Changes in Hematological Parameters of Iron Status and Total Iron Concentrations in Different Biological Matrices during a Sports Season in Women’s Soccer Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Nutritional Intake
2.4. Blood and Urine Sample Collection
2.5. Determination of Hematological Parameters of Iron Status, Female Hormones and Serum Iron
2.6. Determination of Fe in Plasma, Urine, Erythrocytes and Platelets
2.7. Anthropometry, Body Composition and Physical Fitness Tests
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Randell, R.K.; Clifford, T.; Drust, B.; Moss, S.L.; Unnithan, V.B.; De Ste Croix, M.B.A.; Datson, N.; Martin, D.; Mayho, H.; Carter, J.M. Physiological characteristics of female soccer players and health and performance considerations: A narrative review. Sport. Med. 2021, 51, 1377–1399. [Google Scholar] [CrossRef] [PubMed]
- Datson, N.; Hulton, A.; Andersson, H.; Lewis, T.; Weston, M.; Drust, B.; Gregson, W. Applied physiology of female soccer: An update. Sport. Med. 2014, 44, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Saidi, K.; Abderrahman, A.B.; Hackney, A.C.; Bideau, B.; Zouita, S.; Granacher, U.; Zouhal, H. Hematology, hormones, inflammation, and muscle damage in elite and professional soccer players: A systematic review with implications for exercise. Sport. Med. 2021, 51, 2607–2627. [Google Scholar] [CrossRef] [PubMed]
- Heisterberg, M.F.; Fahrenkrug, J.; Krustrup, P.; Storskov, A.; Kjær, M.; Andersen, J.L. Extensive monitoring through multiple blood samples in professional soccer players. J. Strength Cond. Res. 2013, 27, 1260–1271. [Google Scholar] [CrossRef]
- Silva, J.; Rebelo, A.; Marques, F.; Pereira, L.; Seabra, A.; Ascensão, A.; Magalhães, J. Biochemical impact of soccer: An analysis of hormonal, muscle damage, and redox markers during the season. Appl. Physiol. Nutr. Metab. 2014, 39, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; McFadden, B.A.; Sanders, D.J.; Rabideau, M.M.; Hofacker, M.L.; Arent, S.M. Biomarker response to a competitive season in division I female soccer players. J. Strength Cond. Res. 2019, 33, 2622–2628. [Google Scholar] [CrossRef]
- Schumacher, Y.O.; Schmid, A.; Grathwohl, D.; Bültermann, D.; Berg, A. Hematological indices and iron status in athletes of various sports and performances. Med. Sci. Sport. Exerc. 2002, 34, 869–875. [Google Scholar] [CrossRef]
- Landahl, G.; Adolfsson, P.; Börjesson, M.; Mannheimer, C.; Rödjer, S. Iron deficiency and anemia: A common problem in female elite soccer players. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 689–694. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Ahmetovic, Z. Indicators of iron status in elite soccer players during the sports season. Int. J. Lab. Hematol. 2009, 31, 447–452. [Google Scholar] [CrossRef]
- Reinke, S.; Taylor, W.R.; Duda, G.N.; Von Haehling, S.; Reinke, P.; Volk, H.-D.; Anker, S.D.; Doehner, W. Absolute and functional iron deficiency in professional athletes during training and recovery. Int. J. Cardiol. 2012, 156, 186–191. [Google Scholar] [CrossRef]
- Nickerson, H.J.; Holubets, M.C.; Weiler, B.R.; Haas, R.G.; Schwartz, S.; Ellefson, M.E. Causes of iron deficiency in adolescent athletes. J. Pediatr. 1989, 114, 657–663. [Google Scholar] [CrossRef]
- Beard, J.; Tobin, B. Iron status and exercise. Am. J. Clin. Nutr. 2000, 72, 594s–597s. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, J.; Raczynski, G.; Stupnicki, R. Iron Status in Female Endurance Athletes and in Non-Athletes. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S. Iron and the endurance athlete. Appl. Physiol. Nutr. Metab. 2014, 39, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Beals, K.A. Eating behaviors, nutritional status, and menstrual function in elite female adolescent volleyball players. J. Am. Diet. Assoc. 2002, 102, 1293–1296. [Google Scholar] [CrossRef]
- Diehl, D.M.; Lohman, T.G.; Smith, S.C.; Kertzer, R. Effects of physical training and competition on the iron status of female field hockey players. Int. J. Sport. Med. 1986, 7, 264–270. [Google Scholar] [CrossRef]
- Balaban, E.P. Sports anemia. Clin. Sport. Med. 1992, 11, 313–325. [Google Scholar] [CrossRef]
- DellaValle, D.M.; Haas, J.D. Iron status is associated with endurance performance and training in female rowers. Med. Sci. Sport. Exerc. 2012, 44, 1552–1559. [Google Scholar] [CrossRef]
- Newhouse, I.J.; Clement, D.B. Iron status in athletes. Sport. Med. 1988, 5, 337–352. [Google Scholar] [CrossRef]
- Baynes, R.D. Assessment of iron status. Clin. Biochem. 1996, 29, 209–215. [Google Scholar] [CrossRef]
- Malczewska, J.; Bach, W.; Stupnicki, R. The effects of physical exercise on the concentrations of ferritin and transferrin receptor in plasma of female judoists. Int. J. Sport. Med. 2000, 21, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Cowell, B.S.; Rosenbloom, C.A.; Skinner, R.; Summers, S.H. Policies on screening female athletes for iron deficiency in NCAA division IA institutions. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 277–285. [Google Scholar] [CrossRef]
- Dale, J.C.; Burritt, M.F.; Zinsmeister, A.R. Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. Am. J. Clin. Pathol. 2002, 117, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Grijota, F.J.; Toro-Román, V.; Siquier-Coll, J.; Robles-Gil, M.C.; Muñoz, D.; Maynar-Mariño, M. Total Iron Concentrations in Different Biological Matrices—Influence of Physical Training. Nutrients 2022, 14, 3549. [Google Scholar] [CrossRef]
- Kang, H.-S.; Matsuo, T. Effects of 4 weeks iron supplementation on haematological and immunological status in elite female soccer players. Asia Pac. J. Clin. Nutr. 2004, 13, 353–358. [Google Scholar]
- Toro-Román, V.; Muñoz, D.; Maynar-Mariño, M.; Clemente-Gil, S.; Robles-Gil, M.C. Sex Differences in Copper Concentrations during a Sports Season in Soccer Players. Nutrients 2023, 15, 495. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composicion de Alimentos: Guia de Prácticas; Pirámide: Madrid, Spain, 2016; ISBN 978-84-368-3623-3. [Google Scholar]
- Porta, J.; Galiano, D.; Tejedo, A.; González, J.M. Valoración de la composición corporal. Utopías y realidades. In Manual de Cineantropometría; Esparza Ros, F., Ed.; Grupo Español de Cineantropometría: Madrid, Spain, 1993; pp. 113–170. [Google Scholar]
- Yuhasz, M.S. Physical Fitness Manual, 1st ed.; University of Western Ontario: London, ON, Canada, 1974. [Google Scholar]
- Yamamoto, K.; Takita, M.; Kami, M.; Tsubokura, M.; Tanimoto, T.; Kitamura, T.; Takemoto, Y. Profiles of anemia in adolescent students with sports club membership in an outpatient clinic setting: A retrospective study. PeerJ 2022, 10, e13004. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Ahmetovic, Z. Weekly training volume and hematological status in female top-level athletes of different sports. J. Sport. Med. Phys. Fit. 2008, 48, 398. [Google Scholar]
- Lu, Y.; Ahmed, S.; Harari, F.; Vahter, M. Impact of Ficoll density gradient centrifugation on major and trace element concentrations in erythrocytes and blood plasma. J. Trace Elem. Med. Biol. 2015, 29, 249–254. [Google Scholar] [CrossRef]
- Heitland, P.; Köster, H.D. Human Biomonitoring of 73 elements in blood, serum, erythrocytes and urine. J. Trace Elem. Med. Biol. 2021, 64, 126706. [Google Scholar] [CrossRef]
- Heitland, P.; Köster, H.D. Biomonitoring of 37 trace elements in blood samples from inhabitants of northern Germany by ICP–MS. J. Trace Elem. Med. Biol. 2006, 20, 253–262. [Google Scholar] [CrossRef]
- Beard, J. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, 568S–580S. [Google Scholar] [CrossRef]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Magallanes, V.M.; Barba-Moreno, L.; Romero-Parra, N.; Rael, B.; Benito, P.J.; Swinkels, D.W.; Laarakkers, C.M.; Díaz, Á.E.; Peinado, A.B.; Group, I.S. Menstrual cycle affects iron homeostasis and hepcidin following interval running exercise in endurance-trained women. Eur. J. Appl. Physiol. 2022, 122, 2683–2694. [Google Scholar] [CrossRef]
- Kim, I.; Yetley, E.A.; Calvo, M.S. Variations in iron-status measures during the menstrual cycle. Am. J. Clin. Nutr. 1993, 58, 705–709. [Google Scholar] [CrossRef]
- Barba-Moreno, L.; Alfaro-Magallanes, V.M.; de Jonge, X.A.K.J.; Díaz, A.E.; Cupeiro, R.; Peinado, A.B. Hepcidin and interleukin-6 responses to endurance exercise over the menstrual cycle. Eur. J. Sport Sci. 2022, 22, 218–226. [Google Scholar] [CrossRef]
- Michos, C.; Kalfakakou, V.; Karkabounas, S.; Kiortsis, D.; Evangelou, A. Changes in copper and zinc plasma concentrations during the normal menstrual cycle in women. Gynecol. Endocrinol. 2010, 26, 250–255. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Maynar-Mariño, M.; Grijota, F.J.; Bartolomé, I.; Siquier-Coll, J.; Román, V.T.; Muñoz, D. Influence of physical training on erythrocyte concentrations of iron, phosphorus and magnesium. J. Int. Soc. Sport. Nutr. 2020, 17, 1–7. [Google Scholar]
- Ottomano, C.; Franchini, M. Sports anaemia: Facts or fiction? Blood Transfus. 2012, 10, 252. [Google Scholar]
- Calleja, C.A.; Hurtado, M.M.C.; Daschner, Á.; Escámez, P.F.; Abuín, C.M.F.; Pons, R.M.G.; Fandos, M.E.G.; Muñoz, M.J.G.; López-García, E.; Vinuesa, J.M. Informe del Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) sobre Ingestas Nutricionales de Referencia para la población española. Rev. Com. Científico AESAN 2019, 29, 43–68. [Google Scholar]
- Vandevijvere, S.; Michels, N.; Verstraete, S.; Ferrari, M.; Leclercq, C.; Cuenca-García, M.; Grammatikaki, E.; Manios, Y.; Gottrand, F.; Santamaría, J. V Intake and dietary sources of haem and non-haem iron among European adolescents and their association with iron status and different lifestyle and socio-economic factors. Eur. J. Clin. Nutr. 2013, 67, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Lambeth, A.; Scott, D. Nutritional practices of national female soccer players: Analysis and recommendations. J. Sport. Sci. Med. 2006, 5, 130. [Google Scholar]
- Dobrowolski, H.; Włodarek, D. Dietary intake of Polish female soccer players. Int. J. Environ. Res. Public Health 2019, 16, 1134. [Google Scholar] [CrossRef] [PubMed]
- Escanero, J.F.; Villanueva, J.; Rojo, A.; Herrera, A.; del Diego, C.; Guerra, M. Iron stores in professional athletes throughout the sports season. Physiol. Behav. 1997, 62, 811–814. [Google Scholar] [CrossRef]
- Damian, M.-T.; Vulturar, R.; Login, C.C.; Damian, L.; Chis, A.; Bojan, A. Anemia in sports: A narrative review. Life 2021, 11, 987. [Google Scholar] [CrossRef]
- Saidi, K.; Zouhal, H.; Rhibi, F.; Tijani, J.M.; Boullosa, D.; Chebbi, A.; Hackney, A.C.; Granacher, U.; Bideau, B.; Ben Abderrahman, A. Effects of a six-week period of congested match play on plasma volume variations, hematological parameters, training workload and physical fitness in elite soccer players. PLoS ONE 2019, 14, e0219692. [Google Scholar] [CrossRef]
- Gravina, L.; Ruiz, F.; Lekue, J.A.; Irazusta, J.; Gil, S.M. Metabolic impact of a soccer match on female players. J. Sport. Sci. 2011, 29, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.L.; Cossio-Bolaños, M.A.; Dunlop, G.; Rouissi, M.; Chtara, M.; Bragazzi, N.L.; Chamari, K. Stability in post-seasonal hematological profiles in response to high-competitive match-play loads within elite top-level European soccer players: Implications from a pilot study. Open Access J. Sport. Med. 2018, 9, 157. [Google Scholar] [CrossRef]
- Anđelković, M.; Baralić, I.; Đorđević, B.; Stevuljević, J.K.; Radivojević, N.; Dikić, N.; Škodrić, S.R.; Stojković, M. Hematological and biochemical parameters in elite soccer players during a competitive half season. J. Med. Biochem. 2015, 34, 460. [Google Scholar] [CrossRef]
- Gropper, S.S.; Blessing, D.; Dunham, K.; Barksdale, J.M. Iron status of female collegiate athletes involved in different sports. Biol. Trace Elem. Res. 2006, 109, 1–13. [Google Scholar] [CrossRef]
- Fallon, K.E.; Fallon, S.K.; Boston, T. The acute phase response and exercise: Court and field sports. Br. J. Sport. Med. 2001, 35, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Thirup, P. Haematocrit. Sport. Med. 2003, 33, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Montain, S.J. Fluid and electrolyte supplementation for exercise heat stress. Am. J. Clin. Nutr. 2000, 72, 564s–572s. [Google Scholar] [CrossRef]
- Mclnnis, M.D.; Newhouse, I.J.; von Duvillard, S.P.; Thayer, R. The effect of exercise intensity on hematuria in healthy male runners. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 79, 99–105. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur. J. Appl. Physiol. 2009, 106, 51–59. [Google Scholar] [CrossRef]
- Siquier-Coll, J.; Bartolomé, I.; Perez-Quintero, M.; Grijota, F.J.; Arroyo, J.; Muñoz, D.; Maynar-Mariño, M. Serum, erythrocyte and urinary concentrations of iron, copper, selenium and zinc do not change during an incremental test to exhaustion in either normothermic or hyperthermic conditions. J. Therm. Biol. 2019, 86, 102425. [Google Scholar] [CrossRef]
- Terink, R.; Ten Haaf, D.; Bongers, C.W.G.; Balvers, M.G.J.; Witkamp, R.F.; Mensink, M.; Eijsvogels, T.M.H.; Gunnewiek, J.M.T.K.; Hopman, M.T.E. Changes in iron metabolism during prolonged repeated walking exercise in middle-aged men and women. Eur. J. Appl. Physiol. 2018, 118, 2349–2357. [Google Scholar] [CrossRef]
- Mettler, S.; Zimmermann, M.B. Iron excess in recreational marathon runners. Eur. J. Clin. Nutr. 2010, 64, 490–494. [Google Scholar] [CrossRef]
- Ponorac, N.; Popović, M.; Karaba-Jakovljević, D.; Bajić, Z.; Scanlan, A.; Stojanović, E.; Radovanović, D. Professional female athletes are at a heightened risk of iron-deficient erythropoiesis compared with nonathletes. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 48–53. [Google Scholar] [CrossRef]
- Sandström, G.; Börjesson, M.; Rödjer, S. Iron deficiency in adolescent female athletes—Is iron status affected by regular sporting activity? Clin. J. Sport Med. 2012, 22, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Dawson, B.; Peeling, P. Hemolytic effects of a football-specific training session in elite female players. Int. J. Sport. Physiol. Perform. 2012, 7, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Nishiie-Yano, R.; Hirayama, S.; Tamura, M.; Kanemochi, T.; Ueno, T.; Hirayama, A.; Hori, A.; Ai, T.; Hirose, N.; Miida, T. Hemolysis Is Responsible for Elevation of Serum Iron Concentration After Regular Exercises in Judo Athletes. Biol. Trace Elem. Res. 2020, 197, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Rajaram, S. Exercise and Iron Status. J. Nutr. 1992, 122, 782–787. [Google Scholar] [CrossRef]
- Dufaux, B.; Hoederath, A.; Streitberger, I.; Hollmann, W.; Assmann, G. Serum ferritin, transferrin, haptoglobin, and iron in middle-and long-distance runners, elite rowers, and professional racing cyclists. Int. J. Sport. Med. 1981, 2, 43–46. [Google Scholar] [CrossRef]
- Paulev, P.-E.; Jordal, R.; Pedersen, N.S. Dermal excretion of iron in intensely training athletes. Clin. Chim. Acta 1983, 127, 19–27. [Google Scholar] [CrossRef]
- Szygula, Z. Erythrocytic system under the influence of physical exercise and training. Sport. Med. 1990, 10, 181–197. [Google Scholar] [CrossRef]
- Weight, L.M.; Byrne, M.J.; Jacobs, P. Haemolytic effects of exercise. Clin. Sci. 1991, 81, 147–152. [Google Scholar] [CrossRef]
- Córdova-Martínez, A.; Villa, G.; Aguiló, A.; Tur, J.A.; Pons, A. Hand strike-induced hemolysis and adaptations in iron metabolism in Basque ball players. Ann. Nutr. Metab. 2006, 50, 206–213. [Google Scholar] [CrossRef]
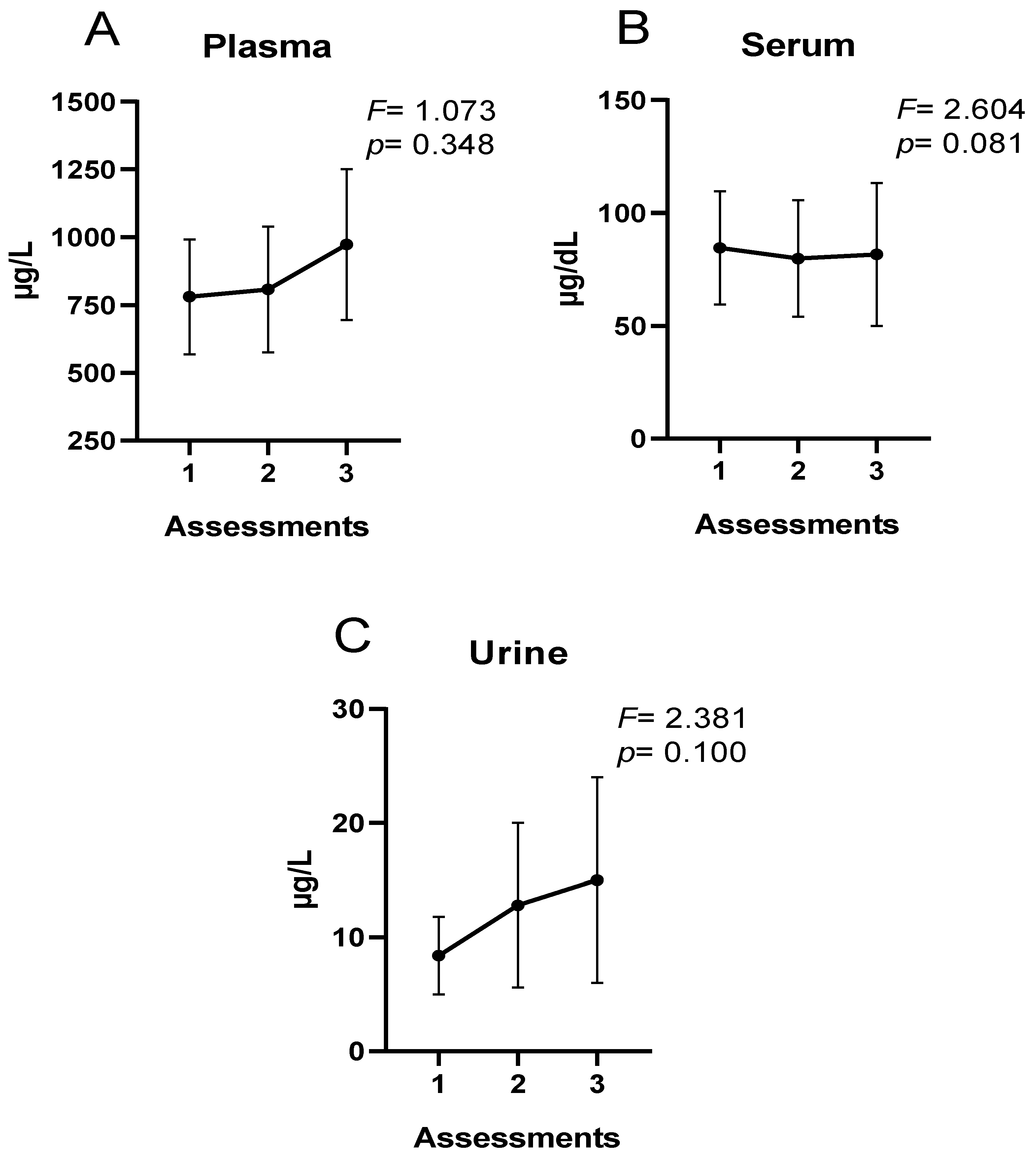
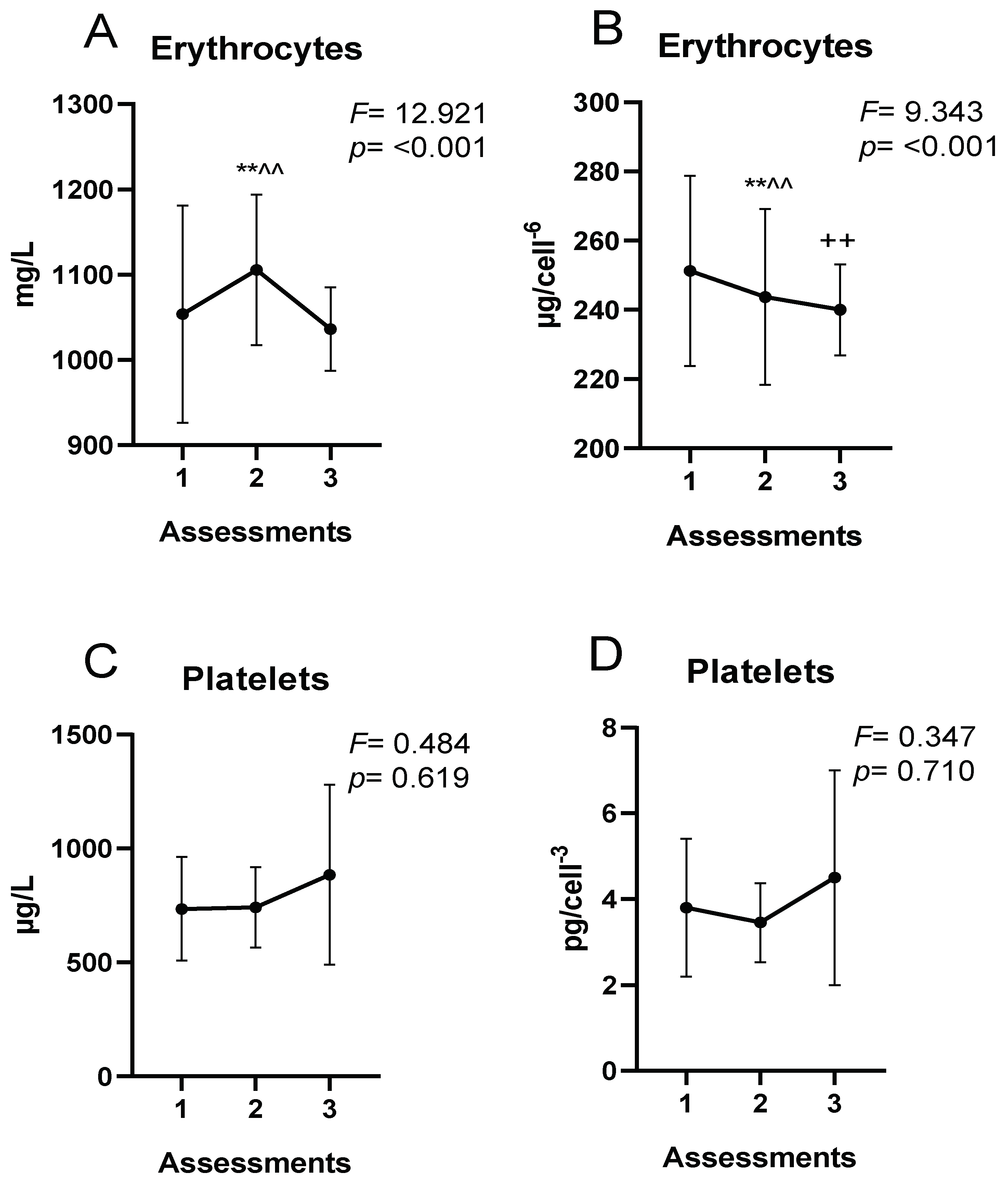
| Women’s Soccer Players | |
|---|---|
| N | 24 |
| Age (years) | 23.37 ± 3.95 |
| Experience (years) | 14.51 ± 4.94 |
| Training (weeks) | 39 |
| Training sessions (n) | 133 ± 25 |
| Training (min) | 10,578 ± 3227 |
| Matches played (n) | 36 |
| Injuries (nº) | 8 |
| Absence from training (days) | 14 ± 10 |
| Women’s Soccer Players | ||
|---|---|---|
| Age of first appearance (years) | 13.5 ± 1.15 | |
| Regular menstruation (%) | 100.00 | |
| Duration of bleeding (days) | 4.77 ± 1.47 | |
| Amount of bleeding (%) | Light | 11.11 |
| Moderate | 77.77 | |
| Heavy | 11.11 | |
| Menstrual cycle (days) | 27.93 ± 2.78 | |
| Cessation of menstruation (%) | Never | 88.88 |
| Sometimes | 12.22 |
| Women’s Soccer Players | |
|---|---|
| Height (m) | 1.65 ± 0.06 |
| Weight (kg) | 59.58 ± 7.17 |
| Σ6 skinfolds (mm) | 94.62 ± 18.54 |
| Fat (%) | 18.16 ± 2.74 |
| SJ (s) | 0.539 ± 0.045 |
| CMJ (s) | 0.569 ± 0.055 |
| Incremental test time (min) | 9.18 ± 1.12 |
| VO2max (mL/min/kg) | 39.72 ± 6.22 |
| VO2max (L/min) | 2.28 ± 0.40 |
| Women’s Soccer Players | F | p | ||
|---|---|---|---|---|
| Energy (kcal/day) | Assessment 1 | 1578.1 ± 316.2 | 1.188 | 0.307 |
| Assessment 2 | 1681.5 ± 427.3 | |||
| Assessment 3 | 1697.3 ± 386.1 | |||
| Proteins (g/day) | Assessment 1 | 90.4 ± 21.6 | 0.841 | 0.473 |
| Assessment 2 | 96.2 ± 18.3 | |||
| Assessment 3 | 92.6 ± 20.4 | |||
| Lipids (g/day) | Assessment 1 | 48.3 ± 12.3 | 1.467 | 0.219 |
| Assessment 2 | 55.6 ± 15.3 | |||
| Assessment 3 | 60.3 ± 20.6 | |||
| Carbohydrates (g/day) | Assessment 1 | 206.1 ± 81.3 | 1.956 | 0.156 |
| Assessment 2 | 241.5 ± 56.1 | |||
| Assessment 3 | 235.8 ± 61.7 | |||
| Folic acid (µg/day) | Assessment 1 | 521.4 ± 61.8 | 1.145 | 0.391 |
| Assessment 2 | 511.6 ± 49.1 | |||
| Assessment 3 | 500.8 ± 61.8 | |||
| B12 (µg/day) | Assessment 1 | 5.8 ± 1.7 | 0.378 | 0.550 |
| Assessment 2 | 6.1 ± 1.9 | |||
| Assessment 3 | 6.4 ± 2.1 | |||
| Fe (mg/day) | Assessment 1 | 12.5 ± 2.5 | 0.247 | 0.713 |
| Assessment 2 | 12.6 ± 1.8 | |||
| Assessment 3 | 12.7 ± 2.6 |
| Women’s Soccer Players | F | p | ||
|---|---|---|---|---|
| Progesterone (ng/mL) | Assessment 1 | 2.65 ± 3.88 | 0.052 | 0.998 |
| Assessment 2 | 2.38 ± 3.21 | |||
| Assessment 3 | 2.31 ± 2.89 | |||
| Estradiol-17β (pg/mL) | Assessment 1 | 74.04 ± 45.30 | 0.165 | 0.894 |
| Assessment 2 | 71.32 ± 39.25 | |||
| Assessment 3 | 68.30 ± 40.93 |
| Women’s Soccer Players | F | p | ||
|---|---|---|---|---|
| Erythrocytes (millions) | Assessment 1 | 4.37 ± 0.22 | 3.767 | 0.028 |
| Assessment 2 | 4.19 ± 0.27 ** | |||
| Assessment 3 | 4.35 ± 0.27 | |||
| Hemoglobin (gr%) | Assessment 1 | 12.79 ± 0.92 | 13.414 | <0.001 |
| Assessment 2 | 12.29 ± 0.93 | |||
| Assessment 3 | 13.82 ± 0.95 ++^^ | |||
| Hematocrit (%) | Assessment 1 | 36.34 ± 2.43 | 2.371 | 0.101 |
| Assessment 2 | 34.89 ± 2.61 | |||
| Assessment 3 | 35.88 ± 2.43 | |||
| MCV (fL) | Assessment 1 | 87.01 ± 3.48 | 0.184 | 0.832 |
| Assessment 2 | 86.55 ± 3.22 | |||
| Assessment 3 | 87.04 ± 3.17 | |||
| MCH (Pg) | Assessment 1 | 29.90 ± 1.39 | 12.507 | <0.001 |
| Assessment 2 | 31.43 ± 1.45 ** | |||
| Assessment 3 | 31.75 ± 1.38 ++ | |||
| Platelets (miles) | Assessment 1 | 196.00 ± 38.01 | 2.631 | 0.079 |
| Assessment 2 | 219.08 ± 34.19 | |||
| Assessment 3 | 204.39 ± 31.52 | |||
| B12 (pg/mL) | Assessment 1 | 447.95 ± 137.16 | 0.249 | 0.780 |
| Assessment 2 | 443.28 ± 103.48 | |||
| Assessment 3 | 466.34 ± 96.96 | |||
| Folic acid (ng/mL) | Assessment 1 | 5.24 ± 2.08 | 0.968 | 0.385 |
| Assessment 2 | 6.15 ± 2.16 | |||
| Assessment 3 | 5.72 ± 1.67 | |||
| Ferritin (mcg/L) | Assessment 1 | 32.26 ± 15.31 | 1.452 | 0.241 |
| Assessment 2 | 21.62 ± 7.80 | |||
| Assessment 3 | 28.00 ± 13.23 | |||
| Transferrin (mcg/L) | Assessment 1 | 270.82 ± 37.92 | 1.003 | 0.372 |
| Assessment 2 | 273.16 ± 36.60 | |||
| Assessment 3 | 259.08 ± 26.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro-Román, V.; Robles-Gil, M.C.; Bartolomé, I.; Grijota, F.J.; Muñoz, D.; Maynar-Mariño, M. Changes in Hematological Parameters of Iron Status and Total Iron Concentrations in Different Biological Matrices during a Sports Season in Women’s Soccer Players. Nutrients 2023, 15, 1833. https://doi.org/10.3390/nu15081833
Toro-Román V, Robles-Gil MC, Bartolomé I, Grijota FJ, Muñoz D, Maynar-Mariño M. Changes in Hematological Parameters of Iron Status and Total Iron Concentrations in Different Biological Matrices during a Sports Season in Women’s Soccer Players. Nutrients. 2023; 15(8):1833. https://doi.org/10.3390/nu15081833
Chicago/Turabian StyleToro-Román, Víctor, María C. Robles-Gil, Ignacio Bartolomé, Francisco J. Grijota, Diego Muñoz, and Marcos Maynar-Mariño. 2023. "Changes in Hematological Parameters of Iron Status and Total Iron Concentrations in Different Biological Matrices during a Sports Season in Women’s Soccer Players" Nutrients 15, no. 8: 1833. https://doi.org/10.3390/nu15081833
APA StyleToro-Román, V., Robles-Gil, M. C., Bartolomé, I., Grijota, F. J., Muñoz, D., & Maynar-Mariño, M. (2023). Changes in Hematological Parameters of Iron Status and Total Iron Concentrations in Different Biological Matrices during a Sports Season in Women’s Soccer Players. Nutrients, 15(8), 1833. https://doi.org/10.3390/nu15081833










