Effects of Intra-Amniotic Administration of the Hydrolyzed Protein of Chia (Salvia hispanica L.) and Lacticaseibacillus paracasei on Intestinal Functionality, Morphology, and Bacterial Populations, In Vivo (Gallus gallus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Material
2.1.1. Hydrolyzed Chia protein
2.1.2. Probiotic
2.2. The Intra-Amniotic Administration
2.3. Extraction of the Total RNA from the Duodenum Tissue Samples
2.4. Real-Time Polymerase Chain Reaction (RT-PCR) and Prime Design
2.5. Real-Time qPCR Design
2.6. Collection of Microbial Samples and DNA Extraction of Intestinal Content
2.7. Primer Design and PCR Amplification of Bacterial 16S rDNA
2.8. Morphological Examination of Duodenal Tissue
2.9. Statistical Analysis
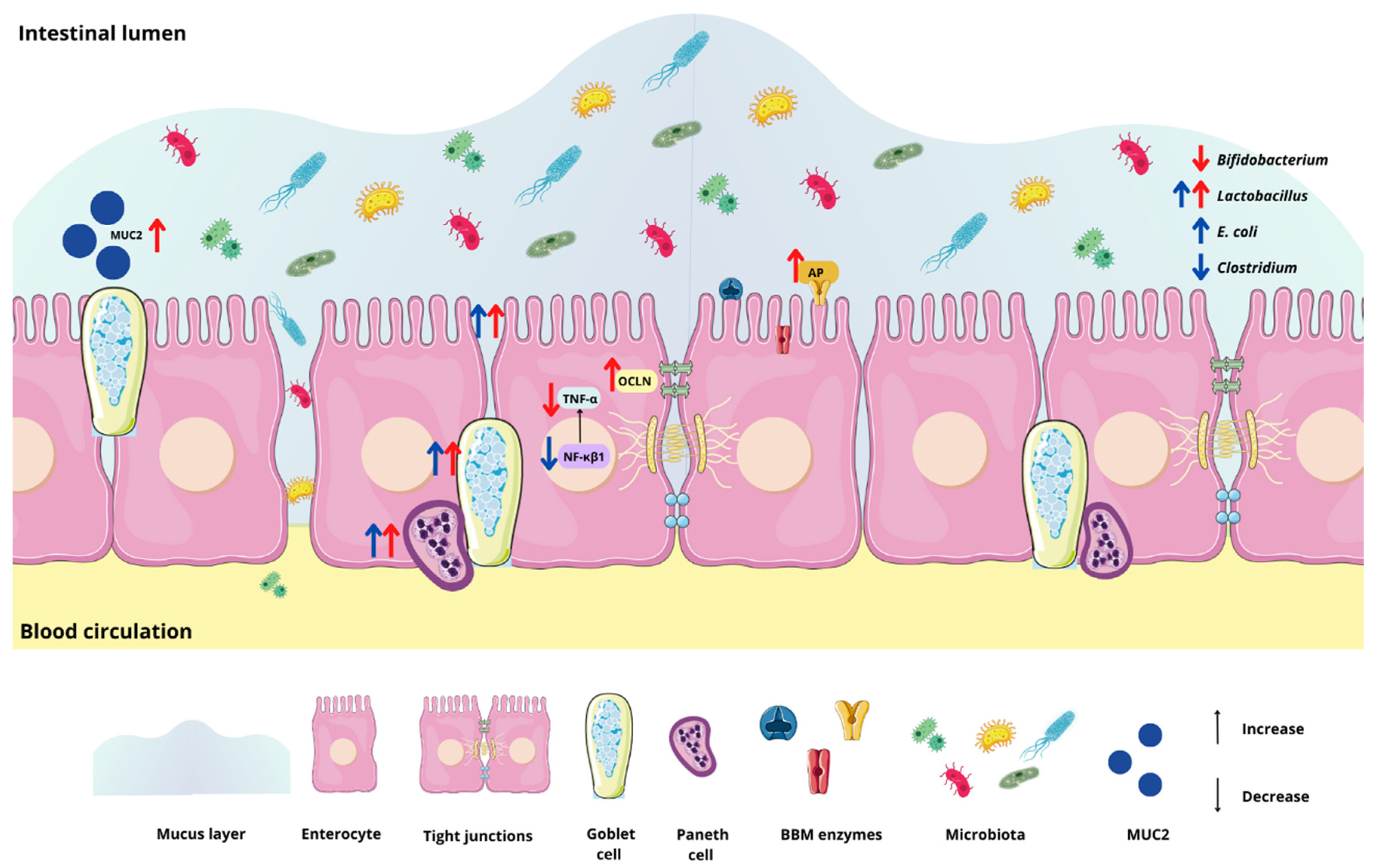
3. Results
3.1. Body Weight
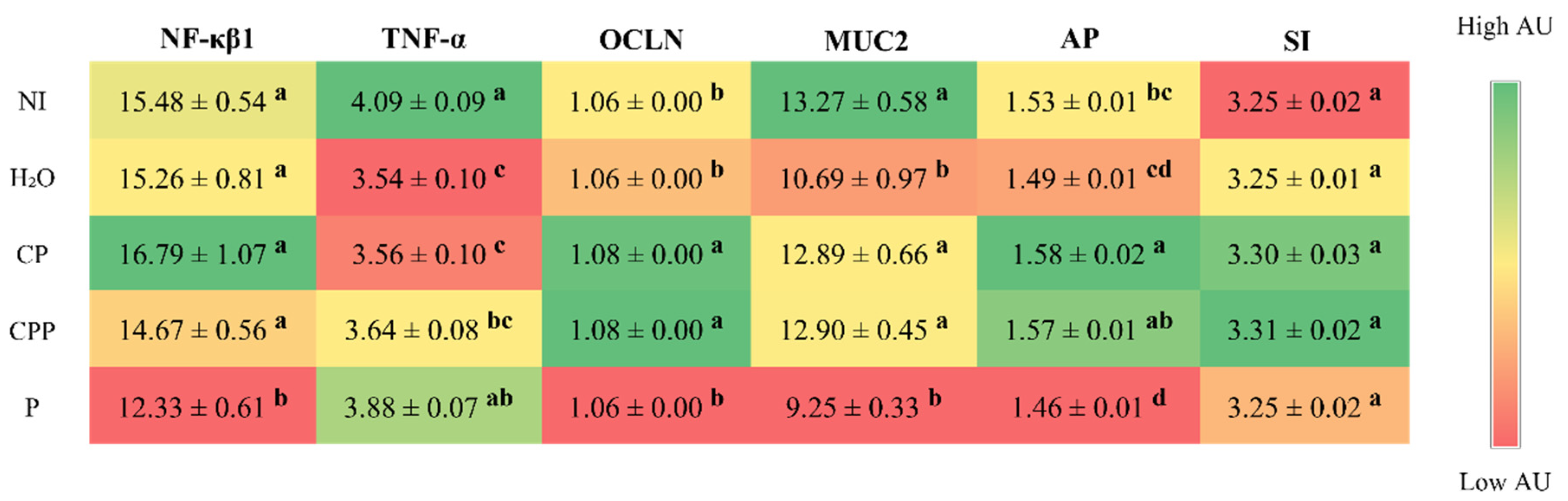
3.2. Effect of the Chia Protein and/or Probiotic on the Gene Expression of Intestinal Inflammation, Intestinal Barrier Proteins, and Brush Border Membrane Functional Proteins
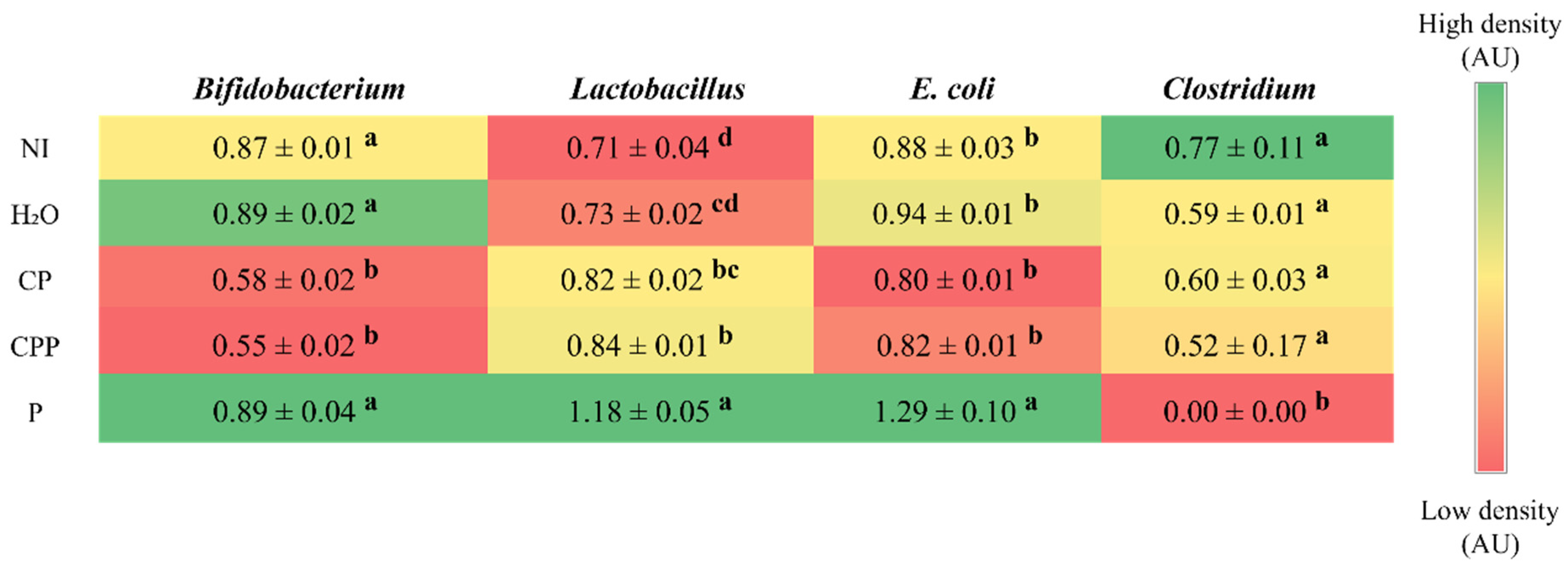
3.3. Effect of Chia Protein and/or a Probiotic on the Bacterial Population in Cecum Contents
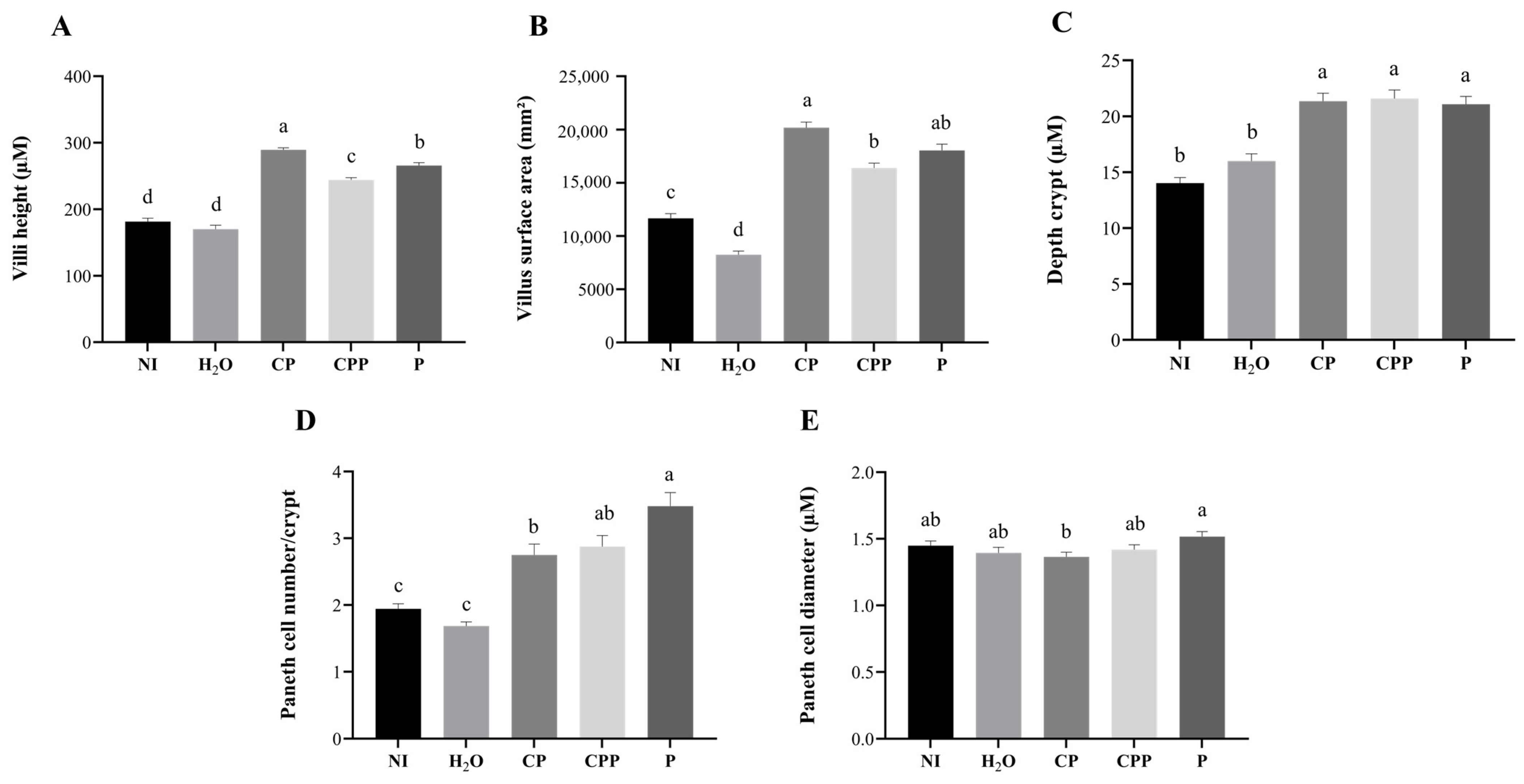
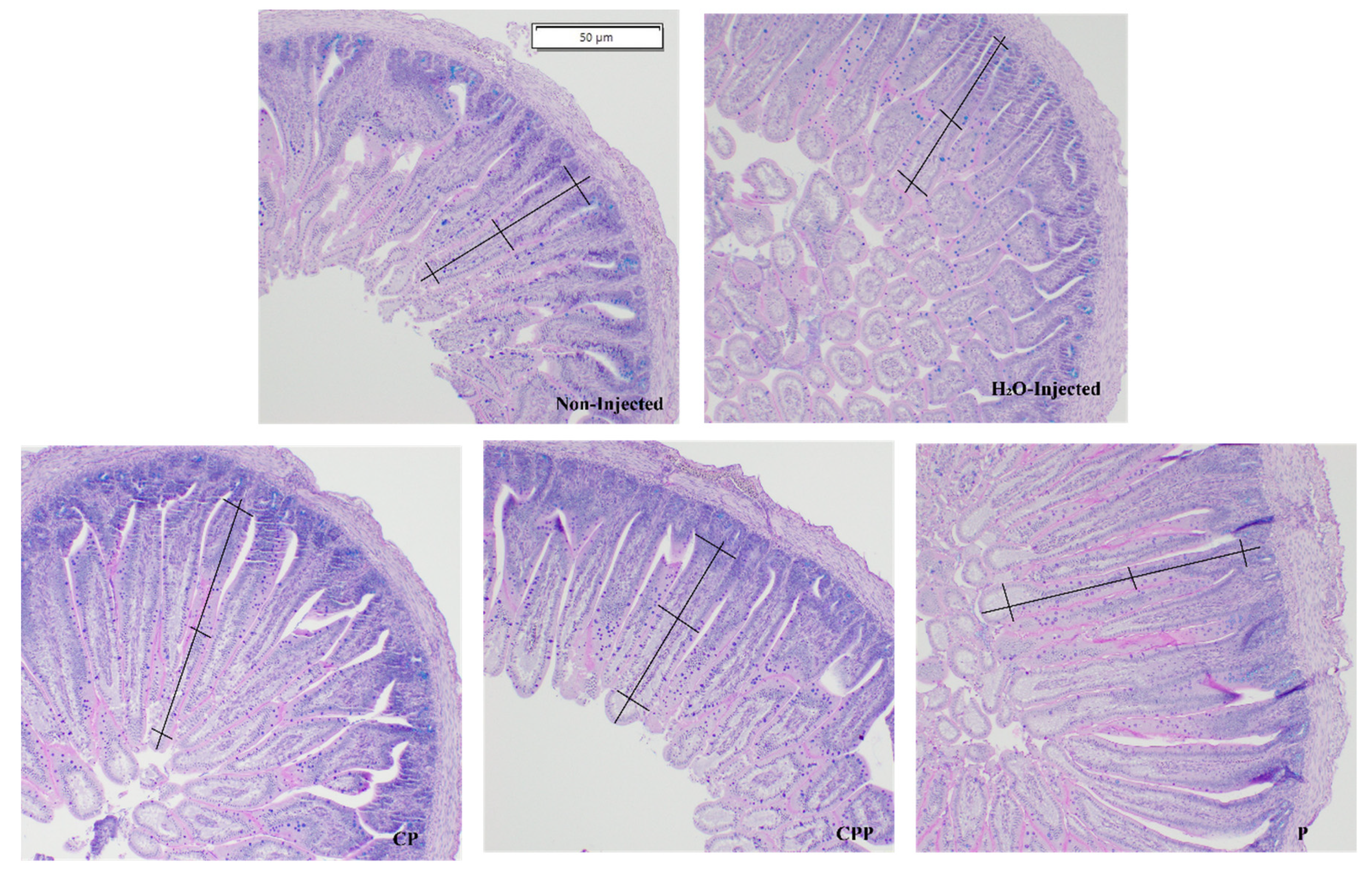
3.4. Effect of Chia Protein and/or a Probiotic on Duodenal Morphological Parameters
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enes, B.N.; Moreira, L.D.P.D.; Toledo, R.C.L.; Moraes, É.A.; de Castro Moreira, M.E.; Hermsdorff, H.H.M.; Noratto, G.; Mertens-Talcott, S.U.; Talcott, S.; Martino, H.S.D. Effect of Different Fractions of Chia (Salvia hispanica L.) on Glucose Metabolism, in Vivo and in Vitro. J. Funct. Foods 2020, 71, 157–164. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Dias, D.M.; de Castro Moreira, M.E.; Toledo, R.C.L.; da Matta, S.L.P.; Della Lucia, C.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chia Seed Shows Good Protein Quality, Hypoglycemic Effect and Improves the Lipid Profile and Liver and Intestinal Morphology of Wistar Rats. Plant Foods Hum. Nutr. 2016, 71, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Toledo, R.C.L.; Grancieri, M.; de Castro Moreira, M.E.; Medina, N.R.; Silva, R.R.; Costa, N.M.B.; Martino, H.S.D. Effects of Chia (Salvia hispanica L.) on Calcium Bioavailability and Inflammation in Wistar Rats. Food Res. Int. 2019, 116, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Toledo, R.C.L.; Mishima, M.D.V.; de Castro Moreira, M.E.; Vasconcelos, C.M.; Pereira, C.E.R.; Favarato, L.S.C.; Costa, N.M.B.; Martino, H.S.D. Effects of Chia (Salvia hispanica L.) on Oxidative Stress and Inflammation in Ovariectomized Adult Female Wistar Rats. Food Funct. 2019, 10, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.D.A.; Verneque, B.J.F.; Mota, A.P.L.; Duarte, C.K. Chia Seed (Salvia hispanica L.) Consumption and Lipid Profile: A Systematic Review and Meta-Analysis. Food Funct. 2021, 12, 8835–8849. [Google Scholar] [CrossRef]
- Vega Joubert, M.B.; Degrave, V.; Ingaramo, P.; Oliva, M.E.; D’Alessandro, M.E. Salvia hispanica L. (Chia) Seed Improves Liver Inflammation and Endothelial Dysfunction in an Experimental Model of Metabolic Syndrome. Food Funct. 2022, 13, 11249–11261. [Google Scholar] [CrossRef] [PubMed]
- Mishima, M.D.V.; Ladeira, L.C.M.; da Silva, B.P.; Toledo, R.C.L.; de Oliveira, T.V.; Costa, N.M.B.; Martino, H.S.D. Cardioprotective Action of Chia (Salvia hispanica L.) in Ovariectomized Rats Fed a High Fat Diet. Food Funct. 2021, 12, 3069–3082. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Kolba, N.; Martino, H.S.D.; Hart, J.; Tako, E. Soluble Extracts from Chia Seed (Salvia hispanica L.) Affect Brush Border Membrane Functionality, Morphology and Intestinal Bacterial Populations in Vivo (Gallus gallus). Nutrients 2019, 11, 2457. [Google Scholar] [CrossRef]
- Mishima, M.D.V.; Da Silva, B.P.; Gomes, M.J.C.; Toledo, R.C.L.; Mantovani, H.C.; de São José, V.P.B.; Costa, N.M.B.; Tako, E.; Martino, H.S.D. Effect of Chia (Salvia hispanica L.) Associated with High-Fat Diet on the Intestinal Health of Wistar Rats. Nutrients 2022, 14, 4924. [Google Scholar] [CrossRef]
- Mishima, M.D.V.; Da Silva, B.P.; Gomes, M.J.C.; Toledo, R.C.L.; Pereira, C.E.R.; Costa, N.M.B.; Martino, H.S.D. Effect of Chia Flour Associated with High Fat Diet on Intestinal Health in Female Ovariectomized Wistar Rats. Eur. J. Nutr. 2022, 62, 905–919. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Anunciação, P.C.; da Silva Matyelka, J.C.; Della Lucia, C.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chemical Composition of Brazilian Chia Seeds Grown in Different Places. Food Chem. 2017, 221, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Stampini, H.; Martino, D.; Mejia, E.G. De Chia Seed (Salvia hispanica L.) as a Source of Proteins and Bioactive Peptides with Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Stampini, H.; Martino, D.; Gonzalez, E.; Mejia, D. Digested Total Protein and Protein Fractions from Chia Seed (Salvia hispanica L.) Had High Scavenging Capacity and Inhibited 5-LOX, COX-1-2, and INOS Enzymes. Food Chem. 2019, 289, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Verediano, T.A.; Sant’Ana, C.T.; de Assis, A.; Toledo, R.L.; de Mejia, E.G.; Martino, H.S.D. Digested Protein from Chia Seed (Salvia hispanica L) Prevents Obesity and Associated Inflammation of Adipose Tissue in Mice Fed a High-Fat Diet. PharmaNutrition 2022, 21, 100298. [Google Scholar] [CrossRef]
- Hou, T.; Kolba, N.; Glahn, R.P.; Tako, E. Intra-Amniotic Administration (Gallus gallus) of Cicer Arietinum and Lens Culinaris Prebiotics Extracts and Duck Egg White Peptides Affects Calcium Status and Intestinal Functionality. Nutrients 2017, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Requena, T.; Miguel, M.; Garcés-Rimón, M.; Martínez-Cuesta, M.C.; López-Fandiño, R.; Peláez, C. Pepsin Egg White Hydrolysate Modulates Gut Microbiota in Zucker Obese Rats. Food Funct. 2017, 8, 437–443. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Liu, G.; Fang, J.; Yan, W.; Duraipandiyan, V.; Al-Dhabi, N.A.; Ali Esmail, G.; Jiang, H. Egg Protein Transferrin-Derived Peptides IRW and IQW Regulate Citrobacter Rodentium-Induced, Inflammation-Related Microbial and Metabolomic Profiles. Front. Microbiol. 2019, 10, 643. [Google Scholar] [CrossRef]
- Jiao, H.; Zhang, Q.; Lin, Y.; Gao, Y.; Zhang, P. The Ovotransferrin-Derived Peptide IRW Attenuates Lipopolysaccharide-Induced Inflammatory Responses. Biomed Res. Int. 2019, 2019, 8676410. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J. Impact of Food-Derived Bioactive Peptides on Gut Function and Health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Ballan, R.; Battistini, C.; Xavier-santos, D.; Marta, S.; Saad, I. Interactions of Probiotics and Prebiotics with the Gut Microbiota, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 171. [Google Scholar]
- Araújo, M.M.; Botelho, P.B. Probiotics, Prebiotics, and Synbiotics in Chronic Constipation: Outstanding Aspects to Be Considered for the Current Evidence. Front. Nutr. 2022, 9, 2941. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Duan, A.Y.; Ju, A.Q.; Zhang, Y.N.; Qin, Y.J.; Xue, L.G.; Ma, X.; Luan, W.M.; Yang, S.B. The Effects of In Ovo Injection of Synbiotics on the Early Growth Performance and Intestinal Health of Chicks. Front. Vet. Sci. 2021, 8, 658301. [Google Scholar] [CrossRef]
- Duar, R.M.; Lin, X.B.; Zheng, J.; Martino, M.E.; Grenier, T.; Pérez-Muñoz, M.E.; Leulier, F.; Gänzle, M.; Walter, J. Lifestyles in Transition: Evolution and Natural History of the Genus Lactobacillus. FEMS Microbiol. Rev. 2017, 41, S27–S48. [Google Scholar] [CrossRef]
- Ren, S.; Wang, C.; Chen, A.; Lv, W.; Gao, R. The Probiotic Lactobacillus Paracasei Ameliorates Diarrhea Cause by Escherichia coli O8 via Gut Microbiota Modulation1. Front. Nutr. 2022, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef]
- Hou, T.; Tako, E. The in Ovo Feeding Administration (Gallus gallus)—An Emerging in Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients 2018, 10, 418. [Google Scholar] [CrossRef]
- Reicher, N.; Melkman-Zehavi, T.; Dayan, J.; Wong, E.A.; Uni, Z. Nutritional Stimulation by In-Ovo Feeding Modulates Cellular Proliferation and Differentiation in the Small Intestinal Epithelium of Chicks. Anim. Nutr. 2022, 8, 91–101. [Google Scholar] [CrossRef]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (Direct-fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef]
- Shehata, A.M.; Paswan, V.K.; Attia, Y.A.; Abougabal, M.S.; Khamis, T.; Alqosaibi, A.I.; Alnamshan, M.M.; Elmazoudy, R.; Abaza, M.A.; Salama, E.A.A.; et al. In Ovo Inoculation of Bacillus Subtilis and Raffinose Affects Growth Performance, Cecal Microbiota, Volatile Fatty Acid, Ileal Morphology and Gene Expression, and Sustainability of Broiler Chickens (Gallus gallus). Front. Nutr. 2022, 9, 3847. [Google Scholar] [CrossRef]
- Orona-Tamayo, D.; Valverde, M.E.; Nieto-Rendón, B.; Paredes-López, O. Inhibitory Activity of Chia (Salvia hispanica L.) Protein Fractions against Angiotensin I-Converting Enzyme and Antioxidant Capacity. LWT-Food Sci. Technol. 2015, 64, 236–242. [Google Scholar] [CrossRef]
- Megías, C.; Del Mar Yust, M.; Pedroche, J.; Lquari, H.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Purification of an ACE Inhibitory Peptide after Hydrolysis of Sunflower (Helianthus annuus L.) Protein Isolates. J. Agric. Food Chem. 2004, 52, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Verediano, T.A.; Stampini Duarte Martino, H.; Kolba, N.; Fu, Y.; Cristina Dias Paes, M.; Tako, E. Black Corn (Zea mays L.) Soluble Extract Showed Anti-Inflammatory Effects and Improved the Intestinal Barrier Integrity in Vivo (Gallus gallus). Food Res. Int. 2022, 157, 111227. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.C.; Martino, H.S.D.; Tako, E. Effects of Iron and Zinc Biofortified Foods on Gut Microbiota in Vivo (Gallus gallus): A Systematic Review. Nutrients 2021, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Martino, H.S.D.; Kolba, N.; Tako, E. Yacon (Smallanthus sonchifolius) Flour Soluble Extract Improve Intestinal Bacterial Populations, Brush Border Membrane Functionality and Morphology in Vivo (Gallus gallus). Food Res. Int. 2020, 137, 109705. [Google Scholar] [CrossRef]
- Agarwal, N.; Kolba, N.; Jung, Y.; Cheng, J.; Tako, E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus gallus). Nutrients 2022, 14, 220. [Google Scholar] [CrossRef]
- Dias, D.M.; Kolba, N.; Hart, J.J.; Ma, M.; Sha, S.T.; Lakshmanan, N.; Nutti, M.R.; Martino, H.S.D.; Glahn, R.P.; Tako, E. Soluble Extracts from Carioca Beans (Phaseolus vulgaris L.) Affect the Gut Microbiota and Iron Related Brush Border Membrane Protein Expression in Vivo (Gallus gallus). Food Res. Int. 2019, 123, 172–180. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Kolba, N.; Agarwal, N.; Kim, D.; Eshel, A.; Koren, O.; Tako, E. Modifications in the Intestinal Functionality, Morphology and Microbiome Following Intra-Amniotic Administration (Gallus gallus) of Grape (Vitis vinifera) Stilbenes (Resveratrol and Pterostilbene). Nutrients 2021, 13, 3247. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Welch, R.M.; Lei, X.; Yasuda, K.; Miller, D.D. Dietary Inulin Affects the Expression of Intestinal Enterocyte Iron Transporters, Receptors and Storage Protein and Alters the Microbiota in the Pig Intestine. Br. J. Nutr. 2008, 99, 472–480. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Knez, M.; Stangoulis, J.C. The Effect of Wheat Prebiotics on the Gut Bacterial Population and Iron Status of Iron Deficient Broiler Chickens. Nutr. J. 2014, 13, 58. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-ΚB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-ΚB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; de Mejia, E.G. Chia (Salvia hispanica L.) Seed Total Protein and Protein Fractions Digests Reduce Biomarkers of Inflammation and Atherosclerosis in Macrophages In Vitro. Mol. Nutr. Food Res. 2019, 63, 1900021. [Google Scholar] [CrossRef] [PubMed]
- Yeşilyurt, N.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Involvement of Probiotics and Postbiotics in the Immune System Modulation. Biologics 2021, 1, 89–110. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a Source of Peptides with Remarkable Biological Activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Kou, X.; Gao, J.; Zhang, Z.; Wang, H.; Wang, X. Purification and Identification of Antioxidant Peptides from Chickpea (Cicer arietinum L.) Albumin Hydrolysates. LWT-Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Pedersen, J.; Coskun, M.; Soendergaard, C.; Salem, M.; Nielsen, O.H. Inflammatory Pathways of Importance for Management of Inflammatory Bowel Disease. World J. Gastroenterol. 2014, 20, 64–77. [Google Scholar] [CrossRef]
- Kaminsky, L.W.; Al-sadi, R.; Ma, T.Y. IL-1 b and the Intestinal Epithelial Tight Junction Barrier. Front. Immunol. 2021, 12, 6–9. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the Intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Kang, Y.; Park, H.; Choe, B.H.; Kang, B. The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease. Front. Med. 2022, 9, 848344. [Google Scholar] [CrossRef]
- Pawłowska, B.; Sobieszczańska, B.M. Intestinal Epithelial Barrier: The Target for Pathogenic Escherichia coli. Adv. Clin. Exp. Med. 2017, 26, 1437–1445. [Google Scholar] [CrossRef]
- Tarabova, L.; Makova, Z.; Piesova, E.; Szaboova, R.; Faixova, Z. Intestinal Mucus Layer and Mucins (A Review). Folia Vet. 2016, 60, 21–25. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and Related Bacterial Factors: Therapeutic Targets for Ulcerative Colitis. EBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Martino, H.S.D.; Tako, E. Plant Origin Prebiotics Affect Duodenal Brush Border Membrane Functionality and Morphology, in Vivo (Gallus gallus). Food Funct. 2021, 12, 6157–6166. [Google Scholar] [CrossRef]
- Potten, C.S.; Loeffler, M. A Comprehensive Model of the Crypts of the Small Intestine of the Mouse Provides Insight into the Mechanisms of Cell Migration and the Proliferation Hierarchy. J. Theor. Biol. 1987, 127, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Wallaeys, C.; Garcia-Gonzalez, N.; Libert, C. Paneth Cells as the Cornerstones of Intestinal and Organismal Health: A Primer. EMBO Mol. Med. 2022, 15, e16427. [Google Scholar] [CrossRef]
- Witten, J.; Samad, T.; Ribbeck, K. Selective Permeability of Mucus Barriers. Curr. Opin. Biotechnol. 2018, 52, 124–133. [Google Scholar] [CrossRef]
- Verediano, T.A.; Agarwal, N.; Martino, H.S.D.; Grancieri, M.; Paes, M.C.D.; Tako, E. Effect of Black Corn Anthocyanin-Rich Extract (Zea mays L.) on Cecal Microbial Populations In Vivo (Gallus gallus). Nutrients 2022, 14, 4679. [Google Scholar] [CrossRef]

| Gene | Oligonucleotides (5′-3′) | |
|---|---|---|
| Forward Primer (5′-3′) | Reverse Primer (5′-3′) | |
| BBM functionality | ||
| AP | CGTCAGCCAGTTTGACTATGTA | CTCTCAAAGAAGCTGAGGATGG |
| SI | CCAGCAATGCCAGCATATTG | CGGTTTCTCCTTACCACTTCTT |
| 18S rRNA | GCAAGACGAACTAAAGCGAAAG | TCGGAACTACGACGGTATCT |
| Inflammation | ||
| TNF-α | GACAGCCTATGCCAACAAGTA | TTACAGGAAGGGCAACTCATC |
| NF-κβ1 | CACAGCTGGAGGGAAGTAAAT | TTGAGTAAGGAAGTGAGGTTGAG |
| Intestinal Barrier | ||
| MUC2 | CCTGCTGCAAGGAAGTAGAA | GGAAGATCAGAGTGGTGCATAG |
| OCLN | GTCTGTGGGTTCCTCATCGT | GTTCTTCACCCACTCCTCCA |
| NI | H2O | CP | CPP | P | |
|---|---|---|---|---|---|
| Villi Goblet Cell Number | 24.68 ± 0.74 b | 38.38 ± 0.91 a | 26.40 ± 0.72 b | 27.46 ± 0.71 b | 25.79 ± 0.83 b |
| Villi Goblet Cell Diameter (μM) | 2.45 ± 0.06 c | 2.20 ± 0.05 d | 2.60 ± 0.06 bc | 2.94 ± 0.10 ab | 3.13 ± 0.08 a |
| Villi Goblet Cell Type Number | |||||
| Acidic | 15.28 ± 0.71 b | 26.71 ± 1.12 a | 10.29 ± 0.59 c | 8.49 ± 0.43 c | 8.82 ± 0.52 c |
| Neutral | 0.79 ± 0.13 a | 0.10 ± 0.04 b | 0.28 ± 0.09 b | 0.43 ± 0.12 b | 0.47 ± 0.12 b |
| Mixed | 8.68 ± 0.57 c | 11.57 ± 0.66 a | 15.83 ± 0.66 a | 18.54 ± 0.65 a | 16.51 ± 0.73 a |
| Crypt Goblet Cell Number | 12.67 ± 0.55 a | 10.95 ± 0.62 b | 10.03 ± 0.36 b | 8.04 ± 0.38 c | 10.18 ± 0.40 b |
| Crypt Goblet Cell Diameter (μM) | 2.92 ± 0.05 d | 3.13 ± 0.05 cd | 3.20 ± 0.06 bc | 3.40 ± 0.06 ab | 3.62 ± 0.07 a |
| Crypt Goblet Cell Type Number | |||||
| Acidic | 8.53 ± 0.43 a | 7.88 ± 0.51 ab | 7.63 ± 0.30 a | 5.98 ± 0.30 b | 7.14 ± 0.33 ab |
| Neutral | 0.41 ± 0.06 a | 0.50 ± 0.07 a | 0.09 ± 0.03 b | 0.08 ± 0.03 b | 0.04 ± 0.02 b |
| Mixed | 3.73 ± 0.27 a | 2.58 ± 0.21 bc | 2.33 ± 0.15 bc | 1.98 ± 0.17 c | 3.00 ± 0.17 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishima, M.D.V.; Martino, H.S.D.; Kolba, N.; Shah, D.D.; Grancieri, M.; Dos Santos, K.M.O.; Lima, J.P.; Da Silva, B.P.; Gonzalez de Mejia, E.; Tako, E. Effects of Intra-Amniotic Administration of the Hydrolyzed Protein of Chia (Salvia hispanica L.) and Lacticaseibacillus paracasei on Intestinal Functionality, Morphology, and Bacterial Populations, In Vivo (Gallus gallus). Nutrients 2023, 15, 1831. https://doi.org/10.3390/nu15081831
Mishima MDV, Martino HSD, Kolba N, Shah DD, Grancieri M, Dos Santos KMO, Lima JP, Da Silva BP, Gonzalez de Mejia E, Tako E. Effects of Intra-Amniotic Administration of the Hydrolyzed Protein of Chia (Salvia hispanica L.) and Lacticaseibacillus paracasei on Intestinal Functionality, Morphology, and Bacterial Populations, In Vivo (Gallus gallus). Nutrients. 2023; 15(8):1831. https://doi.org/10.3390/nu15081831
Chicago/Turabian StyleMishima, Marcella Duarte Villas, Hércia Stampini Duarte Martino, Nikolai Kolba, Drashti Dhirenkumar Shah, Mariana Grancieri, Karina Maria Olbrich Dos Santos, Janine Passos Lima, Bárbara Pereira Da Silva, Elvira Gonzalez de Mejia, and Elad Tako. 2023. "Effects of Intra-Amniotic Administration of the Hydrolyzed Protein of Chia (Salvia hispanica L.) and Lacticaseibacillus paracasei on Intestinal Functionality, Morphology, and Bacterial Populations, In Vivo (Gallus gallus)" Nutrients 15, no. 8: 1831. https://doi.org/10.3390/nu15081831
APA StyleMishima, M. D. V., Martino, H. S. D., Kolba, N., Shah, D. D., Grancieri, M., Dos Santos, K. M. O., Lima, J. P., Da Silva, B. P., Gonzalez de Mejia, E., & Tako, E. (2023). Effects of Intra-Amniotic Administration of the Hydrolyzed Protein of Chia (Salvia hispanica L.) and Lacticaseibacillus paracasei on Intestinal Functionality, Morphology, and Bacterial Populations, In Vivo (Gallus gallus). Nutrients, 15(8), 1831. https://doi.org/10.3390/nu15081831








