Relationship of the Behavior of Older Participants with Body Composition Change: Results of the Second Wave of the Cognition of Older People, Education, Recreational Activities, Nutrition, Comorbidities, and Functional Capacity Studies (COPERNICUS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Assessment Methods
2.2.1. Body Composition Analysis
2.2.2. Activity Level Questionnaire
2.2.3. Depression Severity and Health Assessment
2.2.4. Cognitive Function Assessment
2.2.5. Demographic and Occupation-Related Data
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Body Composition Trajectory in Older People
4.2. Physical Activities and Product Consumption Frequency as Predictors of Skeletal Muscle Mass
4.3. Effects of Food Product Consumption Frequency on Changes in Body Composition within Two Years
4.4. Study Limitations
5. Conclusions
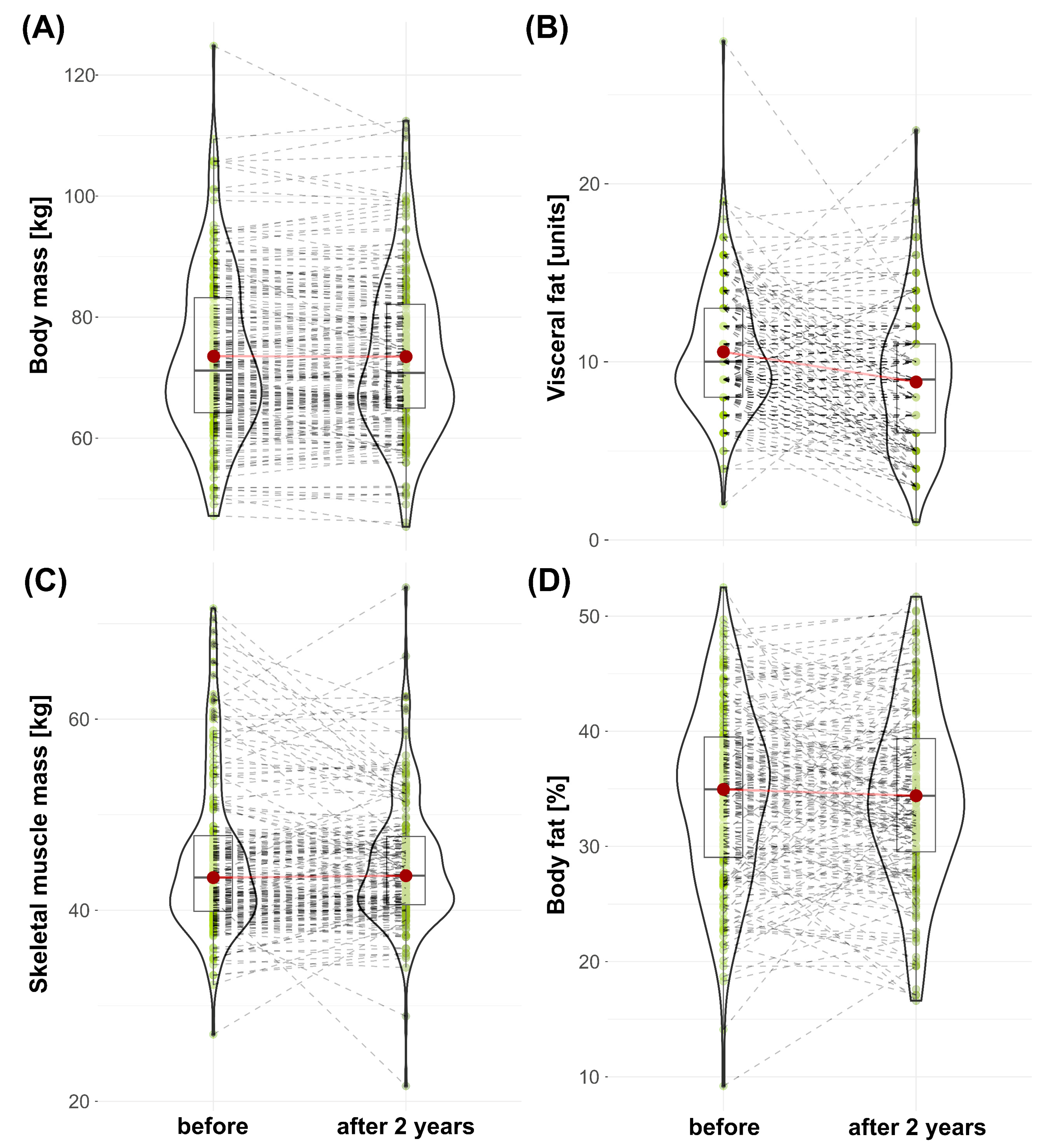
- No significant changes in body weight, muscle mass, or body fat percentage were observed.
- A significant reduction in visceral fat level was noted. Changes in all body composition measurements were characterized by relatively high variance.
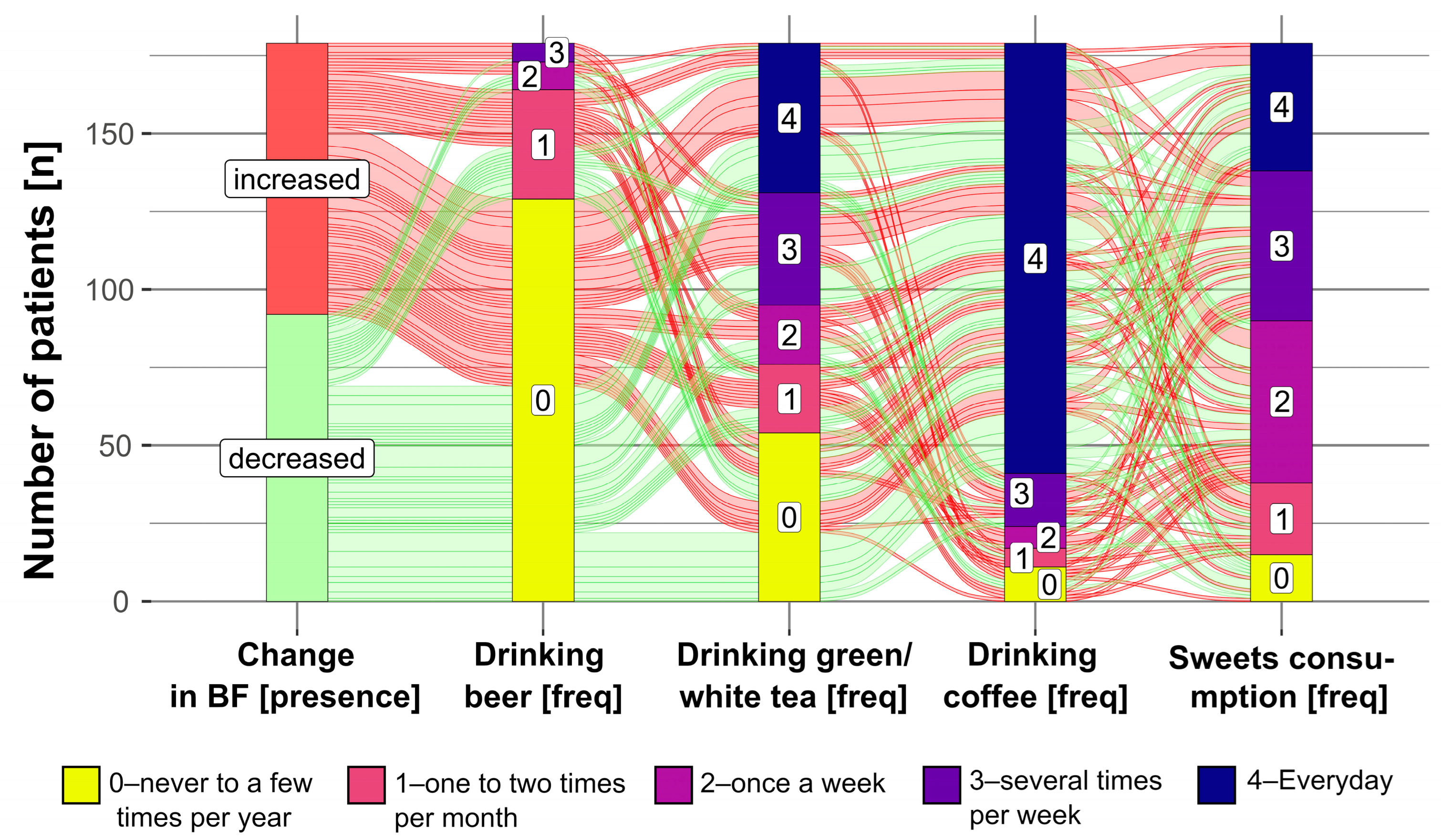
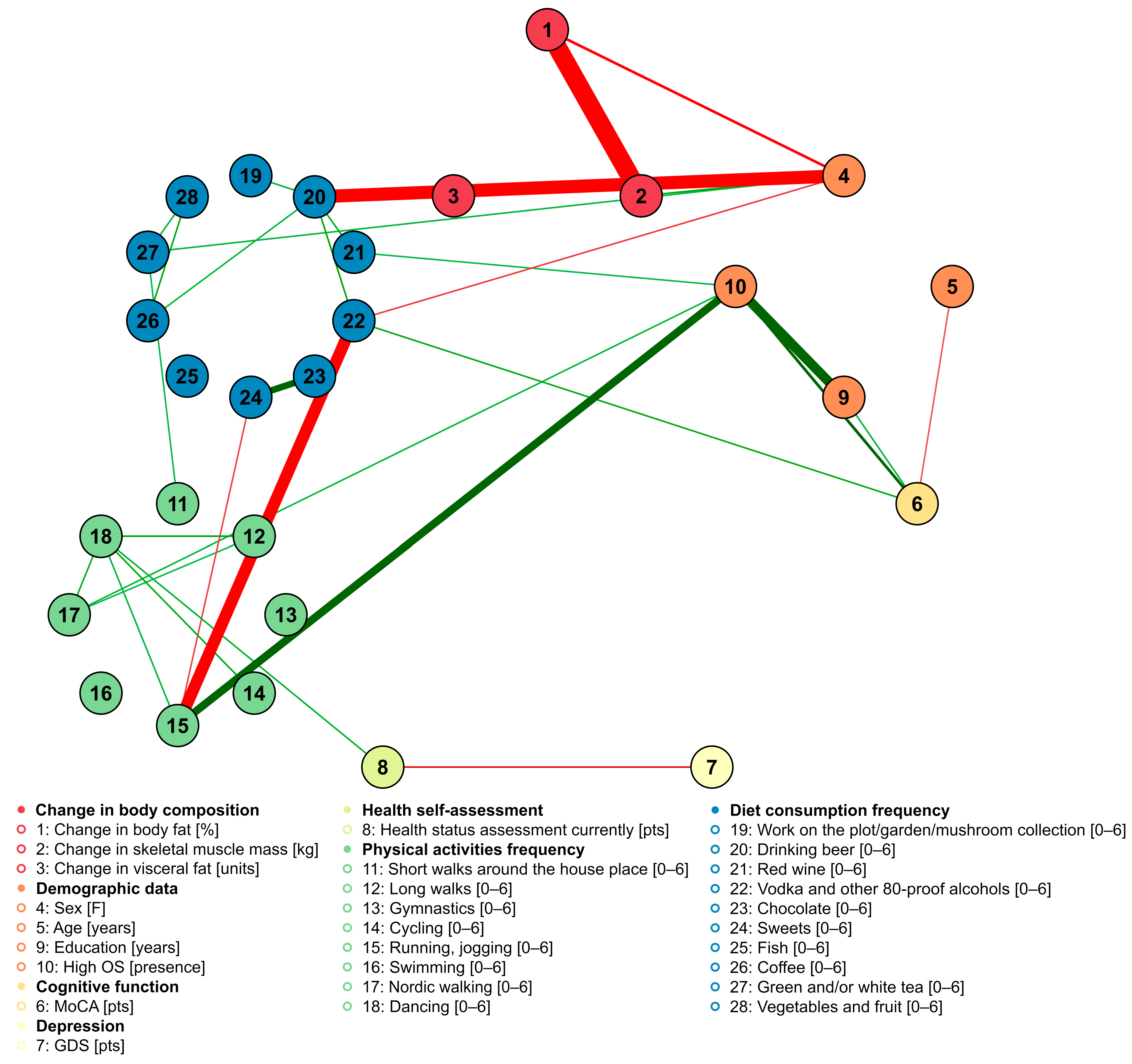
- More frequent drinking of beer, drinking of green or white tea, and consumption of sweets were related to a higher increase in body fat percentage within two years. Daily coffee consumption was related to a lower increase in body fat percentage in comparison to consumers who drank coffee a few times per week.
- More frequent drinking of beer and consumption of sweets was related to a decrease in skeletal muscle mass within two years. Contrarily, drinking red wine one to two times per month was related to an increase in muscle mass in comparison to drinking red wine never to a few times per year.
- Drinking beer a few times per week was related to a decrease in muscle mass in comparison to drinking beer never to a few times per year. Consuming sweets one to two times per month was related to a decrease in muscle mass in comparison to consuming sweets never to a few times per year. Contrarily, drinking red wine one to two times per month was related to an increase in muscle mass in comparison to drinking red wine never to a few times per year
- Frequency of physical activities was not related to changes in body composition.
- The frequencies of food product consumption are interrelated; for instance, subjects who ate sweets the most frequently (once a week or more frequently) were more often daily consumers of coffee.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Peralta, M.; Ramos, M.; Lipert, A.; Martins, J.; Marques, A. Prevalence and trends of overweight and obesity in older adults from 10 European countries from 2005 to 2013. Scand. J. Public Health 2018, 46, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Flatt, T.; Partridge, L. Horizons in the evolution of aging. BMC Biol. 2018, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Al Snih, S.; Ottenbacher, K.J.; Markides, K.S.; Kuo, Y.F.; Eschbach, K.; Goodwin, J.S. The effect of obesity on disability vs mortality in older Americans. Arch. Intern. Med. 2007, 167, 774–780. [Google Scholar] [CrossRef]
- Barzilay, J.I.; Blaum, C.; Moore, T.; Xue, Q.L.; Hirsch, C.H.; Walston, J.D.; Fried, L.P. Insulin resistance and inflammation as precursors of frailty: The cardiovascular health study. Arch. Intern. Med. 2007, 167, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Frisardi, V.; Solfrizzi, V.; Seripa, D.; Capurso, C.; Santamato, A.; Sancarlo, D.; Vendemiale, G.; Pilotto, A.; Panza, F. Metabolic-cognitive syndrome: Across-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res. Rev. 2010, 9, 399–417. [Google Scholar] [CrossRef]
- Cevenini, E.; Monti, D.; Franceschi, C. Inflammageing. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Verdijk, L.; van der Hoeven, L.; Adam, J.J.; van Kranenburg, J.; Nilwik, R.; van Loon, L.J. Patients with type 2diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J. Am. Med. Dir. Assoc. 2013, 14, 585–592. [Google Scholar] [CrossRef]
- Yamauchi, T.; Midorikawa, T.; Hagihara, J.; Sasaki, K. Quality of life nutritional status, physical activity, and their interrelationships of elderly living on an underpopulated island in Japan. Geriatr. Gerontol. Int. 2007, 7, 26–33. [Google Scholar] [CrossRef]
- Hu, Z.; Qin, L.; Kaminga, A.C.; Xu, H. Relationship between multiple lifestyle behaviors and health-related quality of life among elderly individuals with prediabetes in rural communities in China: A STROBE-compliant article. Medicine 2020, 99, e19560. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.J.; Spring, B.; Nigg, C.R. Multiple health behavior change research: An introduction and overview. Prev. Med. 2008, 46, 181–188. [Google Scholar] [CrossRef]
- Mkhitaryan, S.; Crutzen, R.; Steenaart, E.; de Vries, N.K. Network approach in health behavior research: How can we explore new questions? Health Psychol. Behav. Med. 2019, 7, 362–384. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Vidal-Alaball, J.; Lopez Segui, F.; Moreno-Sánchez, P.A. A Social Network Analysis of Tweets Related to Masks during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 8235. [Google Scholar] [CrossRef] [PubMed]
- Hevey, D. Network analysis: A brief overview and tutorial. Health Psychol. Behav. Med. 2018, 6, 301–328. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Reznick, A.Z.; Keidar, Z.; Karkabi, K.; Aizenbud, D. Body composition in heavy smokers: Comparison of segmental bioelectrical impedance analysis and dual-energy X-ray absorptiometry. Adv. Exp. Med. Biol. 2015, 840, 1–11. [Google Scholar] [CrossRef]
- Stahn, A.; Terblanche, E.; Gunga, H.-C. Use of bioelectrical impedance: General principles and overview. In Handbook of Anthropometry: Physical Measures of Human form in Health and Disease; Preedy, V.R., Ed.; Springer: New York, NY, USA, 2012; pp. 49–90. [Google Scholar]
- Bailey, K.V.; Ferro-Luzzi, A. Use of body mass index of adults in assessing individual and community nutritional status. Bull. World Health Organ. 1995, 73, 673–680. [Google Scholar]
- Kujawski, S.; Kujawska, A.; Gajos, M.; Topka, W.; Perkowski, R.; Androsiuk-Perkowska, J.; Newton, J.L.; Zalewski, P.; Kędziora-Kornatowska, K. Cognitive functioning in older people. results of the first wave of cognition of older people, education, recreational activities, nutrition, comorbidities, and functional capacity studies (COPERNICUS). Front. Aging Neurosci. 2018, 10, 421. [Google Scholar] [CrossRef]
- Almeida, O.P.; Almeida, S.A. Short versions of the geriatric depression scale: A study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int. J. Geriatr. Psychiatry 1999, 14, 858–865. [Google Scholar] [CrossRef]
- Conradsson, M.; Rosendahl, E.; Littbrand, H.; Gustafson, Y.; Olofsson, B.; Lövheim, H. Usefulness of the Geriatric Depression Scale 15-item version among very old people with and without cognitive impairment. Aging Ment. Health 2013, 17, 638–645. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment; MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriat Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Patil, I. Ggstatsplot: ‘ggplot2’ Based Plots with Statistical Details. CRAN. 2018. Available online: https://mran.revolutionanalytics.com/snapshot/2018-09-28/web/packages/ggstatsplot/ggstatsplot.pdf (accessed on 13 February 2023).
- The Jamovi Project. Jamovi (Version 2.3). [Computer Software]. 2022. Available online: https://www.jamovi.org (accessed on 13 February 2023).
- Bjoern Koneswarakantha. Easyalluvial: Generate Alluvial Plots with a Single Line of Code. [R Package]. 2019. Available online: https://CRAN.R-project.org/package=easyalluvial (accessed on 13 February 2023).
- Serdar Balci. ClinicoPath Jamovi Module. [R Package]. 2022. Available online: https://github.com/sbalci/ClinicoPathJamoviModule (accessed on 13 February 2023).
- Epskamp, S.; Cramer, A.O.J.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. qgraph: Network Visualizations of Relationships in Psychometric Data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 13 February 2023).
- Mattioli, A.V. Effects of caffeine and coffee consumption on cardiovascular disease and risk factors. Future Cardiol. 2007, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Raguso, C.A.; Kyle, U.; Kossovsky, M.P.; Roynette, C.; Paoloni-Giacobino, A.; Hans, D.; Genton, L.; Pichard, C. A 3-year longitudinal study on body composition changes in the elderly: Role of physical exercise. Clin. Nutr. 2006, 25, 573–580. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Tylavsky, F.; Kritchevsky, S.B.; Cauley, J.A.; Newman, A.B.; Blunt, B.A.; Harris, T.B. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J. Appl. Physiol. 2003, 94, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Chopp-Hurley, J.N.; Wiebenga, E.G.; Keller, H.H.; Maly, M.R. Nutrition risk, physical activity and fibre intake are associated with body composition in OA: Analysis of the Canadian Longitudinal Study on Aging. BMJ Nutr. Prev. Health 2022, 5, 191–200. [Google Scholar] [CrossRef]
- Grgic, J.; Garofolini, A.; Orazem, J.; Sabol, F.; Schoenfeld, B.J.; Pedisic, Z. Effects of resistance training on muscle size and strength in very elderly adults: A systematic review and meta-analysis of randomized controlled trials. Sports Med. 2020, 50, 1983–1999. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation 2014, 129 (Suppl. 2), S102–S138. [Google Scholar] [CrossRef]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.; Desai, M.; King, A.C. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: The DIETFITS randomized clinical trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kolesarova, A. The anti-obesity and health-promoting effects of tea and coffee. Physiol. Res. 2021, 70, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.Y.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Masi, D.; Spoltore, M.E.; Rossetti, R.; Watanabe, M.; Tozzi, R.; Caputi, A.; Risi, R.; Balena, A.; Gandini, O.; Mariani, S.; et al. The Influence of Ketone Bodies on Circadian Processes Regarding Appetite, Sleep and Hormone Release: A Systematic Review of the Literature. Nutrients 2022, 14, 1410. [Google Scholar] [CrossRef] [PubMed]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Ostfeld, R.J.; McMacken, M. The ketogenic diet for obesity and diabetes—Enthusiasm outpaces evidence. JAMA Intern. Med. 2019, 179, 1163–1164. [Google Scholar] [CrossRef]
- Bae, J.H.; Park, J.H.; Im, S.S.; Song, D.K. Coffee and health. Integr. Med. Res. 2014, 3, 189–191. [Google Scholar] [CrossRef]
- Przybysz, M.A.; Widla, G.; Dluzewska, E. Preferencje konsumenckie picia kawy. Wpływ temperatury i czasu prażenia ziarna kawy na ocenę smaku i zapachu espresso. Zesz. Probl. Postępów Nauk. Rol. 2013, 572, 65–79. [Google Scholar]
| 95% Confidence Interval | ||||||
|---|---|---|---|---|---|---|
| Predictor | Estimate | SE | Lower | Upper | t | p |
| Intercept a | 20.34 | 11.91 | −3.22 | 43.89 | 1.71 | 0.090 |
| Sex [F]: | ||||||
| 0–1 | 5.04 | 1.46 | 2.16 | 7.92 | 3.46 | <0.001 |
| High OS [presence]: | ||||||
| 0–1 | −0.72 | 1.77 | −4.23 | 2.78 | −0.41 | 0.684 |
| Age [years] | −0.13 | 0.09 | −0.31 | 0.04 | −1.50 | 0.136 |
| MoCA [pts] | −0.08 | 0.15 | −0.37 | 0.21 | −0.53 | 0.596 |
| GDS [pts] | −0.20 | 0.21 | −0.61 | 0.22 | −0.95 | 0.345 |
| Health status assessment currently [pts] | −0.30 | 0.35 | −0.99 | 0.39 | −0.86 | 0.390 |
| Education [years] | −0.08 | 0.16 | −0.39 | 0.24 | −0.47 | 0.638 |
| Drinking beer [freq]: | ||||||
| 3–0 | 0.41 | 1.39 | −2.35 | 3.16 | 0.29 | 0.771 |
| 4–0 | 0.67 | 2.47 | −4.21 | 5.56 | 0.27 | 0.786 |
| 5–0 | 8.99 | 3.59 | 1.89 | 16.08 | 2.51 | 0.013 |
| Chocolate [freq]: | ||||||
| 3–0 | −3.01 | 1.77 | −6.51 | 0.49 | −1.70 | 0.091 |
| 4–0 | −2.15 | 1.76 | −5.62 | 1.32 | −1.22 | 0.223 |
| 5–0 | −3.41 | 1.80 | −6.98 | 0.15 | −1.89 | 0.060 |
| 6–0 | −0.29 | 2.37 | −4.99 | 4.41 | −0.12 | 0.903 |
| Sweets [freq]: | ||||||
| 3–0 | 5.41 | 2.35 | 0.75 | 10.06 | 2.30 | 0.023 |
| 4–0 | 4.96 | 2.12 | 0.77 | 9.16 | 2.34 | 0.021 |
| 5–0 | 4.45 | 2.13 | 0.24 | 8.66 | 2.09 | 0.038 |
| 6–0 | 4.83 | 2.27 | 0.35 | 9.31 | 2.13 | 0.035 |
| Fish [freq]: | ||||||
| 0–4 | 0.80 | 1.64 | −2.45 | 4.05 | 0.49 | 0.627 |
| 3–4 | −0.58 | 1.31 | −3.17 | 2.01 | −0.44 | 0.658 |
| 5–4 | 0.27 | 1.54 | −2.79 | 3.33 | 0.17 | 0.862 |
| Coffee [freq]: | ||||||
| 0–6 | 2.85 | 2.08 | −1.26 | 6.96 | 1.37 | 0.173 |
| 3–6 | 2.75 | 2.70 | −2.59 | 8.09 | 1.02 | 0.310 |
| 4–6 | −1.29 | 2.88 | −6.99 | 4.41 | −0.45 | 0.654 |
| 5–6 | 3.88 | 1.76 | 0.40 | 7.37 | 2.20 | 0.029 |
| Green and/or white tea [freq]: | ||||||
| 0–6 | −3.20 | 1.42 | −6.02 | −0.39 | −2.25 | 0.026 |
| 3–6 | −1.86 | 1.89 | −5.60 | 1.88 | −0.98 | 0.328 |
| 4–6 | 0.44 | 1.95 | −3.42 | 4.30 | 0.23 | 0.821 |
| 5–6 | −0.49 | 1.48 | −3.41 | 2.43 | −0.33 | 0.738 |
| Red wine [freq]: | ||||||
| 3–0 | −2.48 | 1.26 | −4.97 | 0.01 | −1.97 | 0.051 |
| 4–0 | 1.59 | 1.96 | −2.30 | 5.47 | 0.81 | 0.420 |
| 5–0 | −0.22 | 2.39 | −4.95 | 4.51 | −0.09 | 0.926 |
| 6–0 | −0.07 | 5.00 | −9.96 | 9.81 | −0.01 | 0.988 |
| Vegetables and fruit [freq]: | ||||||
| 4–3 | −5.95 | 6.85 | −19.49 | 7.60 | −0.87 | 0.387 |
| 5–3 | −7.49 | 6.57 | −20.49 | 5.51 | −1.14 | 0.257 |
| 6–3 | −7.55 | 6.40 | −20.21 | 5.11 | −1.18 | 0.240 |
| Vodka and other 80-proof alcohols [freq]: | ||||||
| 3–0 | 0.31 | 1.43 | −2.52 | 3.13 | 0.21 | 0.831 |
| 4–0 | −1.40 | 2.26 | −5.87 | 3.07 | −0.62 | 0.536 |
| 5–0 | 10.20 | 7.00 | −3.64 | 24.05 | 1.46 | 0.147 |
| 6–0 | 6.58 | 7.07 | −7.41 | 20.57 | 0.93 | 0.354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujawska, A.; López Sánchez, G.F.; Hoti, F.; Kujawski, S.; Zalewski, P.; Kędziora-Kornatowska, K. Relationship of the Behavior of Older Participants with Body Composition Change: Results of the Second Wave of the Cognition of Older People, Education, Recreational Activities, Nutrition, Comorbidities, and Functional Capacity Studies (COPERNICUS). Nutrients 2023, 15, 1834. https://doi.org/10.3390/nu15081834
Kujawska A, López Sánchez GF, Hoti F, Kujawski S, Zalewski P, Kędziora-Kornatowska K. Relationship of the Behavior of Older Participants with Body Composition Change: Results of the Second Wave of the Cognition of Older People, Education, Recreational Activities, Nutrition, Comorbidities, and Functional Capacity Studies (COPERNICUS). Nutrients. 2023; 15(8):1834. https://doi.org/10.3390/nu15081834
Chicago/Turabian StyleKujawska, Agnieszka, Guillermo F. López Sánchez, Flaka Hoti, Sławomir Kujawski, Paweł Zalewski, and Kornelia Kędziora-Kornatowska. 2023. "Relationship of the Behavior of Older Participants with Body Composition Change: Results of the Second Wave of the Cognition of Older People, Education, Recreational Activities, Nutrition, Comorbidities, and Functional Capacity Studies (COPERNICUS)" Nutrients 15, no. 8: 1834. https://doi.org/10.3390/nu15081834
APA StyleKujawska, A., López Sánchez, G. F., Hoti, F., Kujawski, S., Zalewski, P., & Kędziora-Kornatowska, K. (2023). Relationship of the Behavior of Older Participants with Body Composition Change: Results of the Second Wave of the Cognition of Older People, Education, Recreational Activities, Nutrition, Comorbidities, and Functional Capacity Studies (COPERNICUS). Nutrients, 15(8), 1834. https://doi.org/10.3390/nu15081834






