rettig, J.; Neher, E.; Sørensen, J.B. Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment. J. Neurosci. 2002, 22, 9278–9286. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; Feregalli, B.; Togni, S.; Cornelli, U.; Giacomelli, L.; Eggenhoffner, R.; Belcaro, G. A novel phospholipid delivery system of curcumin (Meriva®) preserves muscular mass in healthy aging subjects. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 762–766. [Google Scholar] [PubMed]
- Abdelmeguid, N.E.; Hammad, T.M.; Abdel-Moneim, A.M.; Salam, S.A. Effect of epigallocatechin-3-gallate on stress-induced depression in a mouse model: Role of interleukin-1β and brain-derived neurotrophic factor. Neurochem. Res. 2022, 47, 3464–3475. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, Y.; Botchway, B.O.A.; Zhang, J.; Fan, R.; Zhang, Y.; Liu, X. Curcumin can improve Parkinson’s disease via activating BDNF/PI3k/Akt signaling pathways. Food Chem. Toxicol. 2022, 164, 113091. [Google Scholar] [CrossRef]
- Sechi, S.; Chiavolelli, F.; Spissu, N.; Di Cerbo, A.; Canello, S.; Guidetti, G.; Fiore, F.; Cocco, R. An antioxidant dietary supplement improves brain-derived neurotrophic factor levels in serum of aged dogs: Preliminary results. J. Vet. Med. 2015, 2015, 412501. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, Y.; Chen, H.; Meng, L.; Chen, B.; Gong, H.; Zhao, Y.; Qi, R. Resveratrol inhibits MMP3 and MMP9 expression and secretion by suppressing TLR4/NF- κ B/STAT3 activation in Ox-LDL-treated HUVECs. Oxidative Med. Cell. Longev. 2019, 2019, 9013169. [Google Scholar] [CrossRef]
- Tsantarliotou, M.P.; Lavrentiadou, S.N.; Psalla, D.A.; Margaritis, I.E.; Kritsepi, M.G.; Zervos, I.A.; Latsari, M.I.; Sapanidou, V.G.; Taitzoglou, I.A.; Sinakos, Z.M. Suppression of plasminogen activator inhibitor-1 (PAI-1) activity by crocin ameliorates lipopolysaccharide-induced thrombosis in rats. Food Chem. Toxicol. 2019, 125, 190–197. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Sirago, G.; Coelho-Junior, H.J.; Marzetti, E. Molecular routes to sarcopenia and biomarker development: Per aspera ad astra. Curr. Opin. Pharmacol. 2021, 57, 140–147. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kaneko, Y.; Sato, T.; Shimizu, S.; Kanetaka, H.; Hanyu, H. Sarcopenia and muscle functions at various stages of Alzheimer disease. Front. Neurol. 2018, 9, 710. [Google Scholar] [CrossRef]
- Szlejf, C.; Suemoto, C.K.; Brunoni, A.R.; Viana, M.C.; Moreno, A.B.; Matos, S.M.A.; Lotufo, P.A.; Benseñor, I.M. Depression is associated with sarcopenia due to low muscle strength: Results from the ELSA-Brasil study. J. Am. Med. Dir. Assoc. 2019, 20, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, C.; Reed, M.J.; Pham, T.N.; Penn, K.; Bentov, I.; Kaplan, S.J. Association of brain atrophy and masseter sarcopenia with 1-year mortality in older trauma patients. JAMA Surg. 2019, 154, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.C.; Chen, W.L.; Wu, L.W.; Chang, Y.W.; Kao, T.W. Sarcopenia and cognitive impairment: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Liu, L.K.; Chen, W.T.; Lee, W.J.; Peng, L.N.; Wang, P.N.; Chen, L.K. Cognitive function in individuals with physical frailty but without dementia or cognitive complaints: Results from the I-Lan longitudinal aging study. J. Am. Med. Dir. Assoc. 2015, 16, 899.e9–899.e16. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.L.; Blizzard, C.L.; Wood, A.G.; Thrift, A.G.; Wardill, T.; Srikanth, V.K. Longitudinal relationships between cognitive decline and gait slowing: The Tasmanian study of cognition and gait. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Roberts, R.O.; Savica, R.; Cha, R.; Drubach, D.I.; Christianson, T.; Pankratz, V.S.; Geda, Y.E.; Machulda, M.M.; Ivnik, R.J.; et al. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the Mayo Clinic study of aging. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 929–937. [Google Scholar] [CrossRef]
- Callisaya, M.L.; Beare, R.; Phan, T.G.; Blizzard, L.; Thrift, A.G.; Chen, J.; Srikanth, V.K. Brain structural change and gait decline: A longitudinal population-based study. J. Am. Geriatr. Soc. 2013, 61, 1074–1079. [Google Scholar] [CrossRef]
- Taylor, M.E.; Lasschuit, D.A.; Lord, S.R.; Delbaere, K.; Kurrle, S.E.; Mikolaizak, A.S.; Kvelde, T.; Close, J.C.T. Slow gait speed is associated with executive function decline in older people with mild to moderate dementia: A one year longitudinal study. Arch. Gerontol. Geriatr. 2017, 73, 148–153. [Google Scholar] [CrossRef]
- Watson, N.L.; Rosano, C.; Boudreau, R.M.; Simonsick, E.M.; Ferrucci, L.; Sutton-Tyrrell, K.; Hardy, S.E.; Atkinson, H.H.; Yaffe, K.; Satterfield, S.; et al. Executive function, memory, and gait speed decline in well-functioning older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1093–1100. [Google Scholar] [CrossRef]
- Steves, C.J.; Mehta, M.M.; Jackson, S.H.D.; Spector, T.D. Kicking back cognitive ageing: Leg power predicts cognitive ageing after ten years in older female twins. Gerontology 2016, 62, 138–149. [Google Scholar] [CrossRef]
- Kong, S.H.; Park, Y.J.; Lee, J.Y.; Cho, N.H.; Moon, M.K. Insulin resistance is associated with cognitive decline among older Koreans with normal baseline cognitive function: A prospective community-based cohort study. Sci. Rep. 2018, 8, 650. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.N.; Stanford, M.; Jiang, X. Low cholesterol level linked to reduced semantic fluency performance and reduced gray matter volume in the medial temporal lobe. Front. Aging Neurosci. 2020, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Law, C.K.; Lam, F.M.; Chung, R.C.; Pang, M.Y. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: A systematic review. J. Physiother. 2020, 66, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-organ crosstalk: The emerging roles of myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.Y.; Moon, S.; Park, D.H.; Kwak, H.B.; Kang, J.H. Roles of myokines in exercise-induced improvement of neuropsychiatric function. Pflugers Arch. 2019, 471, 491–505. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; You, W.; Shan, T. Myokines mediate the cross talk between skeletal muscle and other organs. J. Cell. Physiol. 2021, 236, 2393–2412. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef]
- Kramer, A.F.; Colcombe, S. Fitness effects on the cognitive function of older adults: A meta-analytic study-revisited. Perspect. Psychol. Sci. 2018, 13, 213–217. [Google Scholar] [CrossRef]
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Kim, J.S.; Alves, H.; Szabo, A.; Phillips, S.M.; Wójcicki, T.R.; Mailey, E.L.; et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain. Behav. Immun. 2013, 28, 90–99. [Google Scholar] [CrossRef]
- Vreugdenhil, A.; Cannell, J.; Davies, A.; Razay, G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: A randomized controlled trial. Scand. J. Caring Sci. 2012, 26, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Sardahaee, F.S.; Anderssen, S.; Ballard, C. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment. Health 2010, 14, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Amboni, M.; Barone, P.; Hausdorff, J.M. Cognitive contributions to gait and falls: Evidence and implications. Mov. Disord. 2013, 28, 1520–1533. [Google Scholar] [CrossRef]
- Iizuka, K.; Machida, T.; Hirafuji, M. Skeletal muscle is an endocrine organ. J. Pharmacol. Sci. 2014, 125, 125–131. [Google Scholar] [CrossRef]
- Yu, L.; Boyle, P.A.; Leurgans, S.E.; Wilson, R.S.; Bennett, D.A.; Buchman, A.S. Incident mobility disability, mild cognitive impairment, and mortality in community-dwelling older adults. Neuroepidemiology 2019, 53, 55–62. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch. Neurol. 2009, 66, 1339–1344. [Google Scholar] [CrossRef]
- Beeri, M.S.; Leugrans, S.E.; Delbono, O.; Bennett, D.A.; Buchman, A.S. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 2021, 69, 1826–1835. [Google Scholar] [CrossRef]
- Aprahamian, I.; Cipolli, G.C.; Yassuda, M.S. Sarcopenia and cognitive impairment: Possible physiopathological causation or just a spurious association? Clin. Nutr. 2020, 39, 1622. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, G.B.; Lourenco, M.V.; De Felice, F.G. Protective actions of exercise-related FNDC5/Irisin in memory and Alzheimer’s disease. J. Neurochem. 2020, 155, 602–611. [Google Scholar] [CrossRef]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef]
- Berger, M.J.; Doherty, T.J. Sarcopenia: Prevalence, mechanisms, and functional consequences. Interdiscip. Top. Gerontol. 2010, 37, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Chastin, S.F.M.; Skelton, D.A. Prevalence of sedentary behavior in older adults: A systematic review. Int. J. Environ. Res. Public Health 2013, 10, 6645–6661. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H. Effects of hindlimb unloading on neurotrophins in the rat spinal cord and soleus muscle. Brain Res. 2016, 1630, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J.; et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef]
- Zampieri, S.; Mammucari, C.; Romanello, V.; Barberi, L.; Pietrangelo, L.; Fusella, A.; Mosole, S.; Gherardi, G.; Höfer, C.; Löfler, S.; et al. Physical exercise in aging human skeletal muscle increases mitochondrial calcium uniporter expression levels and affects mitochondria dynamics. Physiol. Rep. 2016, 4, e13005. [Google Scholar] [CrossRef]
- Joseph, A.M.; Adhihetty, P.J.; Leeuwenburgh, C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J. Physiol. 2016, 594, 5105–5123. [Google Scholar] [CrossRef]
- Nishimune, H.; Stanford, J.A.; Mori, Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve 2014, 49, 315–324. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating irisin in healthy, young individuals: Day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Erden, Y.; Ozyalin, F.; Cigremis, Y.; Colak, C.; Sandal, S. The effects of intracerebroventricular infusion of irisin on feeding behaviour in rats. Neurosci. Lett. 2017, 645, 25–32. [Google Scholar] [CrossRef]
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.H.; Lyu, X.; Zushin, P.J.H.; et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022, 606, 785–790. [Google Scholar] [CrossRef]
- Martins, C.; Morgan, L.M.; Bloom, S.R.; Robertson, M.D. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007, 193, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.Y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Fujimoto, S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J. Endocrinol. 2009, 203, 357–364. [Google Scholar] [CrossRef]
- Ueda, S.Y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Nakao, H.; Fujimoto, S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J. Endocrinol. 2009, 201, 151–159. [Google Scholar] [CrossRef]
- Bates, C.J.; Prentice, A.; Cole, T.J.; Van Der Pols, J.C.; Doyle, W.; Finch, S.; Smithers, G.; Clarke, P.C. Micronutrients: Highlights and research challenges from the 1994-5 national diet and nutrition survey of people aged 65 years and over. Br. J. Nutr. 1999, 82, 7–15. [Google Scholar] [CrossRef]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musarò, A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Ageing Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef]
- Drey, M.; Sieber, C.C.; Bauer, J.M.; Uter, W.; Dahinden, P.; Fariello, R.G.; Vrijbloed, J.W. C-terminal Agrin fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp. Gerontol. 2013, 48, 76–80. [Google Scholar] [CrossRef]
- Chevalley, T.; Brandi, M.L.; Cashman, K.D.; Cavalier, E.; Harvey, N.C.; Maggi, S.; Cooper, C.; Al-Daghri, N.; Bock, O.; Bruyère, O.; et al. Role of vitamin D supplementation in the management of musculoskeletal diseases: Update from an European Society of Clinical and Economical Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) working group. Aging Clin. Exp. Res. 2022, 34, 2603–2623. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Hayhoe, R.P.G.; Luben, R.N.; Welch, A.A. Positive associations of dietary intake and plasma concentrations of vitamin E with skeletal muscle mass, heel bone ultrasound attenuation and fracture risk in the EPIC-Norfolk cohort. Antioxidants 2021, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Pilleron, S.; Weber, D.; Pérès, K.; Colpo, M.; Gomez-Cabrero, D.; Stuetz, W.; Dartigues, J.-F.; Ferrucci, L.; Bandinelli, S.; Garcia-Garcia, F.J.; et al. Patterns of circulating fat-soluble vitamins and carotenoids and risk of frailty in four European cohorts of older adults. Eur. J. Nutr. 2019, 58, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Kochlik, B.; Stuetz, W.; Pérès, K.; Pilleron, S.; Féart, C.; García García, F.J.; Bandinelli, S.; Gomez-Cabrero, D.; Rodriguez-Mañas, L.; Grune, T.; et al. Associations of fat-soluble micronutrients and redox biomarkers with frailty status in the FRAILOMIC initiative. J. Cachexia Sarcopenia Muscle 2019, 10, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Ble, A.; Cherubini, A.; Volpato, S.; Bartali, B.; Walston, J.D.; Windham, B.G.; Bandinelli, S.; Lauretani, F.; Guralnik, J.M.; Ferrucci, L. Lower plasma vitamin E levels are associated with the frailty syndrome: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low nutrient intake is an essential component of frailty in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef]
- Arosio, B.; Rossi, P.D.; Ferri, E.; Cesari, M.; Vitale, G. Characterization of vitamin D status in older persons with cognitive impairment. Nutrients 2022, 14, 1142. [Google Scholar] [CrossRef]
- Casati, M.; Boccardi, V.; Ferri, E.; Bertagnoli, L.; Bastiani, P.; Ciccone, S.; Mansi, M.; Scamosci, M.; Rossi, P.D.; Mecocci, P.; et al. Vitamin E and Alzheimer’s disease: The mediating role of cellular aging. Aging Clin. Exp. Res. 2020, 32, 459–464. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Landi, F.; Picca, A.; Marzetti, E. Protein intake and cognitive function in older adults: A systematic review and meta-analysis. Nutr. Metab. Insights 2021, 14, 11786388211022373. [Google Scholar] [CrossRef]
- Jang, J.; Koh, J.H.; Kim, Y.; Kim, H.J.; Park, S.; Chang, Y.; Jung, J.; Wolfe, R.R.; Kim, I.Y. Balanced free essential amino acids and resistance exercise training synergistically improve dexamethasone-induced impairments in muscle strength, endurance, and insulin sensitivity in mice. Int. J. Mol. Sci. 2022, 23, 9735. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Haramizu, S.; Ota, N.; Minegishi, Y.; Shimotoyodome, A. Continuous supplementation of milk fat globule membrane with habitual exercise from a young age improves motor coordination and skeletal muscle function in aged mice. J. Nutr. Sci. Vitaminol. 2019, 65, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Del Signore, S.; Anker, S.D.; Bejuit, R.; Bordes, P.; Cherubini, A.; Cruz-Jentoft, A.J.; et al. Multicomponent intervention to prevent mobility disability in frail older adults: Randomised controlled trial (SPRINTT project). BMJ 2022, 377, e068788. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Bäckman, L.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lehtisalo, J.; Levälahti, E.; et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimers. Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Li, Y.; Du, J.; Mitch, W.E.; Rosenthal, N.; Delafontaine, P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J. Clin. Investig. 2005, 115, 451–458. [Google Scholar] [CrossRef]
- Burks, T.N.; Andres-Mateos, E.; Marx, R.; Mejias, R.; Van Erp, C.; Simmers, J.L.; Walston, J.D.; Ward, C.W.; Cohn, R.D. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci. Transl. Med. 2011, 3, 82ra37. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Acuña, M.J.; Morales, M.G.; Becerra, A.; Simon, F.; Brandan, E. Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochem. Biophys. Res. Commun. 2011, 410, 665–670. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Morales, M.G.; Rivera, J.C.; Cabrera, D.; Simon, F. Renin-angiotensin system: An old player with novel functions in skeletal muscle. Med. Res. Rev. 2015, 35, 437–463. [Google Scholar] [CrossRef]
- Soto, M.E.; Abellan Van Kan, G.; Nourhashemi, F.; Gillette-Guyonnet, S.; Cesari, M.; Cantet, C.; Rolland, Y.; Vellas, B. Angiotensin-converting enzyme inhibitors and Alzheimer’s disease progression in older adults: Results from the Réseau sur la Maladie d’Alzheimer Français cohort. J. Am. Geriatr. Soc. 2013, 61, 1482–1488. [Google Scholar] [CrossRef]
- Baptista, L.C.; Zumbro, E.L.; Graham, Z.A.; Hernandez, A.R.; Buchanan, T.; Sun, Y.; Yang, Y.; Banerjee, A.; Verma, A.; Li, Q.; et al. Multi-omics profiling of the impact of an angiotensin (1-7)-expressing probiotic combined with exercise training in aged male rats. J. Appl. Physiol. (1985), 2023; Online ahead of print. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arosio, B.; Calvani, R.; Ferri, E.; Coelho-Junior, H.J.; Carandina, A.; Campanelli, F.; Ghiglieri, V.; Marzetti, E.; Picca, A. Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle–Brain Axis. Nutrients 2023, 15, 1853. https://doi.org/10.3390/nu15081853
Arosio B, Calvani R, Ferri E, Coelho-Junior HJ, Carandina A, Campanelli F, Ghiglieri V, Marzetti E, Picca A. Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle–Brain Axis. Nutrients. 2023; 15(8):1853. https://doi.org/10.3390/nu15081853
Chicago/Turabian StyleArosio, Beatrice, Riccardo Calvani, Evelyn Ferri, Hélio José Coelho-Junior, Angelica Carandina, Federica Campanelli, Veronica Ghiglieri, Emanuele Marzetti, and Anna Picca. 2023. "Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle–Brain Axis" Nutrients 15, no. 8: 1853. https://doi.org/10.3390/nu15081853
APA StyleArosio, B., Calvani, R., Ferri, E., Coelho-Junior, H. J., Carandina, A., Campanelli, F., Ghiglieri, V., Marzetti, E., & Picca, A. (2023). Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle–Brain Axis. Nutrients, 15(8), 1853. https://doi.org/10.3390/nu15081853









