Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics
Abstract
1. Introduction
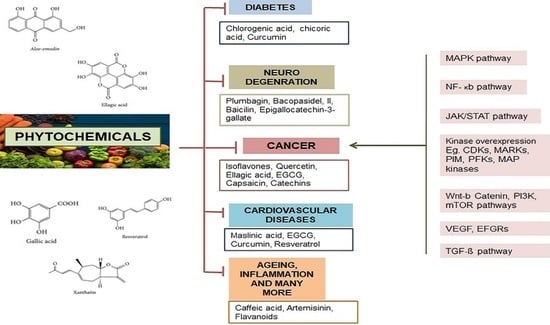
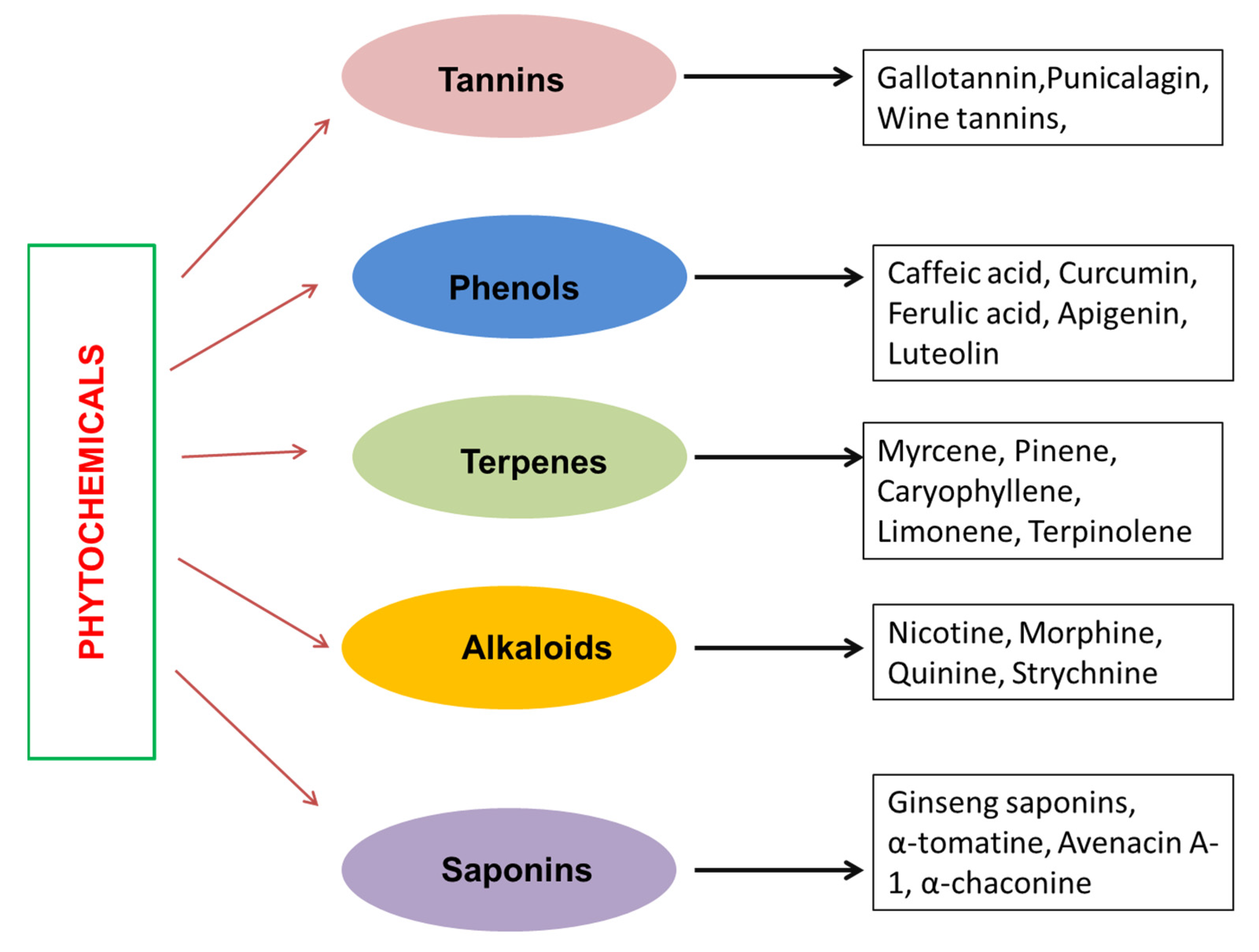
2. Phytochemicals
3. Phenolic Compounds and Their Role in Cancer Management
3.1. Curcumin
3.2. Resveratrol
3.3. Apigenin
3.4. Gingerol
3.5. Thymoquinone
4. Tannins in Cancer Management
4.1. Epigallocatechin Gallate
4.2. Gallic Acid
| Tannins | Cancer | Effect on Cancer | Refs. |
|---|---|---|---|
| Tannic acid (TA) | Breast cancer cell lines (MCF-7, MDA-MB-231, BT474) Prostate Cancer Cells (PC-3 and LNCaP) Head and Neck Cancer (FaDu and YD-38) |
| [135,136,137] [138] [139] |
| Ellagic acid (EA) | Human Bladder Cancer Cell Lines (T24, UM-UC-3, 5637, and HT-1376) Lung Cancer cell line A549 |
| [140] [141,142,143] |
| EGCG | Breast cancer cell line 4T1 Human esophageal squamous carcinoma cells Eca109 Colorectal cancer (DLD-1 and SW480) Oral squamous cell carcinoma (HSC-3) |
| [144] [145] [146] [147] |
| Gallic acid | Prostate cancer cell lines (DU145) Human lung cancer cells. Calu-6 and A549 Leukemia K562 cell line |
| [127] [130] [148] |
| Procyanidins | Human breast cancer cell line MCF7 Non-small cell lung cancer (NSCLC) |
| [149] [150] |
| Green tea catechins | Human lung cancer cell line PC-9 Human prostate cancer DU145 cell line |
| [151] [152] |
| Epicatechin (flavon-3-ol monomer units) | Human bladder cancer TCCSUP cell line |
| [153] |
5. Alkaloids in Cancer Treatment
6. Terpenes in Cancer Treatment
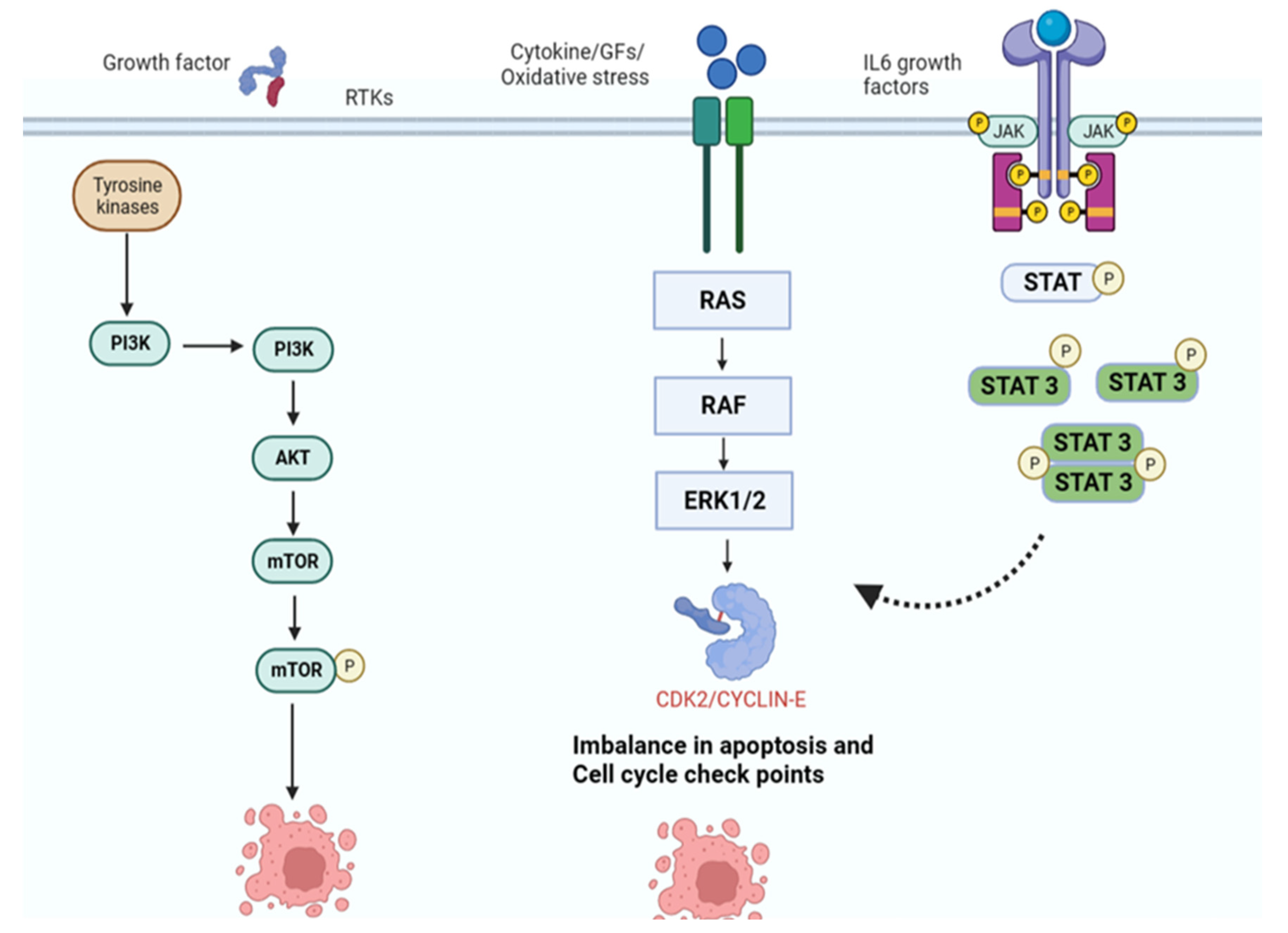
7. Mechanism of Action of Phytochemicals
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.-L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef]
- Judson, P.L.; Abdallah, R.; Xiong, Y.; Ebbert, J.; Lancaster, J.M. Complementary and alternative medicine use in individuals presenting for care at a comprehensive cancer center. Integr. Cancer Ther. 2017, 16, 96–103. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Woo, Y.M.; Shin, Y.; Lee, E.J.; Lee, S.; Jeong, S.H.; Kong, H.K.; Park, E.Y.; Kim, H.K.; Han, J.; Chang, M.; et al. Inhibition of aerobic glycolysis represses Akt/mTOR/HIF-1α axis and restores tamoxifen sensitivity in antiestrogen-resistant breast cancer cells. PLoS ONE 2015, 10, e0132285. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, R.; Yu, J.; Xing, C.; Zhuang, C.; Qu, Z. Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis. Nutrients 2023, 15, 491. [Google Scholar] [CrossRef]
- Mandal, M.K.; Mohammad, M.; Parvin, S.I.; Islam, M.M.; Gazi, H.A.R.; Alberto, A.K.M.; da Costa, M.J.; Carvalho, J.C.T. A Short Review on Anticancer Phytochemicals. Pharmacogn. Rev. 2023, 17, 11–23. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic acid exhibits anticancer effects via MARK4 inhibition. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, S.; Anjum, F.; Shamsi, A.; Khan, P.; Fatima, H.; Shafie, A.; Islam, A.; Hassan, M.I. Myricetin inhibits breast and lung cancer cells proliferation via inhibiting MARK4. J. Cell. Biochem. 2022, 123, 359–374. [Google Scholar] [CrossRef]
- Ogbonna, J.; Kenechukwu, F.; Attama, A.; Chime, S. Different approaches to formulation of herbal extracts/phytopharmaceuticals/bioactive phytochstituents-a review. Int. J. Pharm. Sci. Rev. Res. 2012, 16, 1–8. [Google Scholar]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Breinbauer, R.; Vetter, I.R.; Waldmann, H. From protein domains to drug candidates—Natural products as guiding principles in the design and synthesis of compound libraries. Angew. Chem. Int. Ed. 2002, 41, 2878–2890. [Google Scholar] [CrossRef]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.-J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Anwar, S.; Mohammad, T.; Shahwan, M.; Hassan, M.I.; Islam, A. Therapeutic potential of polyphenols in Alzheimer’s therapy: Broad-spectrum and minimal side effects as key aspects. In Autism Spectrum Disorder and Alzheimer’s Disease: Advances in Research; Springer: Berlin/Heidelberg, Germany, 2022; pp. 111–133. [Google Scholar]
- Panda, A.K.; Chakraborty, D.; Sarkar, I.; Khan, T.; Sa, G. New insights into therapeutic activity and anticancer properties of curcumin. J. Exp. Pharmacol. 2017, 9, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Murugan, D.; Leong, X.-F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. In Proceedings of Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–17. [Google Scholar]
- Anwar, S.; Mohammad, T.; Shamsi, A.; Queen, A.; Parveen, S.; Luqman, S.; Hasan, G.M.; Alamry, K.A.; Azum, N.; Asiri, A.M.; et al. Discovery of Hordenine as a potential inhibitor of pyruvate dehydrogenase kinase 3: Implication in lung Cancer therapy. Biomedicines 2020, 8, 119. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Mohammad, T.; Islam, A.; Hassan, M.I. Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188568. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.S.; Mohanty, S.; Hossain, D.M.S.; Bhattacharyya, S.; Banerjee, S.; Chakraborty, J.; Saha, S.; Ray, P.; Bhattacharjee, P.; Mandal, D. Curcumin enhances the efficacy of chemotherapy by tailoring p65NFκB-p300 cross-talk in favor of p53-p300 in breast cancer. J. Biol. Chem. 2011, 286, 42232–42247. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.; Husain, M. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci. Rep. 2018, 8, 8323. [Google Scholar] [CrossRef] [PubMed]
- Selvaraji, S.; Poh, L.; Natarajan, V.; Mallilankaraman, K.; Arumugam, T.V. Negative conditioning of mitochondrial dysfunction in age-related neurodegenerative diseases. Cond. Med. 2019, 2, 30. [Google Scholar]
- Devasagayam, T.; Sainis, K. Immune System and Antioxidants, Especially Those Derived from Indian Medicinal Plants. IJEB 2002, 40, 639–655. [Google Scholar]
- Akhtar, M.F.; Saleem, A.; Rasul, A.; Baig, M.M.F.A.; Bin-Jumah, M.; Daim, M.M.A. Anticancer natural medicines: An overview of cell signaling and other targets of anticancer phytochemicals. Eur. J. Pharmacol. 2020, 888, 173488. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A. Anticancer potential of medicinal plants and their phytochemicals: A review. Braz. J. Bot. 2015, 38, 199–210. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Thomson, M.; Ali, M. Garlic [Allium sativum]: A review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targets 2003, 3, 67–81. [Google Scholar] [CrossRef]
- Khan, I.; Abbas, T.; Anjum, K.; Abbas, S.Q.; Shagufta, B.I.; Ali Shah, S.A.; Akhter, N. Antimicrobial potential of aqueous extract of Camellia sinensis against representative microbes. Pak. J. Pharm. Sci. 2019, 32, 631–636. [Google Scholar]
- Sharif, T.; Alhosin, M.; Auger, C.; Minker, C.; Kim, J.-H.; Etienne-Selloum, N.; Bories, P.; Gronemeyer, H.; Lobstein, A.; Bronner, C.; et al. Aronia melanocarpa juice induces a redox-sensitive p73-related caspase 3-dependent apoptosis in human leukemia cells. PLoS ONE 2012, 7, e32526. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Mishra, B.; Sangwan, N.S. Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. BioMed Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef] [PubMed]
- Kumar Roy, M.; Nakahara, K.; Na Thalang, V.; Trakoontivakorn, G.; Takenaka, M.; Isobe, S.; Tsushida, T. Baicalein, a flavonoid extracted from a methanolic extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. Die Pharm.-Int. J. Pharm. Sci. 2007, 62, 149–153. [Google Scholar]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Ishikawa, H.; Saeki, T.; Otani, T.; Suzuki, T.; Shimozuma, K.; Nishino, H.; Fukuda, S.; Morimoto, K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006, 136, 816S–820S. [Google Scholar] [CrossRef]
- Tanaka, S.; Haruma, K.; Yoshihara, M.; Kajiyama, G.; Kira, K.; Amagase, H.; Chayama, K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J. Nutr. 2006, 136, 821S–826S. [Google Scholar] [CrossRef]
- Natelson, E.A.; Giovanella, B.C.; Verschraegen, C.F.; Fehir, K.M.; De Ipolyi, P.D.; Harris, N.; Stehlin, J.S. Phase I clinical and pharmacological studies of 20-(S)-camptothecin and 20-(S)-9-nitrocamptothecin as anticancer agents. Ann. N. Y. Acad. Sci. 1996, 803, 224–230. [Google Scholar] [CrossRef]
- Verschraegen, C.F.; Gilbert, B.E.; Loyer, E.; Huaringa, A.; Walsh, G.; Newman, R.A.; Knight, V. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20 (s)-camptothecin in patients with advanced pulmonary malignancies. Clin. Cancer Res. 2004, 10, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Hsu, C.; Lin, J.; Hsu, M.; Ho, Y.; Shen, T.; Ko, T.; Lin, J.; Lin, B.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high risk or pre-malignant lesion. Anti-Cancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Xie, L.P.; Lee, A.H.; Binns, C.W. Protective effect of green tea against prostate cancer: A case-control study in southeast China. Int. J. Cancer 2004, 108, 130–135. [Google Scholar] [CrossRef]
- Gao, Y.T.; McLaughlin, J.K.; Blot, W.J.; Ji, B.T.; Dai, Q.; Fraumeni, J.F. Reduced risk of esophageal cancer associated with green tea consumption. JNCI J. Natl. Cancer Inst. 1994, 86, 855–858. [Google Scholar] [CrossRef]
- Ji, B.T.; Chow, W.H.; Hsing, A.W.; McLaughlin, J.K.; Dai, Q.; Gao, Y.T.; Blot, W.J.; Fraumeni, J.F., Jr. Green tea consumption and the risk of pancreatic and colorectal cancers. Int. J. Cancer 1997, 70, 255–258. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, C.Y.; Lee, S.J. Effects of sun ginseng on subjective quality of life in cancer patients: A double-blind, placebo-controlled pilot trial. J. Clin. Pharm. Ther. 2006, 31, 331–334. [Google Scholar] [CrossRef]
- Miyanaga, N.; Akaza, H.; Hinotsu, S.; Fujioka, T.; Naito, S.; Namiki, M.; Takahashi, S.; Hirao, Y.; Horie, S.; Tsukamoto, T.; et al. Prostate cancer chemoprevention study: An investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Cancer Sci. 2012, 103, 125–130. [Google Scholar] [CrossRef]
- Lazarevic, B.; Boezelijn, G.; Diep, L.M.; Kvernrod, K.; Ogren, O.; Ramberg, H.; Moen, A.; Wessel, N.; Berg, R.E.; Egge-Jacobsen, W.; et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr. Cancer 2011, 63, 889–898. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.M.; Banerjee, S.; Banerjee, S.K.; Holzbeierlein, J.M.; Thrasher, J.B.; Kambhampati, S.; Keighley, J.; Van Veldhuizen, P. Short-term soy isoflavone intervention in patients with localized prostate cancer: A randomized, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e68331. [Google Scholar] [CrossRef]
- Hoensch, H.; Groh, B.; Edler, L.; Kirch, W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J. Gastroenterol. WJG 2008, 14, 2187. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Pridham, J.B. Phenolics in plants in health and disease. Proceedings of a Plant Phenolics Group Symposium held at Bristol, April 1959. In Proceedings of Phenolics in Plants in Health and Disease. Proceedings of a Plant Phenolics Group Symposium Held at Bristol, April 1959; Pergamon Press: Oxford, UK; London, UK, 1960. [Google Scholar]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Rahaiee, S.; Assadpour, E.; Esfanjani, A.F.; Silva, A.S.; Jafari, S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, I.; Puoci, F.; Chimento, A.; Sirianni, R.; Ruggiero, C.; Avena, P.; Pezzi, V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2013, 57, 71–83. [Google Scholar] [CrossRef]
- Jafari, S.; Saeidnia, S.; Abdollahi, M. Role of natural phenolic compounds in cancer chemoprevention via regulation of the cell cycle. Curr. Pharm. Biotechnol. 2014, 15, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Tsakiroglou, P.; VandenAkker, N.E.; Del Bo’, C.; Riso, P.; Klimis-Zacas, D. Role of berry anthocyanins and phenolic acids on cell migration and angiogenesis: An updated overview. Nutrients 2019, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.-Y.; Sayed, K.A.E. Olive phenolics as c-Met inhibitors:(-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. WJG 2013, 19, 7726. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Zhu, S.t.; Harris, P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005, 49, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Murad, L.D.; Soares, N.d.C.P.; Brand, C.; Monteiro, M.C.; Teodoro, A.J. Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr. Cancer 2015, 67, 532–542. [Google Scholar] [CrossRef]
- Rezaei-Tavirani, M.; Tavirani, M.R.; Azodi, M.Z. The bioinformatics aspects of gene screening of HT-29, human colon cell line treated with caffeic acid. Gastroenterol. Hepatol. Bed Bench 2019, 12, 246. [Google Scholar]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Venkata Reddy, B. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Kurata, R.; Adachi, M.; Yamakawa, O.; Yoshimoto, M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J. Agric. Food Chem. 2007, 55, 185–190. [Google Scholar] [CrossRef]
- Sourani, Z.; Pourgheysari, B.; Rafieian-Kopaei, M.; Shirzad, H.; Shirzad, M. The effect of gallic acid on Jurkat cell line. J. Herbmed Pharmacol. 2015, 4, 129–132. [Google Scholar]
- Zhao, B.; Hu, M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol. Lett. 2013, 6, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Niho, N.; Shibutani, M.; Tamura, T.; Toyoda, K.; Uneyama, C.; Takahashi, N.; Hirose, M. Subchronic toxicity study of gallic acid by oral administration in F344 rats. Food Chem. Toxicol. 2001, 39, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Rao, C.V. Chemoprevention of cancer by curcumin. In Cancer Chemoprevention: Promising Cancer Chemopreventive Agents; Humana Press Inc.: Totowa, NJ, USA, 2004; pp. 169–175. [Google Scholar]
- Lai, C.-S.; Ho, C.-T.; Pan, M.-H. The cancer chemopreventive and therapeutic potential of tetrahydrocurcumin. Biomolecules 2020, 10, 831. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Prakobwong, S.; Khoontawad, J.; Yongvanit, P.; Pairojkul, C.; Hiraku, Y.; Sithithaworn, P.; Pinlaor, P.; Aggarwal, B.B.; Pinlaor, S. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int. J. Cancer 2011, 129, 88–100. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Banerjee, S.; Stafford, L.J.; Xia, C.; Liu, M.; Aggarwal, B.B. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene 2002, 21, 8852–8861. [Google Scholar] [CrossRef] [PubMed]
- Gururaj, A.E.; Belakavadi, M.; Venkatesh, D.A.; Marmé, D.; Salimath, B.P. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem. Biophys. Res. Commun. 2002, 297, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. Rev. Mutat. Res. 2008, 658, 68–94. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, J.; Ge, M.; Yu, M.; Liu, L.; Zhang, Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem. Toxicol. 2013, 51, 87–92. [Google Scholar] [CrossRef]
- Upadhyay, G.; Singh, A.K.; Kumar, A.; Prakash, O.; Singh, M.P. Resveratrol modulates pyrogallol-induced changes in hepatic toxicity markers, xenobiotic metabolizing enzymes and oxidative stress. Eur. J. Pharmacol. 2008, 596, 146–152. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Aziz, M.H.; Afaq, F.; Ahmad, N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in Survivin. Photochem. Photobiol. 2005, 81, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Reagan-Shaw, S.; Wu, J.; Longley, B.J.; Ahmad, N. Chemoprevention of skin cancer by grape constituent resveratrol: Relevance to human disease? FASEB J. 2005, 19, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of ultraviolet B exposure-mediated activation of NF-κB in normal human keratinocytes by resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Afaq, F.; Aziz, M.H.; Ahmad, N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene 2004, 23, 5151–5160. [Google Scholar] [CrossRef]
- Zhu, Y.; Mao, Y.; Chen, H.; Lin, Y.; Hu, Z.; Wu, J.; Xu, X.; Xu, X.; Qin, J.; Xie, L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013, 13, 54. [Google Scholar] [CrossRef]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; MacLennan, G.T.; Gupta, S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Mafuvadze, B.; Liang, Y.; Besch-Williford, C.; Zhang, X.; Hyder, S.M. Apigenin induces apoptosis and blocks growth of medroxyprogesterone acetate-dependent BT-474 xenograft tumors. Horm. Cancer 2012, 3, 160–171. [Google Scholar] [CrossRef]
- Shukla, S.; Kanwal, R.; Shankar, E.; Datt, M.; Chance, M.R.; Fu, P.; MacLennan, G.T.; Gupta, S. Apigenin blocks IKKα activation and suppresses prostate cancer progression. Oncotarget 2015, 6, 31216. [Google Scholar] [CrossRef]
- Nielsen, S.; Young, J.; Daneshvar, B.; Lauridsen, S.; Knuthsen, P.; Sandström, B.; Dragsted, L.O. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br. J. Nutr. 1999, 81, 447–455. [Google Scholar] [CrossRef]
- Thiery-Vuillemin, A.; Nguyen, T.; Pivot, X.; Spano, J.; Dufresnne, A.; Soria, J. Molecularly targeted agents: Their promise as cancer chemopreventive interventions. Eur. J. Cancer 2005, 41, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C.B.; Fuzer, A.M.; Becceneri, A.B.; da Silva, J.A.; Tomasin, R.; Denoyer, D.; Kim, S.-H.; McIntyre, K.A.; Pearson, H.B.; Yeo, B.; et al. [10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo. Oncotarget 2017, 8, 72260. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.-H.; Hong, S.-S.; Cho, Y.-R.; Seo, D.-W. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of Akt and p38MAPK activity. Oncol. Rep. 2016, 35, 779–784. [Google Scholar] [CrossRef]
- Zhu, W.-Q.; Wang, J.; Guo, X.-F.; Liu, Z.; Dong, W.-G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016, 22, 4149. [Google Scholar] [CrossRef]
- Mostofa, A.; Hossain, M.K.; Basak, D.; Bin Sayeed, M.S. Thymoquinone as a potential adjuvant therapy for cancer treatment: Evidence from preclinical studies. Front. Pharmacol. 2017, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Odeh, L.H.; Talib, W.H.; Basheti, I.A. Synergistic effect of thymoquinone and melatonin against breast cancer implanted in mice. J. Cancer Res. Ther. 2018, 14, S324–S330. [Google Scholar]
- De Jesus, N.Z.T.; de Souza Falcão, H.; Gomes, I.F.; de Almeida Leite, T.J.; de Morais Lima, G.R.; Barbosa-Filho, J.M.; Tavares, J.F.; Silva, M.S.d.; de Athayde-Filho, P.F.; Batista, L.M. Tannins, peptic ulcers and related mechanisms. Int. J. Mol. Sci. 2012, 13, 3203–3228. [Google Scholar] [CrossRef]
- Lamy, E.; Pinheiro, C.; Rodrigues, L.; Capela-Silva, F.; Lopes, O.; Tavares, S.; Gaspar, R. Determinants of Tannin-Rich Food and Beverage Consumption: Oral Perception vs. Psychosocial Aspects. 2016. Available online: https://dspace.uevora.pt/rdpc/handle/10174/18018 (accessed on 15 March 2023).
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Monteleone, D.; Trombetta, D. Health effects of Vaccinium myrtillus L.: Evaluation of efficacy and technological strategies for preservation of active ingredients. Mini Rev. Med. Chem. 2014, 14, 567–584. [Google Scholar] [CrossRef]
- Bimonte, S.; Cascella, M.; Schiavone, V.; Mehrabi-Kermani, F.; Cuomo, A. The roles of epigallocatechin-3-gallate in the treatment of neuropathic pain: An update on preclinical in vivo studies and future perspectives. Drug Des. Dev. Ther. 2017, 11, 2737–2742. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S.M. Oral administration of copper to rats leads to increased lymphocyte cellular DNA degradation by dietary polyphenols: Implications for a cancer preventive mechanism. Biometals 2011, 24, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.; Mullen, W.; Hara, Y.; Crozier, A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J. Nutr. 2008, 138, 1535S–1542S. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Troufflard, S.; Serafini, M.; Crozier, A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 2009, 53, S44–S53. [Google Scholar] [CrossRef] [PubMed]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, E.; Suzuki, H.; Menegazzi, M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann. N. Y. Acad. Sci. 2002, 973, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Dutta, A.; Chatterjee, A. Epigallocatechin-3-gallate (EGCG) downregulates gelatinase-B (MMP-9) by involvement of FAK/ERK/NFκB and AP-1 in the human breast cancer cell line MDA-MB-231. Anti-Cancer Drugs 2010, 21, 632–644. [Google Scholar] [CrossRef]
- Kang, S.U.; Lee, B.-S.; Lee, S.-H.; Baek, S.J.; Shin, Y.S.; Kim, C.-H. Expression of NSAID-activated gene-1 by EGCG in head and neck cancer: Involvement of ATM-dependent p53 expression. J. Nutr. Biochem. 2013, 24, 986–999. [Google Scholar] [CrossRef]
- Bhatia, N.; Agarwal, C.; Agarwal, R. Differential responses of skin cancer-chemopreventive agents silibinin, quercetin, and epigallocatechin 3-gallate on mitogenic signaling and cell cycle regulators in human epidermoid carcinoma A431 cells. Nutr. Cancer 2001, 39, 292–299. [Google Scholar] [CrossRef]
- Liu, K.-C.; Huang, A.-C.; Wu, P.-P.; Lin, H.-Y.; Chueh, F.-S.; Yang, J.-S.; Lu, C.-C.; Chiang, J.-H.; Meng, M.; Chung, J.-G. Gallic acid suppresses the migration and invasion of PC-3 human prostate cancer cells via inhibition of matrix metalloproteinase-2 and-9 signaling pathways. Oncol. Rep. 2011, 26, 177–184. [Google Scholar]
- Wang, R.; Ma, L.; Weng, D.; Yao, J.; Liu, X.; Jin, F. Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol. Rep. 2016, 35, 3075–3083. [Google Scholar] [CrossRef]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Sherin, L.; Sohail, A.; Shujaat, S. Time-dependent AI-modeling of the anticancer efficacy of synthesized gallic acid analogues. Comput. Biol. Chem. 2019, 79, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pei, J.; Zheng, Y.; Miao, Y.-J.; Duan, B.-Z.; Huang, L.-F. Gallic acid: A potential anti-cancer agent. Chin. J. Integr. Med. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Yeh, R.-D.; Chen, J.-C.; Lai, T.-Y.; Yang, J.-S.; Yu, C.-S.; Chiang, J.-H.; Lu, C.-C.; Yang, S.-T.; Yu, C.-C.; Chang, S.-J. Gallic acid induces G0/G1 phase arrest and apoptosis in human leukemia HL-60 cells through inhibiting cyclin D and E, and activating mitochondria-dependent pathway. Anticancer. Res. 2011, 31, 2821–2832. [Google Scholar]
- Ji, B.-C.; Hsu, W.-H.; Yang, J.-S.; Hsia, T.-C.; Lu, C.-C.; Chiang, J.-H.; Yang, J.-L.; Lin, C.-H.; Lin, J.-J.; Suen, L.-J.W.; et al. Gallic acid induces apoptosis via caspase-3 and mitochondrion-dependent pathways in vitro and suppresses lung xenograft tumor growth in vivo. J. Agric. Food Chem. 2009, 57, 7596–7604. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Tyagi, A.; Agarwal, R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol. Cancer Ther. 2006, 5, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- Kawada, M.; Ohno, Y.; Ri, Y.; Ikoma, T.; Yuugetu, H.; Asai, T.; Watanabe, M.; Yasuda, N.; Akao, S.; Takemura, G.; et al. Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice. Anti-Cancer Drugs 2001, 12, 847–852. [Google Scholar] [CrossRef]
- Liang, C.-Z.; Zhang, X.; Li, H.; Tao, Y.-Q.; Tao, L.-J.; Yang, Z.-R.; Zhou, X.-P.; Shi, Z.-L.; Tao, H.-M. Gallic acid induces the apoptosis of human osteosarcoma cells in vitro and in vivo via the regulation of mitogen-activated protein kinase pathways. Cancer Biother. Radiopharm. 2012, 27, 701–710. [Google Scholar] [CrossRef]
- Booth, B.W.; Inskeep, B.D.; Shah, H.; Park, J.P.; Hay, E.J.; Burg, K.J. Tannic acid preferentially targets estrogen receptor-positive breast cancer. Int. J. Breast Cancer 2013, 2013, 369609. [Google Scholar] [CrossRef]
- Ngobili, T.A.; Shah, H.; Park, J.P.; Kwist, K.W.; Inskeep, B.; Burg, K.J.; Booth, B.W. Remodeling of tannic acid crosslinked collagen type I induces apoptosis in ER+ breast cancer cells. Anticancer Res. 2015, 35, 1285–1290. [Google Scholar]
- Jordan, L.G.; Booth, B.W. HER2+ breast cancer cells undergo apoptosis upon exposure to tannic acid released from remodeled cross-linked collagen type I. J. Biomed. Mater. Res. Part A 2018, 106, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, S.; Adali, O. Tannic acid inhibits proliferation, migration, invasion of prostate cancer and modulates drug metabolizing and antioxidant enzymes. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2016, 16, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.T.; Nguyen, T.T.K.; Yoo, H. Tannic acid-induced apoptosis in FaDu hypopharyngeal squamous cell carcinoma. Int. J. Oral Biol. 2019, 44, 43–49. [Google Scholar] [CrossRef]
- Ceci, C.; Tentori, L.; Atzori, M.G.; Lacal, P.M.; Bonanno, E.; Scimeca, M.; Cicconi, R.; Mattei, M.; De Martino, M.G.; Vespasiani, G.; et al. Ellagic acid inhibits bladder cancer invasiveness and in vivo tumor growth. Nutrients 2016, 8, 744. [Google Scholar] [CrossRef]
- Dahiya, R.; Mohammad, T.; Gupta, P.; Haque, A.; Alajmi, M.F.; Hussain, A.; Hassan, M.I. Molecular interaction studies on ellagic acid for its anticancer potential targeting pyruvate dehydrogenase kinase 3. RSC Adv. 2019, 9, 23302–23315. [Google Scholar] [CrossRef]
- Gupta, P.; Mohammad, T.; Khan, P.; Alajmi, M.F.; Hussain, A.; Rehman, M.T.; Hassan, M.I. Evaluation of ellagic acid as an inhibitor of sphingosine kinase 1: A targeted approach towards anticancer therapy. Biomed. Pharmacother. 2019, 118, 109245. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, X.; Niu, C.; Wang, X. Ellagic acid promotes A549 cell apoptosis via regulating the phosphoinositide 3-kinase/protein kinase B pathway. Exp. Ther. Med. 2018, 16, 347–352. [Google Scholar] [CrossRef]
- Wei, R.; Mao, L.; Xu, P.; Zheng, X.; Hackman, R.M.; Mackenzie, G.G.; Wang, Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018, 9, 5682–5696. [Google Scholar] [CrossRef]
- Liu, L.; Ju, Y.; Wang, J.; Zhou, R. Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells. Pathol. -Res. Pract. 2017, 213, 1242–1250. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.-Q.; Zhang, Q.; Zhu, J.-Y.; Li, Y.; Xie, C.-F.; Li, X.-T.; Wu, J.-S.; Geng, S.-S.; Zhong, C.-Y.; et al. (−)-Epigallocatechin-3-gallate inhibits colorectal cancer stem cells by suppressing Wnt/β-catenin pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef]
- Yoshimura, H.; Yoshida, H.; Matsuda, S.; Ryoke, T.; Ohta, K.; Ohmori, M.; Yamamoto, S.; Kiyoshima, T.; Kobayashi, M.; Sano, K. The therapeutic potential of epigallocatechin-3-gallate against human oral squamous cell carcinoma through inhibition of cell proliferation and induction of apoptosis: In vitro and in vivo murine xenograft study. Mol. Med. Rep. 2019, 20, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.C.; Reddy, D.B.; Aparna, A.; Arunasree, K.M.; Gupta, G.; Achari, C.; Reddy, G.; Lakshmipathi, V.; Subramanyam, A.; Reddanna, P. Anti-leukemic effects of gallic acid on human leukemia K562 cells: Downregulation of COX-2, inhibition of BCR/ABL kinase and NF-κB inactivation. Toxicol. In Vitro 2012, 26, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-T.; He, C.-X.; Kong, F.-L.; Han, S.-F.; Kong, X.-S.; Han, W.-Q.; Yang, L.-X. Grape Seed Procyanidins Inhibit the Growth of Breast Cancer MCF-7 Cells by Down-Regulating the EGFR/VEGF/MMP9 Pathway. Nat. Prod. Commun. 2021, 16, 1934578X21991691. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Cao, T.-T.; Liu, C.-L. Combined effect of vorinostat and grape seed proanthocyanidins on modulation of thymidine phosphorylase in non-small cell lung cancer. Trop. J. Pharm. Res. 2015, 14, 953–959. [Google Scholar] [CrossRef]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. Oncol. 2015, 141, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Chiou, Y.-S.; Wang, Y.-J.; Ho, C.-T.; Lin, J.-K. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011, 2, 101–110. [Google Scholar] [CrossRef]
- Philips, B.J.; Coyle, C.H.; Morrisroe, S.N.; Chancellor, M.B.; Yoshimura, N. Induction of apoptosis in human bladder cancer cells by green tea catechins. Biomed. Res. 2009, 30, 207–215. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231. [Google Scholar]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Trybus, W.; Trybus, E.; Król, T. Emodin Sensitizes Cervical Cancer Cells to Vinblastine by Inducing Apoptosis and Mitotic Death. Int. J. Mol. Sci. 2022, 23, 8510. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A. Happy Accidents: Serendipity in Modern Medical Breakthroughs; Arcade Publishing: New York, NY, USA, 2007. [Google Scholar]
- Arora, R.; Malhotra, P.; Mathur, A.K.; Mathur, A.; Govil, C.; Ahuja, P. Anticancer alkaloids of Catharanthus roseus: Transition from traditional to modern medicine. Herbal Medicine: A Cancer Chemopreventive and Therapeutic Perspective; Jaypee Brothers Medical Publishers Pvt. Ltd.: New Delhi, India, 2010; pp. 292–310. [Google Scholar]
- Škubník, J.; Pavlíčková, V.S.; Ruml, T.; Rimpelová, S. Vincristine in combination therapy of cancer: Emerging trends in clinics. Biology 2021, 10, 849. [Google Scholar] [CrossRef]
- Goa, K.L.; Faulds, D. Vinorelbine: A review of its pharmacological properties and clinical use in cancer chemotherapy. Drugs Aging 1994, 5, 200–234. [Google Scholar] [CrossRef]
- Bennouna, J.; Delord, J.-P.; Campone, M.; Nguyen, L. Vinflunine: A new microtubule inhibitor agent. Clin. Cancer Res. 2008, 14, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, B.; Panda, D.; Gupta, S.; Banerjee, M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 2008, 28, 155–183. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Wu, C.-C.; Chuang, Y.-H.; Chuang, W.-L. Anti-cancer mechanisms of clinically acceptable colchicine concentrations on hepatocellular carcinoma. Life Sci. 2013, 93, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khan, S.; Jairajpuri, D.S.; Hussain, A.; Alajmi, M.F.; Islam, A.; Luqman, S.; Parvez, S.; Hassan, M.I. Investigation of sphingosine kinase 1 inhibitory potential of cinchonine and colcemid targeting anticancer therapy. J. Biomol. Struct. Dyn. 2022, 40, 6350–6362. [Google Scholar] [CrossRef]
- Alim, A.; Goze, I.; Goze, H.M.; Tepe, B.; Serkedjieva, J. In vitro antimicrobial and antiviral activities of the essential oil and various extracts of Salvia cedronella Boiss. J. Med. Plants Res. 2009, 3, 413–419. [Google Scholar]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Chung, K.-S.; Hong, J.Y.; Lee, J.-H.; Lee, H.-J.; Park, J.Y.; Choi, J.-H.; Park, H.-J.; Hong, J.; Lee, K.-T. β-caryophyllene in the essential oil from chrysanthemum boreale induces G1 phase cell cycle arrest in human lung cancer cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef]
- Arul, S.; Rajagopalan, H.; Ravi, J.; Dayalan, H. Beta-caryophyllene suppresses ovarian cancer proliferation by inducing cell cycle arrest and apoptosis. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2020, 20, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, V.; Kotakonda, M.; Periyannan, V. JAK1/STAT3 regulatory effect of β-caryophyllene on MG-63 osteosarcoma cells via ROS-induced apoptotic mitochondrial pathway by DNA fragmentation. J. Biochem. Mol. Toxicol. 2020, 34, e22514. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.-P.; Wang, M.-J.; Zeng, X.; Chen, G.G.; Huang, R.-Y. Effects of glycyrrhizin in a mouse model of lung adenocarcinoma. Cell. Physiol. Biochem. 2017, 41, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Horie, N.; Hashimoto, K.; Satoh, K.; Shimoyama, T.; Kaneko, T.; Kusama, K.; Sakagami, H. Bimodal effect of glycyrrhizin on macrophage nitric oxide and prostaglandin E2 production. In Vivo 2008, 22, 583–586. [Google Scholar]
- Saleh, M.; Hashem, F.; Glombitza, K. Cytotoxicity and in vitro effects on human cancer cell lines of volatiles of Apium graveolens var. filicinum. Pharm. Pharmacol. Lett. 1998, 8, 98. [Google Scholar]
- Ferraz, R.P.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.; da Silva, T.B.; Machado, W.J.; Prata, A.P.N.; Costa, E.V.; Moraes, V.R.S.; Nogueira, P.C.L.; et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine 2013, 20, 615–621. [Google Scholar] [CrossRef]
- Silva, S.L.D.; Figueiredo, P.M.; Yano, T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amaz. 2007, 37, 281–286. [Google Scholar] [CrossRef]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; de Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 2014, 953451. [Google Scholar] [CrossRef]
- Ye, Z.; Liang, Z.; Mi, Q.; Guo, Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. BUON./Off. J. Balk. Union. Oncol. 2020, 25, 280–285. [Google Scholar]
- Jia, S.-S.; Xi, G.-P.; Zhang, M.; Chen, Y.-B.; Lei, B.; Dong, X.-S.; Yang, Y.-M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2013, 29, 349–354. [Google Scholar] [CrossRef]
- Hafidh, R.R.; Hussein, S.Z.; MalAllah, M.Q.; Abdulamir, A.S.; Abu Bakar, F. A high-throughput quantitative expression analysis of cancer-related genes in human HepG2 cells in response to limonene, a potential anticancer agent. Curr. Cancer Drug Targets 2018, 18, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Berliocchi, L.; Chiappini, C.; Adornetto, A.; Gentile, D.; Cerri, S.; Russo, R.; Bagetta, G.; Corasaniti, M.T. Early LC3 lipidation induced by d-limonene does not rely on mTOR inhibition, ERK activation and ROS production and it is associated with reduced clonogenic capacity of SH-SY5Y neuroblastoma cells. Phytomedicine 2018, 40, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Manuele, M.G.; Barreiro Arcos, M.L.; Davicino, R.; Ferraro, G.; Cremaschi, G.; Anesini, C. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: Relationship with oxidative stress. Cancer Investig. 2009, 28, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Uedo, N.; Tatsuta, M.; Iishi, H.; Baba, M.; Sakai, N.; Yano, H.; Otani, T. Inhibition by d-limonene of gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1999, 137, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Pappan, K.; Thompson, P.A.; Want, E.J.; Siskos, A.P.; Keun, H.C.; Wulff, J.; Hu, C.; Lang, J.E.; Chow, H.-H.S. Plasma Metabolomic Profiles of Breast Cancer Patients after Short-term Limonene InterventionMetabolomics of Limonene Intervention. Cancer Prev. Res. 2015, 8, 86–93. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 6631. [Google Scholar] [CrossRef]
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. α-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018, 38, BSR20180980. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. α-Pinene inhibits human prostate cancer growth in a mouse xenograft model. Chemotherapy 2018, 63, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic antitumor effect of α-pinene and β-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). Drug Res. 2015, 65, 214–218. [Google Scholar] [CrossRef]
- Yao, Y.-Q.; Ding, X.; Jia, Y.-C.; Huang, C.-X.; Wang, Y.-Z.; Xu, Y.-H. Anti-tumor effect of β-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008, 264, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Huang, F.; Zhao, J.; Ding, H.; Cunningham, C.; Coad, J.; Flynn, D.; Reed, E.; Li, Q. Antitumor effect of β-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell. Mol. Life Sci. CMLS 2005, 62, 881–893. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Zhao, J.; Ding, H.; Cunningham, C.; Chen, F.; Flynn, D.; Reed, E.; Li, Q. Antiproliferative effect of β-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell. Mol. Life Sci. CMLS 2005, 62, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, R.; Xu, L.; Xie, S.; Dong, J.; Jing, Y. β-Elemene piperazine derivatives induce apoptosis in human leukemia cells through downregulation of c-FLIP and generation of ROS. PLoS ONE 2011, 6, e15843. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Wang, G.; Huang, F.; Banda, M.; Reed, E. Antineoplastic effect of β-elemene on prostate cancer cells and other types of solid tumour cells. J. Pharm. Pharmacol. 2010, 62, 1018–1027. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.; Wu, J.; Gao, M.; Wang, A.; Xu, B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011, 67, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ling, C.; Li, W.; Jiang, H.; Zhi, Q.; Jiang, M. Molecular mechanisms of anti-cancer activities of β-elemene: Targeting hallmarks of cancer. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2016, 16, 1426–1434. [Google Scholar] [CrossRef]
- Assmann, C.E.; Cadoná, F.C.; Bonadiman, B.d.S.R.; Dornelles, E.B.; Trevisan, G.; da Cruz, I.B.M. Tea tree oil presents in vitro antitumor activity on breast cancer cells without cytotoxic effects on fibroblasts and on peripheral blood mononuclear cells. Biomed. Pharmacother. 2018, 103, 1253–1261. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Ardestani, S.K. In-vitro and in-vivo anti-breast cancer activity of OEO (Oliveria decumbens vent essential oil) through promoting the apoptosis and immunomodulatory effects. J. Ethnopharmacol. 2020, 248, 112313. [Google Scholar] [CrossRef]
- Döll-Boscardin, P.M.; Sartoratto, A.; Sales Maia, B.H.L.d.N.; Padilha de Paula, J.; Nakashima, T.; Farago, P.V.; Kanunfre, C.C. In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evid.-Based Complement. Altern. Med. 2012, 2012, 342652. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Arnold, N.A.; Menichini, F.; Senatore, F. Composition, antibacterial, antioxidant and antiproliferative activities of essential oils from three Origanum species growing wild in Lebanon and Greece. Nat. Prod. Res. 2016, 30, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Ambrož, M.; Matoušková, P.; Skarka, A.; Zajdlová, M.; Žáková, K.; Skálová, L. The effects of selected sesquiterpenes from myrica rubra essential oil on the efficacy of doxorubicin in sensitive and resistant cancer cell lines. Molecules 2017, 22, 1021. [Google Scholar] [CrossRef] [PubMed]
- Ambrož, M.; Boušová, I.; Skarka, A.; Hanušová, V.; Králová, V.; Matoušková, P.; Szotáková, B.; Skálová, L. The influence of sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules 2015, 20, 15343–15358. [Google Scholar] [CrossRef] [PubMed]
- Ryabchenko, B.; Tulupova, E.; Schmidt, E.; Wlcek, K.; Buchbauer, G.; Jirovetz, L. Investigation of anticancer and antiviral properties of selected aroma samples. Nat. Prod. Commun. 2008, 3, 1934578X0800300710. [Google Scholar] [CrossRef]
- Boris, R.; Elena, T.; Erich, S.; Walter, J.; Gerhard, B.; Leopold, J. Cytotoxic properties of selected sesquiterpene alcohols on human cervix carcinoma cell lines. J. Essent. Oil Bear. Plants 2011, 14, 316–319. [Google Scholar] [CrossRef]
- Tatman, D.; Mo, H. Volatile isoprenoid constituents of fruits, vegetables and herbs cumulatively suppress the proliferation of murine B16 melanoma and human HL-60 leukemia cells. Cancer Lett. 2002, 175, 129–139. [Google Scholar] [CrossRef]
- Wang, H.-L.; Chang, J.-C.; Fang, L.-W.; Hsu, H.-F.; Lee, L.-C.; Yang, J.-F.; Liang, M.-T.; Hsiao, P.-C.; Wang, C.-P.; Wang, S.-W.; et al. Bulnesia sarmientoi supercritical fluid extract exhibits necroptotic effects and anti-metastatic activity on lung cancer cells. Molecules 2018, 23, 3304. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, J.; Luo, Y.; Huang, N.; Zhen, N.; Zhou, Y.; Sun, F.; Li, Z.; Pan, Q.; Li, Y. (−)-Guaiol regulates RAD51 stability via autophagy to induce cell apoptosis in non-small cell lung cancer. Oncotarget 2016, 7, 62585. [Google Scholar] [CrossRef]
- Ovais, M.; Hoque, M.Z.; Khalil, A.T.; Ayaz, M.; Ahmad, I. Mechanisms underlying the anticancer applications of biosynthesized nanoparticles. In Biogenic Nanoparticles for Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 229–248. [Google Scholar]
- Ayaz, M.; Nawaz, A.; Ahmad, S.; Mosa, O.F.; Eisa Hamdoon, A.A.; Khalifa, M.A.; Sadiq, A.; Ullah, F.; Wadood, A.; Kabra, A.; et al. Underlying anticancer mechanisms and synergistic combinations of phytochemicals with cancer chemotherapeutics: Potential benefits and risks. J. Food Qual. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Kowshik, J.; Giri, H.; Kranthi Kiran Kishore, T.; Kesavan, R.; Naik Vankudavath, R.; Bhanuprakash Reddy, G.; Dixit, M.; Nagini, S. Ellagic acid inhibits VEGF/VEGFR2, PI3K/Akt and MAPK signaling cascades in the hamster cheek pouch carcinogenesis model. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2014, 14, 1249–1260. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Yarla, N.S.; Ambra, R.; Pastore, G.; Perry, G. MAPK signalling pathway in cancers: Olive products as cancer preventive and therapeutic agents. In Proceedings of Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–195. [Google Scholar]
- Liao, X.Z.; Gao, Y.; Sun, L.L.; Liu, J.H.; Chen, H.R.; Yu, L.; Chen, Z.Z.; Chen, W.H.; Lin, L.Z. Rosmarinic acid reverses non-small cell lung cancer cisplatin resistance by activating the MAPK signaling pathway. Phytother. Res. 2020, 34, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, F.; Jiang, H.; Wu, K.; Zheng, X.; Cai, Y.; Katakowski, M.; Chopp, M.; To, S.-S.T. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur. J. Pharmacol. 2010, 641, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Chandra, V.; Sankhwar, P.; Popli, P.; Kaushal, J.B.; Sirohi, V.K.; Dwivedi, A. Phytoestrogen genistein inhibits EGFR/PI3K/NF-kB activation and induces apoptosis in human endometrial hyperplasial cells. RSC Adv. 2015, 5, 56075–56085. [Google Scholar] [CrossRef]
- Anwar, S.; Shahwan, M.; Hasan, G.M.; Islam, A.; Hassan, M.I. Microtubule-affinity regulating kinase 4: A potential drug target for cancer therapy. Cell. Signal. 2022, 99, 110434. [Google Scholar] [CrossRef]
- Tung, N.H.; Du, G.-J.; Yuan, C.-S.; Shoyama, Y.; Wang, C.-Z. Isolation and chemopreventive evaluation of novel naphthoquinone compounds from Alkanna tinctoria. Anti-Cancer Drugs 2013, 24. [Google Scholar] [CrossRef]
- Zhong, Y.; Krisanapun, C.; Lee, S.-H.; Nualsanit, T.; Sams, C.; Peungvicha, P.; Baek, S.J. Molecular targets of apigenin in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur. J. Cancer 2010, 46, 3365–3374. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Jang, J.-Y.; Shim, J.-H.; Myung, P.K.; Chae, J.-I. Esculetin, a coumarin derivative, exhibits anti-proliferative and pro-apoptotic activity in G361 human malignant melanoma. J. Cancer Prev. 2015, 20, 106. [Google Scholar] [CrossRef]
- Anand, J.R.; Rijhwani, H.; Malapati, K.; Kumar, P.; Saikia, K.; Lakhar, M. Anticancer activity of esculetin via-modulation of Bcl-2 and NF-κB expression in benzo [a] pyrene induced lung carcinogenesis in mice. Biomed. Prev. Nutr. 2013, 3, 107–112. [Google Scholar] [CrossRef]
- Raju, J.; Patlolla, J.M.; Swamy, M.V.; Rao, C.V. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1392–1398. [Google Scholar] [CrossRef]
- Khan, M.; Xiao, Y.; Yu, B.; Wang, N.; Rasul, A.; Yi, F.; Yang, L.; Yang, H.; Ma, T. Artabotryside A, a constituent from Descurainia sophia (L.) induces cell death in U87 glioma cells through apoptosis and cell cycle arrest at G2/M phase. J. Med. Plants Res. 2012, 6, 3754–3765. [Google Scholar]
- Kampa, M.; Alexaki, V.-I.; Notas, G.; Nifli, A.-P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, E.; Kouimtzoglou, E.; Blekas, G.; Boskou, D.; et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004, 6, R63. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Sayed, S.; Bello, M.; Hussain, N.; Chando, R.K.; Alam, S.; Hasan, M.K. CDK4 as a phytochemical based anticancer drug target. Inform. Med. Unlocked 2022, 28, 100826. [Google Scholar] [CrossRef]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.-L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Chakraborty, S.; Singh, S. Berberine: A potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs 2010, 19, 1297–1307. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018, 194, 75–87. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Önning, G.; Åkesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef]
- Stan, S.D.; Zeng, Y.; Singh, S.V. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr. Cancer 2008, 60, 51–60. [Google Scholar] [CrossRef]
- Bennett, R.L.; Licht, J.D. Targeting epigenetics in cancer. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 187–207. [Google Scholar] [CrossRef] [PubMed]


| Phytochemical | Cancer | Interventions | Effect | References |
|---|---|---|---|---|
| Allium sativum | Colorectal, liver, and pancreatic cancer patients Colorectal ademas | 500 mg of aged garlic extract (GE) in 4 capsules for 12 weeks 2.4 mL GE in 3 capsules twice a day for 1 year | Natural killer (NK) cells increased in number and activity. Reduced size and number of colon adenomas. | [40] [41] |
| Camptothecin (Ct) | Patients with refractory cancer Primary/metastatic lung cancer patients | Ct: 3 weeks drug-1-week rest; Nitro-Ct: 5 day drug- 2 days rest 6.7–26.6 µg/kg of Ct in the form of aerosolized liposomes were given 5 days a week for 6 weeks, followed by a gap of 2 weeks. | Both the compounds showed tumor regression in patients with breast cancer, prostate cancer, and melanomas. 3 lung patients stabilized upon dosage. | [42] [43] |
| Curcumin | Urinary bladder cancer, uterine cervical neoplasm, and intestinal metaplasia Advanced pancreatic cancer | 500 mg/day, orally, for 3 months Dosage was 8 g/day for one month | Improvement in 1 out of every 2 patients with bladder cancer and 1 out of 6 patients with intestinal Metaplasia, and 1 out of 4 patients with uterine cervical neoplasm. Study was conducted on 21 patients, of whom 1 had stable disease for >18 months and 1 had tumor reversion. | [44] [45] |
| Green tea | Patients with high-grade prostate intraepithelial neoplasia Patients with adenocarcinoma of the prostate Esophageal cancer Patients with colon, rectum and pancreas cancer | Green tea catechins (600 mg) were given daily, orally, for one year Tea consumption as a daily routine Usual green tea consumption Non-regular tea consumption | Improved quality of life Risk declination of prostate cancer with increased consumption of green tea. Reduced risk of Esophageal cancer. Inverse relation was associated with cancer and green tea consumption. | [46] [47] [48] [49] |
| Panax ginseng | Patients with cancer of uterine, ovary, rectum, stomach, etc | 3000 mg/day of the heat-processed ginseng for 12 weeks | Improvement of mental and physical functioning, and hence improved quality of life. | [50] |
| Isoflavones | Prostate cancer | (60 mg) daily for 12 months | Reducing prostate cancer incidence for patients aged 65 or more. | [51] |
| Synthetic genistein | Prostate cancer | 54 patients with localized prostate cancer. (30 mg) daily for 3–6 weeks | Decreasing level of serum prostate specific antigen (PSA). | [52] |
| Soy isoflavone | Prostate cancer | 86 patients with localized prostate cancer. (80 mg total isoflavones, 51 mg aglucon units) daily for 6 weeks | No significant change in serum hormone levels, total cholesterol, or PSA. | [53] |
| Flavonoid mixture | Colorectal cancer | (20 mg apigenin and 20 mg EGCG) for 3–4 years. 87 patients with resected colorectal cancer or polypectomy | Reducing the recurrence rate of colon neoplasia in patients with resected colon cancer. | [54] |
| Isoflavones and curcumin | Prostate cancer | Isoflavones (40 mg) and curcumin (100 mg) daily for 6 months | decreasing level of serum PSA. | [55] |
| Alkaloid | Pharmacological Mechanism | Therapeutic Effect | Refs. |
|---|---|---|---|
| Vinblastine | -Binds to tubulin and prevents microtubules from binding. -Induce apoptosis and mitotic death. | Cervical cancer Breast cancer Lung cancer Head and neck cancer Hodgkin’s lymphoma Testicular cancer | [156,157] |
| Vincristine | -Binds tubulin dimer. -Prevents microtubule structure formation. | Acute myeloid leukemia (AML, ANLL) Acute_lymphoblastic leukemia (ALL) Hodgkin’s_lymphoma Non-Hodgkin’s lymphoma | [158,159] |
| Vindesine | Possess anti-mitotic activity | Melanoma Lung cancers Uterine malignancies | [154] |
| Vinorelbine | Exhibits broad-spectrum antitumor activity. Antineoplastic activity | Breast cancer Non-small cell lung cancer (NSCLC) | [159,160] |
| Vinflunine | Decreases metaphase to anaphase transition, Prevents cancer cells from entering mitosis. Increases apoptosis | Metastatic Urothelial carcinoma Transitional cell carcinoma Breast cancer | [161] |
| Colchicine | Microtubule destabilizers perturb the assembly dynamics of microtubules. | Gastric cancer | [162,163] |
| Colcemid | Mitotic arrest Kinase inhibition | Lung Cancer | [164] |
| Terpene | In Vitro Effects | In Vivo Effects | Clinical Trials | Refs. |
|---|---|---|---|---|
| Myrcene | Cytotoxic effects on cancer cell lines Reduced DNA damage | Carcinogenic at higher doses | N/A | [172,173,174,175] |
| Limonene | Shown cytotoxic effects Mediates cell cycle arrest Decreased migration and invasion of cancer cells Apoptosis and autophagy induction Inhibition of the PI3K/Akt pathway | Decreased tumor growth and metastasis, c-jun, and c-myc expression Induced apoptosis and latency period. | Decreased the expression of proteins involved in tumor progression. | [176,177,178,179,180,181,182,183] |
| Pinene | Reduced cell viability. Induced apoptosis, ROS production, and cell cycle arrest | Reduced the number and growth of tumors. | N/A | [184,185,186,187] |
| Elemene | Induced cell cycle arrest and apoptosis Inhibited MAPK pathway Reduced tumor migration and invasion Inhibited angiogenesis | N/A | Effective agents in chemotherapy. Reduced toxicity of chemotherapy. | [188,189,190,191,192,193,194] |
| Terpinene isomers | Reduced proliferation and induced apoptosis in cancer cells | N/A | N/A | [195,196,197,198] |
| Valencene | Reduced cellular proliferation and acted efficiently synergistically with doxorubicin | N/A | N/A | [199,200] |
| Nerolidol | Exhibited cytotoxic effects and induced apoptosis and cell cycle arrest. Acted synergistically with doxorubicin | Inhibited cancer growth | N/A | [201,202,203,204,205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Muhsinah, A.B.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients 2023, 15, 1704. https://doi.org/10.3390/nu15071704
Majrashi TA, Alshehri SA, Alsayari A, Muhsinah AB, Alrouji M, Alshahrani AM, Shamsi A, Atiya A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients. 2023; 15(7):1704. https://doi.org/10.3390/nu15071704
Chicago/Turabian StyleMajrashi, Taghreed A., Saad Ali Alshehri, Abdulrhman Alsayari, Abdullatif Bin Muhsinah, Mohammad Alrouji, Asma M. Alshahrani, Anas Shamsi, and Akhtar Atiya. 2023. "Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics" Nutrients 15, no. 7: 1704. https://doi.org/10.3390/nu15071704
APA StyleMajrashi, T. A., Alshehri, S. A., Alsayari, A., Muhsinah, A. B., Alrouji, M., Alshahrani, A. M., Shamsi, A., & Atiya, A. (2023). Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients, 15(7), 1704. https://doi.org/10.3390/nu15071704