Dietary Trehalose as a Bioactive Nutrient
Abstract
1. Introduction
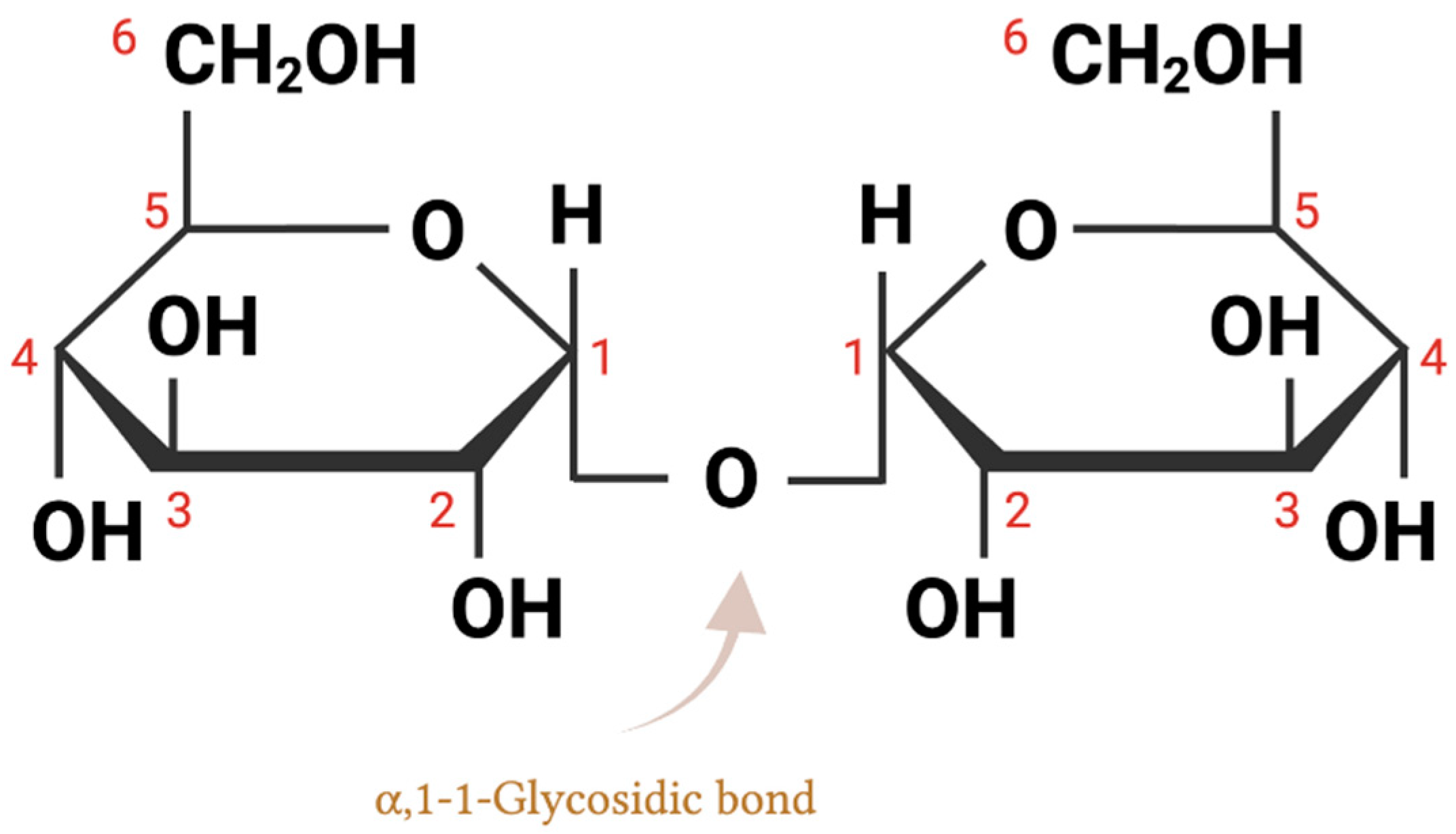
2. Trehalose Chemical Structure and Biochemical Properties
3. Trehalose as an Increasingly Consumed Dietary Sugar
4. Trehalose as a Low Glycemic-Index Sugar for Diabetes Mitigation
5. Trehalose Effects on the Gut Microbiome
5.1. Alterations in Gut Microbiome in Response to Trehalose and Other Sugars
5.2. Trehalose and Clostridioides difficile
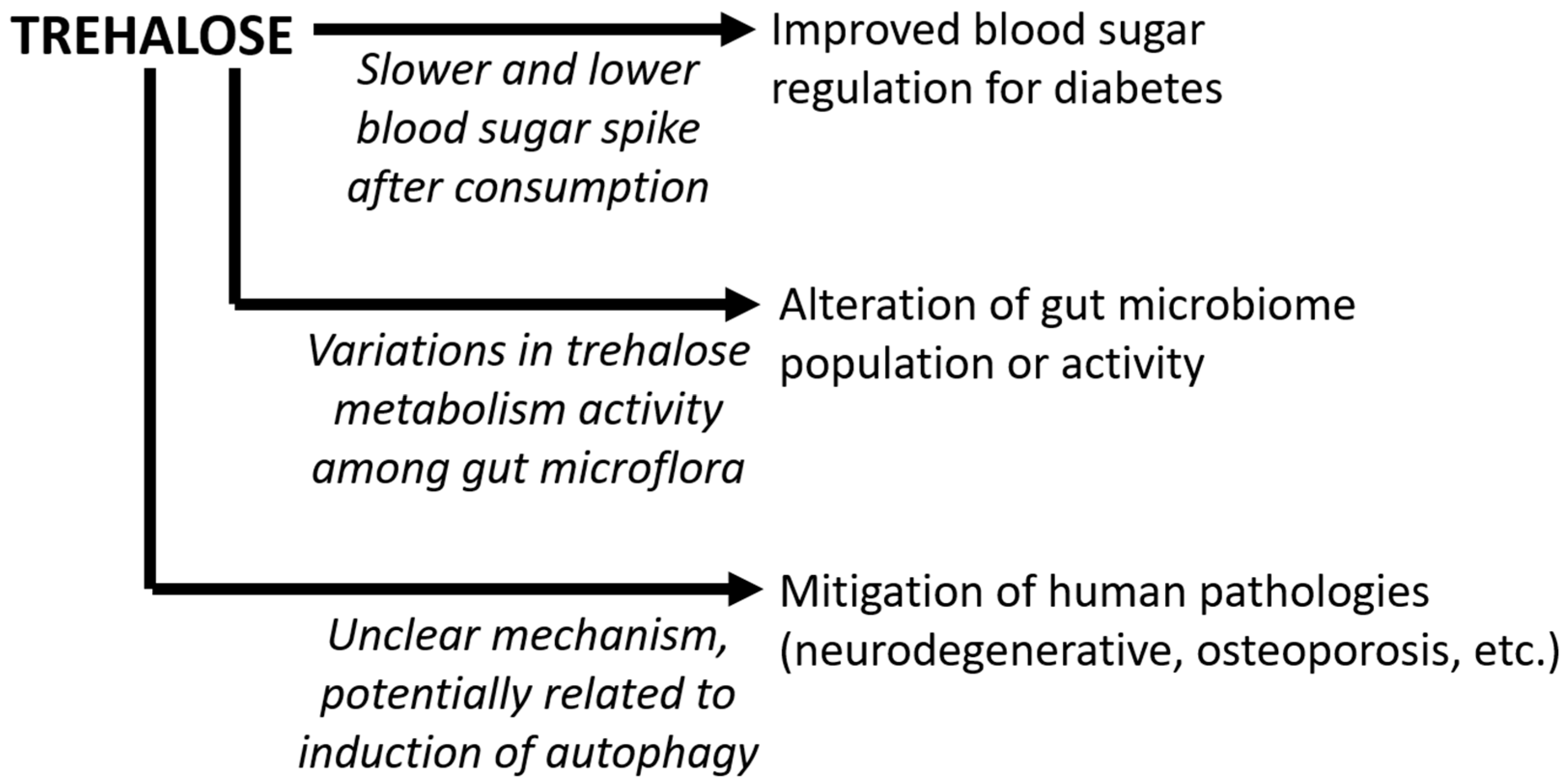
6. Summary and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Q.; Schmidt, R.K.; Teo, B.; Karplus, P.A.; Brady, J.W. Molecular dynamics studies of the hydration of α,α-trehalose. J. Am. Chem. Soc. 1997, 119, 7851–7862. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, S.; Wang, Y.J. Trehalose: Current Use and Future Applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, H. The Importance of Trehalose Sugar. Biomed. J. Sci. Tech. Res. 2019, 21, 15917–15918. [Google Scholar] [CrossRef]
- Tapia, H.; Young, L.; Fox, D.; Bertozzi, C.R.; Koshland, D. Increasing intracellular trehalose is sufficient to confer desiccation tolerance to Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2015, 112, 6122–6127. [Google Scholar] [CrossRef]
- Chen, A.; Gibney, P.A. Intracellular trehalose accumulation via the Agt1 transporter promotes freeze–thaw tolerance in Saccharomyces cerevisiae. J. Appl. Microbiol. 2022, 133, 2390–2402. [Google Scholar] [CrossRef]
- Chen, A.; Tapia, H.; Goddard, J.M.; Gibney, P.A. Trehalose and its applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5004–5037. [Google Scholar] [CrossRef]
- Laihia, J.; Kaarniranta, K. Trehalose for Ocular Surface Health. Biomolecules 2020, 10, 809. [Google Scholar] [CrossRef]
- Cai, X.; Seitl, I.; Mu, W.; Zhang, T.; Stressler, T.; Fischer, L.; Jiang, B. Biotechnical production of trehalose through the trehalose synthase pathway: Current status and future prospects. Appl. Microbiol. Biotechnol. 2018, 102, 2965–2976. [Google Scholar] [CrossRef]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chrétien, D.; Jegou, D.; Bach, J.-M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef]
- O’Neill, M.K.; Piligian, B.F.; Olson, C.D.; Woodruff, P.J.; Swarts, B.M. Tailoring trehalose for biomedical and biotechnological applications. Pure Appl. Chem. 2017, 89, 1223–1249. [Google Scholar] [CrossRef]
- Sahebkar, A.; Khalifeh, M.; Barreto, G.E. Therapeutic potential of trehalose in neurodegenerative diseases: The knowns and unknowns. Neural Regen. Res. 2021, 16, 2026–2027. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, D.; Chen, X.; Bian, F.; Qin, W.; Gao, N.; Xiao, Y.; Li, J.; Pflugfelder, S.C.; Li, D.-Q. Trehalose Induces Autophagy Against Inflammation by Activating TFEB Signaling Pathway in Human Corneal Epithelial Cells Exposed to Hyperosmotic Stress. Investig. Opthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef]
- Lee, H.-J.; Yoon, Y.-S.; Lee, S.-J. Mechanism of neuroprotection by trehalose: Controversy surrounding autophagy induction. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Helferich, B.; Weis, K. Zur Synthese von Glucosiden und von nicht-reduzierenden Disacchariden. Eur. J. Inorg. Chem. 1956, 89, 314–321. [Google Scholar] [CrossRef]
- Stick, R.; Spencer, W. Disaccharides, oligosaccharides and polysaccharides. In Carbohydrates: The Essential Molecules of Life; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 321–341. [Google Scholar] [CrossRef]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose Metabolism: From Osmoprotection to Signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.L.; Panek, A.D. Biotechnological Applications of the Disaccharide Trehalose. Biotechnol. Annu. Rev. 1996, 2, 293–314. [Google Scholar] [PubMed]
- Patist, A.; Zoerb, H. Preservation mechanisms of trehalose in food and biosystems. Colloids Surf. B Biointerfaces 2005, 40, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M.; Hoekstra, F.A.; Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D.; Berne, B.J.; et al. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Olsson, C.; Swenson, J. Structural Comparison between Sucrose and Trehalose in Aqueous Solution. J. Phys. Chem. B 2020, 124, 3074–3082. [Google Scholar] [CrossRef]
- Roe, K.D.; Labuza, T.P. Glass Transition and Crystallization of Amorphous Trehalose-sucrose Mixtures. Int. J. Food Prop. 2005, 8, 559–574. [Google Scholar] [CrossRef]
- Collins, J.; Danhof, H.; Britton, R.A. The role of trehalose in the global spread of epidemic Clostridium difficile. Gut Microbes 2019, 10, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Colaço, C.; Sen, S.; Thangavelu, M.; Pinder, S.; Roser, B. Extraordinary Stability of Enzymes Dried in Trehalose: Simplified Molecular Biology. Nat. Biotechnol. 1992, 10, 1007–1011. [Google Scholar] [CrossRef]
- Richards, A.; Krakowka, S.; Dexter, L.; Schmid, H.; Wolterbeek, A.; Waalkens-Berendsen, D.; Shigoyuki, A.; Kurimoto, M. Trehalose: A review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2002, 40, 871–898. [Google Scholar] [CrossRef]
- Portmann, M.-O.; Birch, G. Sweet taste and solution properties of α,α-trehalose. J. Sci. Food Agric. 1995, 69, 275–281. [Google Scholar] [CrossRef]
- GRAS Notice No GRN 912. 2019. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (accessed on 19 February 2023).
- Elbein, A.D. The Metabolism of α,α-Trehalose. Adv. Carbohydr. Chem. Biochem. 1974, 30, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Buckley, A.M.; Moura, I.B.; Arai, N.; Spittal, W.; Clark, E.; Nishida, Y.; Harris, H.C.; Bentley, K.; Davis, G.; Wang, D.; et al. Trehalose-Induced Remodelling of the Human Microbiota Affects Clostridioides difficile Infection Outcome in an In Vitro Colonic Model: A Pilot Study. Front. Cell. Infect. Microbiol. 2021, 11, 670935. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Argüelles, J.C. Physiological roles of trehalose in bacteria and yeasts: A comparative analysis. Arch. Microbiol. 2000, 174, 217–224. [Google Scholar]
- Horlacher, R.; Boos, W. Characterization of TreR, the major regulator of the Escherichia coli trehalose system. J. Biol. Chem. 1997, 272, 13026–13032. [Google Scholar] [CrossRef] [PubMed]
- Boos, W.; Ehmann, U.; Forkl, H.; Klein, W.; Rimmele, M.; Postma, P. Trehalose transport and metabolism in Escherichia coli. J. Bacteriol. 1990, 172, 3450–3461. [Google Scholar] [CrossRef] [PubMed]
- Arola, H.; Koivula, T.; Karvonen, A.L.; Jokela, H.; Ahola, T.; Isokoski, M. Low trehalase activity is associated with abdominal symptoms caused by edible mushrooms. Scand. J. Gastroenterol. 1999, 34, 898–903. [Google Scholar] [PubMed]
- Gudmand-Høsyer, E.; Fenger, H.J.; Skovbjerg, H.; Kern-Hansen, P.; Madsen, P.R. Trehalase deficiency in Greenland. Scand. J. Gastroenterol. 1988, 23, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Angelopoulos, T.J. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients 2016, 8, 697. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Kalra, S.; Choudhary, N.; Unnikrishnan, A.G.; Ajish, T. Preventive pharmacotherapy in type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2012, 16, 33–43. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Yaribeygi, A.; Sathyapalan, T.; Sahebkar, A. Molecular mechanisms of trehalose in modulating glucose homeostasis in diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2214–2218. [Google Scholar] [CrossRef]
- Yoshizane, C.; Mizote, A.; Yamada, M.; Arai, N.; Arai, S.; Maruta, K.; Mitsuzumi, H.; Ariyasu, T.; Ushio, S.; Fukuda, S. Glycemic, insulinemic and incretin responses after oral trehalose ingestion in healthy subjects. Nutr. J. 2017, 16, 9. [Google Scholar] [CrossRef]
- Oku, T.; Nakamura, S. Estimation of intestinal trehalase activity from a laxative threshold of trehalose and lactulose on healthy female subjects. Eur. J. Clin. Nutr. 2000, 54, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Keyhani-Nejad, F.; Kemper, M.; Schueler, R.; Pivovarova, O.; Rudovich, N.; Pfeiffer, A.F. Effects of Palatinose and Sucrose Intake on Glucose Metabolism and Incretin Secretion in Subjects with Type 2 Diabetes. Diabetes Care 2015, 39, e38–e39. [Google Scholar] [CrossRef]
- Mizote, A.; Yamada, M.; Yoshizane, C.; Arai, N.; Maruta, K.; Arai, S.; Endo, S.; Ogawa, R.; Mitsuzumi, H.; Ariyasu, T.; et al. Daily Intake of Trehalose Is Effective in the Prevention of Lifestyle-Related Diseases in Individuals with Risk Factors for Metabolic Syndrome. J. Nutr. Sci. Vitaminol. 2016, 62, 380–387. [Google Scholar] [CrossRef] [PubMed]
- DeBosch, B.J.; Heitmeier, M.R.; Mayer, A.L.; Higgins, C.B.; Crowley, J.R.; Kraft, T.E.; Chi, M.; Newberry, E.P.; Chen, Z.; Finck, B.N.; et al. Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci. Signal. 2016, 9, ra21. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, P.D. Microbial evolution and ecological opportunity in the gut environment. Proc. R. Soc. B Boil. Sci. 2019, 286, 20191964. [Google Scholar] [CrossRef]
- Di Rienzi, S.C.; Britton, R.A. Adaptation of the Gut Microbiota to Modern Dietary Sugars and Sweeteners. Adv. Nutr. Int. Rev. J. 2019, 11, 616–629. [Google Scholar] [CrossRef]
- Ervin, R.B.; Ogden, C.L. Consumption of Added Sugars among U.S. Adults, 2005–2010; NCHS Data Brief: Hyattsville, MD, USA, 2013; pp. 1–8. [Google Scholar]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners—A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Natural sweeteners: The relevance of food naturalness for consumers, food security aspects, sustainability and health impacts. Int. J. Environ. Res. Public Health 2020, 17, 6285. [Google Scholar] [CrossRef]
- Townsend, G.E.; Han, W.; Schwalm, N.D., III; Raghavan, V.; Barry, N.A.; Goodman, A.L.; Groisman, E.A. Dietary sugar silences a colonization factor in a mammalian gut symbiont. Proc. Natl. Acad. Sci. USA 2019, 116, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.P.; Mota, M.; Martins, F.O.; Nogueira, C.; Gonçalves, T.; Carneiro, T.; Pinto, J.; Duarte, D.; Barros, A.S.; Jones, J.G.; et al. Intestinal Microbial and Metabolic Profiling of Mice Fed with High-Glucose and High-Fructose Diets. J. Proteome Res. 2018, 17, 2880–2891. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Srionnual, S.; Onda, T.; Yanagida, F. Effects of prebiotic oligosaccharides and trehalose on growth and production of bacteriocins by lactic acid bacteria. Lett. Appl. Microbiol. 2007, 45, 190–193. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Ramne, S.; Brunkwall, L.; Ericson, U.; Gray, N.; Kuhnle, G.G.; Nilsson, P.M.; Orho-Melander, M.; Sonestedt, E. Gut microbiota composition in relation to intake of added sugar, sugar-sweetened beverages and artificially sweetened beverages in the Malmö Offspring Study. Eur. J. Nutr. 2021, 60, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Azeez, O.H.; Alkass, S.Y.; Persike, D.S. Long-Term Saccharin Consumption and Increased Risk of Obesity, Diabetes, Hepatic Dysfunction, and Renal Impairment in Rats. Medicina 2019, 55, 681. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Ramiro, R.S.; Barroso-Batista, J.; Güleresi, D.; Lourenço, M.; Gordo, I. Recurrent Reverse Evolution Maintains Polymorphism after Strong Bottlenecks in Commensal Gut Bacteria. Mol. Biol. Evol. 2017, 34, 2879–2892. [Google Scholar] [CrossRef]
- Peuranen, S.; Tiihonen, K.; Apajalahti, J.; Kettunen, A.; Saarinen, M.; Rautonen, N. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. Br. J. Nutr. 2004, 91, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Robinson, C.; Danhof, H.; Knetsch, C.W.; Van Leeuwen, H.C.; Lawley, T.D.; Auchtung, J.; Britton, R.A. Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature 2018, 553, 291–294. [Google Scholar] [CrossRef]
- Saund, K.; Rao, K.; Young, V.; Snitkin, E.S. Genetic Determinants of Trehalose Utilization Are Not Associated With Severe Clostridium difficile Infection Outcome. Open Forum Infect. Dis. 2020, 7, ofz548. [Google Scholar] [CrossRef]
- Danielson, N.D.; Collins, J.; Stothard, A.I.; Dong, Q.Q.; Kalera, K.; Woodruff, P.J.; DeBosch, B.J.; Britton, R.A.; Swarts, B.M. Degradation-resistant trehalose analogues block utilization of trehalose by hypervirulent Clostridioides difficile. Chem. Commun. 2019, 55, 5009–5012. [Google Scholar] [CrossRef]
- Freeman, J.; O’Neill, F.J.; Wilcox, M.H. Effects of cefotaxime and desacetylcefotaxime upon Clostridium difficile proliferation and toxin production in a triple-stage chemostat model of the human gut. J. Antimicrob. Chemother. 2003, 52, 96–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaikh, N.; Ferey, J.L.; Wankhade, U.D.; Chintapalli, S.V.; Higgins, C.B.; Crowley, J.R.; Heitmeier, M.R.; Stothard, A.I.; Mihi, B.; et al. Lactotrehalose, an Analog of Trehalose, Increases Energy Metabolism Without Promoting Clostridioides difficile Infection in Mice. Gastroenterology 2020, 158, 1402–1416.e2. [Google Scholar] [CrossRef]
- Eyre, D.W.; Didelot, X.; Buckley, A.M.; Freeman, J.; Moura, I.B.; Crook, D.W.; Peto, T.E.; Walker, A.S.; Wilcox, M.H.; Dingle, K.E. Clostridium difficile trehalose metabolism variants are common and not associated with adverse patient outcomes when variably present in the same lineage. Ebiomedicine 2019, 43, 347–355. [Google Scholar] [CrossRef]
- Borren, N.; Ghadermarzi, S.; Hutfless, S.; Ananthakrishnan, A.N. The emergence of Clostridium difficile infection in Asia: A systematic review and meta-analysis of incidence and impact. PLoS ONE 2017, 12, e0176797. [Google Scholar] [CrossRef]
- Chaitanya, N.S.N.; Devi, A.; Sahu, S.; Alugoju, P. Molecular mechanisms of action of Trehalose in cancer: A comprehensive review. Life Sci. 2021, 269, 118968. [Google Scholar] [CrossRef]
- Khalifeh, M.; Barreto, G.E.; Sahebkar, A. Trehalose as a promising therapeutic candidate for the treatment of Parkinson’s disease. Br. J. Pharmacol. 2019, 176, 1173–1189. [Google Scholar] [CrossRef]
- Portbury, S.D.; Hare, D.J.; Finkelstein, D.I.; Adlard, P.A. Trehalose improves traumatic brain injury-induced cognitive impairment. PLoS ONE 2017, 12, e0183683. [Google Scholar] [CrossRef] [PubMed]
- Martinon, D.; Borges, V.F.; Gomez, A.C.; Shimada, K. Potential Fast COVID-19 Containment With Trehalose. Front. Immunol. 2020, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.M.; Moura, I.B.; Wilcox, M.H. Is there a causal relationship between trehalose consumption and Clostridioides difficile infection? Curr. Opin. Gastroenterol. 2021, 37, 9–14. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Gibney, P.A. Dietary Trehalose as a Bioactive Nutrient. Nutrients 2023, 15, 1393. https://doi.org/10.3390/nu15061393
Chen A, Gibney PA. Dietary Trehalose as a Bioactive Nutrient. Nutrients. 2023; 15(6):1393. https://doi.org/10.3390/nu15061393
Chicago/Turabian StyleChen, Anqi, and Patrick A. Gibney. 2023. "Dietary Trehalose as a Bioactive Nutrient" Nutrients 15, no. 6: 1393. https://doi.org/10.3390/nu15061393
APA StyleChen, A., & Gibney, P. A. (2023). Dietary Trehalose as a Bioactive Nutrient. Nutrients, 15(6), 1393. https://doi.org/10.3390/nu15061393






