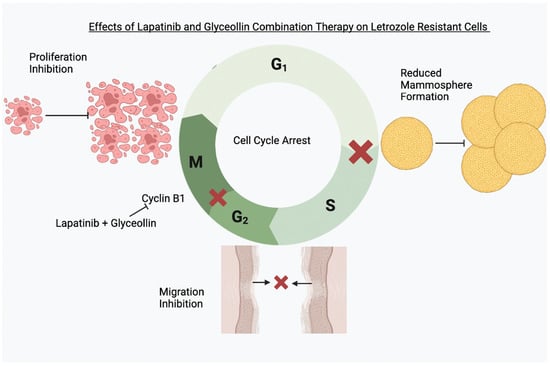
Novel Therapeutic Combination Targets the Growth of Letrozole-Resistant Breast Cancer through Decreased Cyclin B1
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
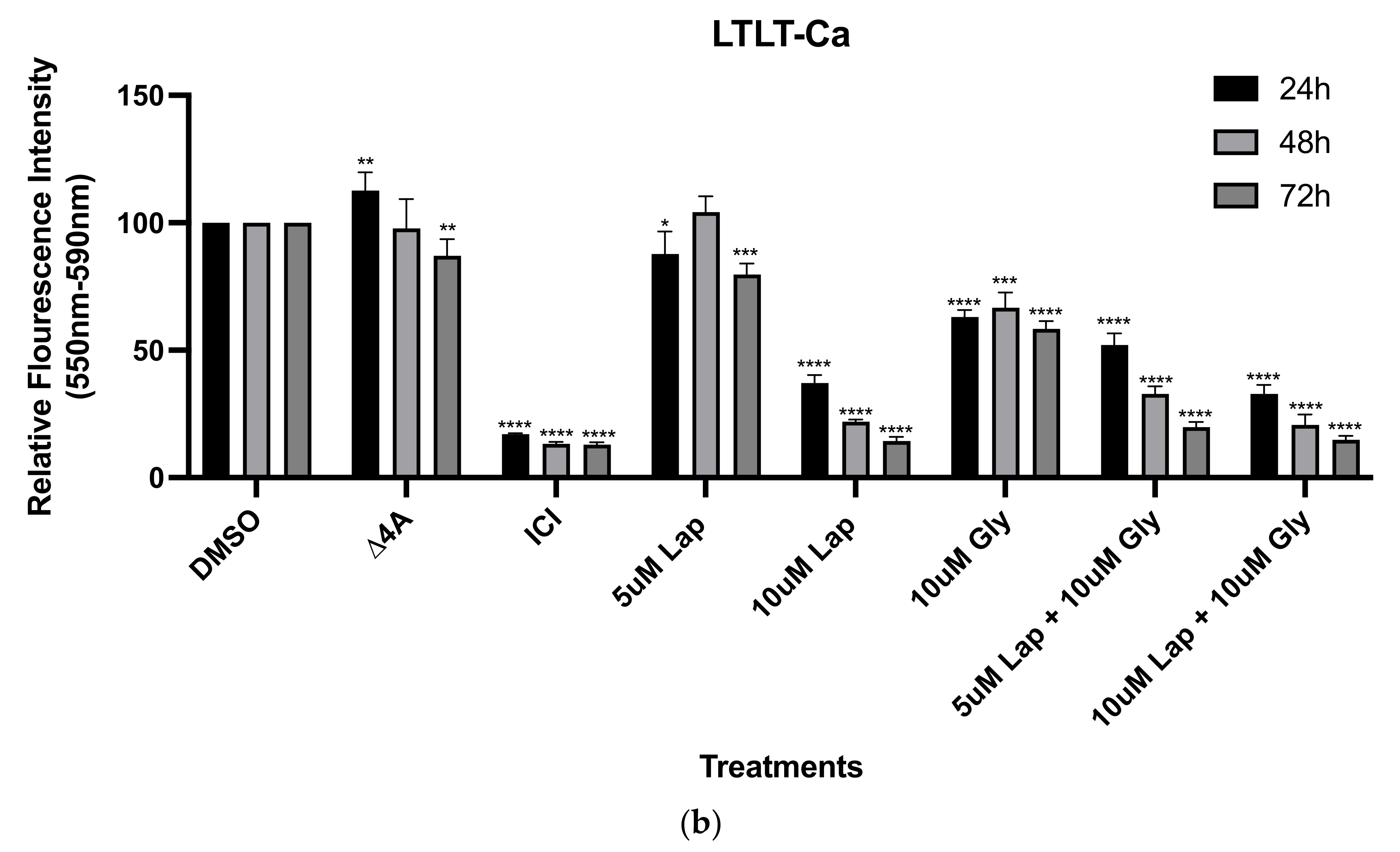
2.2. Viability Assays
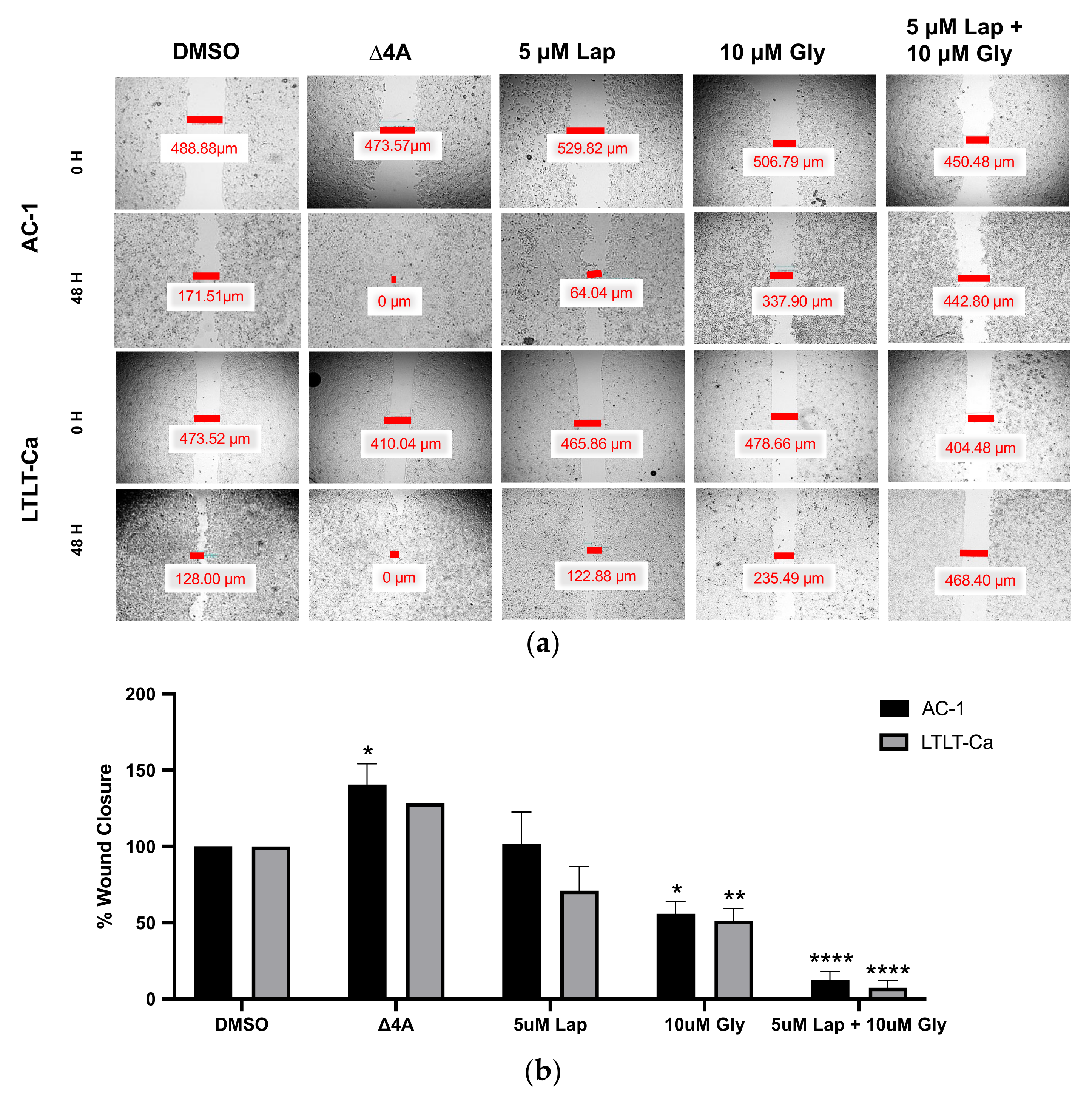
2.3. Wound Healing Assay
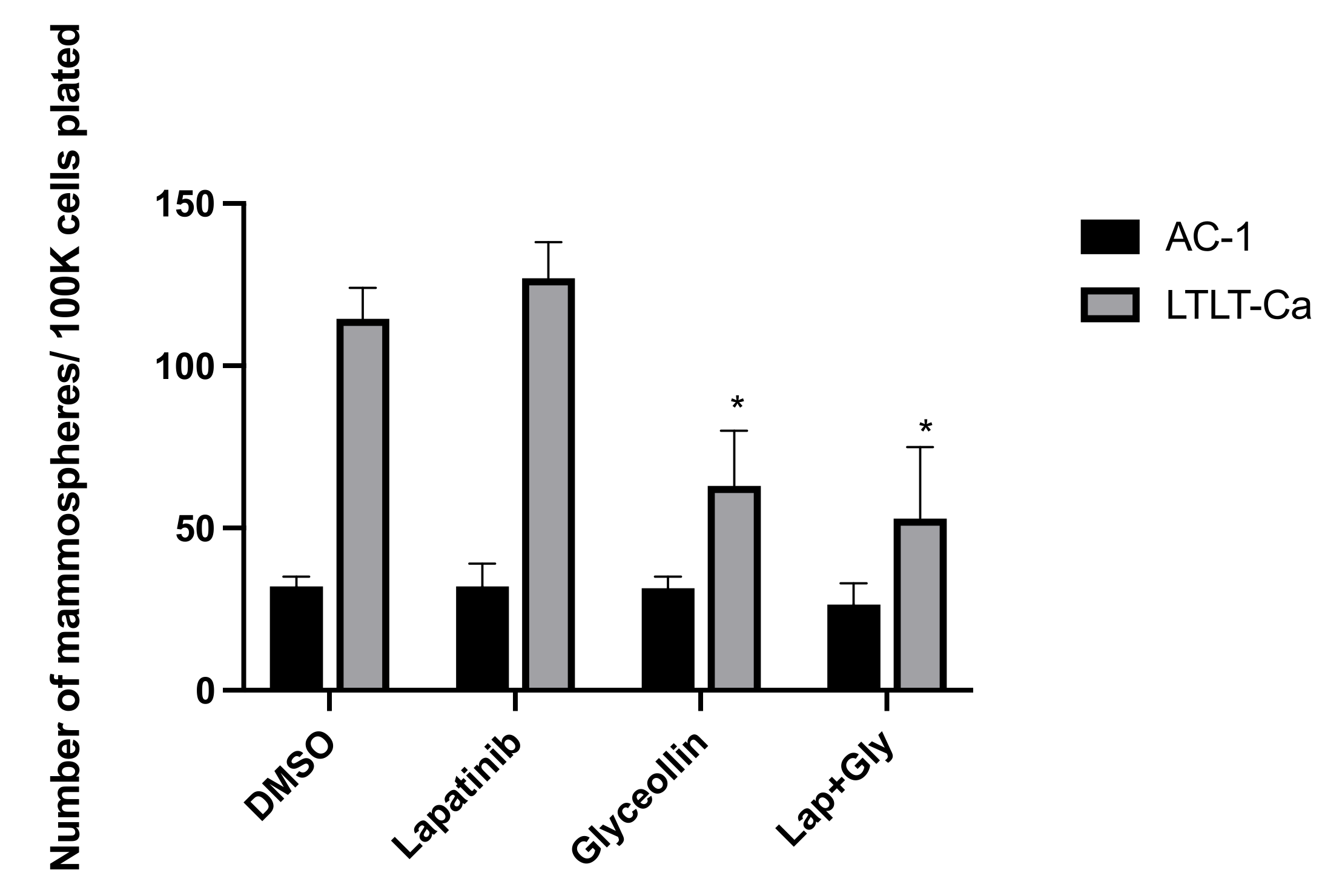
2.4. Mammosphere Formation Assay
2.5. Cell Cycle Analysis
2.6. Western Blot Analysis
3. Results
3.1. Increased Growth Factor Expression in Hormone-Independent Aromatase-Inhibitor-Resistant Breast Cancer Cells
3.2. Glyceollin and Lapatinib Inhibit the Migratory Behavior of Letrozole-Sensitive and Letrozole-Resistant Breast Cancer Cells
3.3. Glyceollin Inhibits Mammosphere Formation in Letrozole-Resistant Breast Cancer Cells
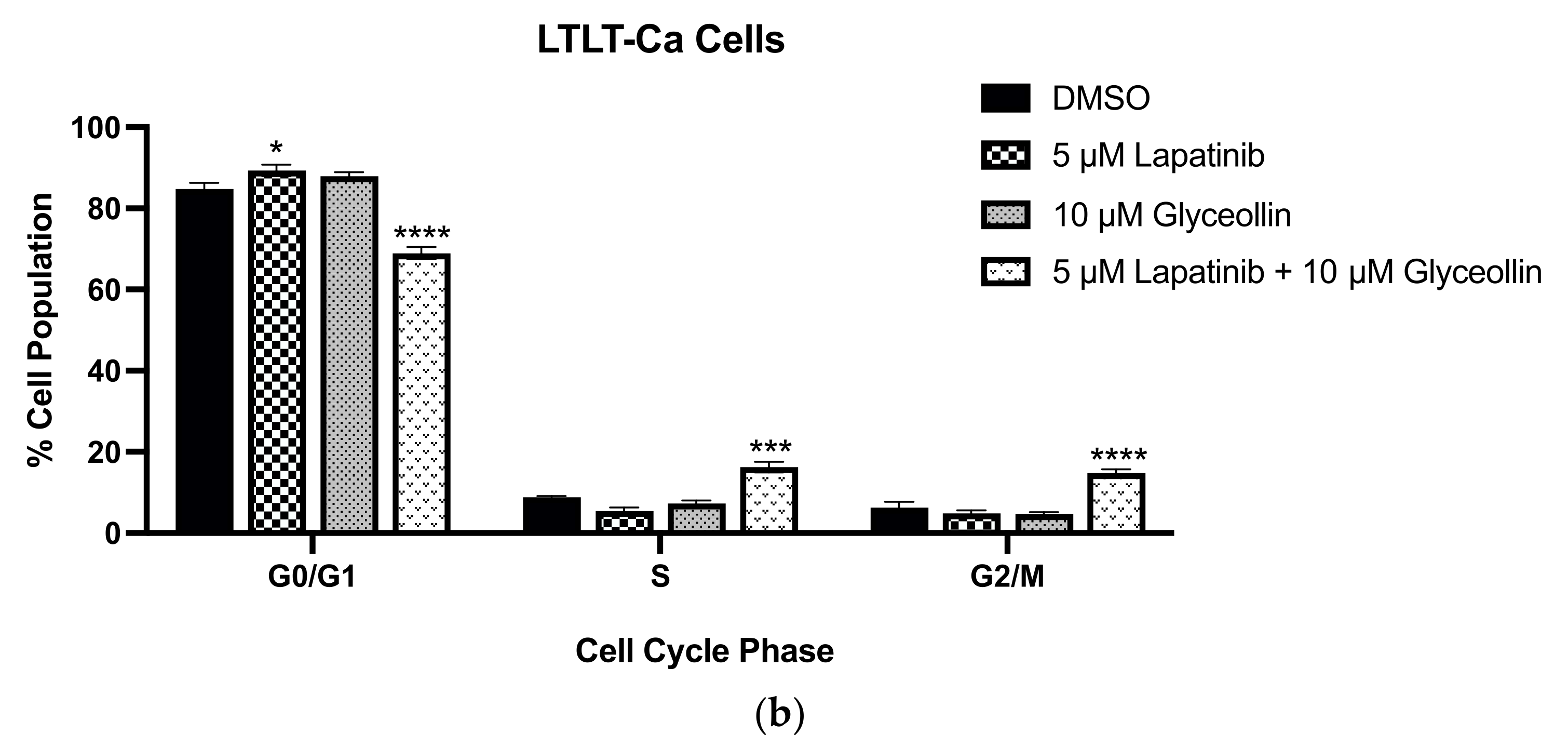
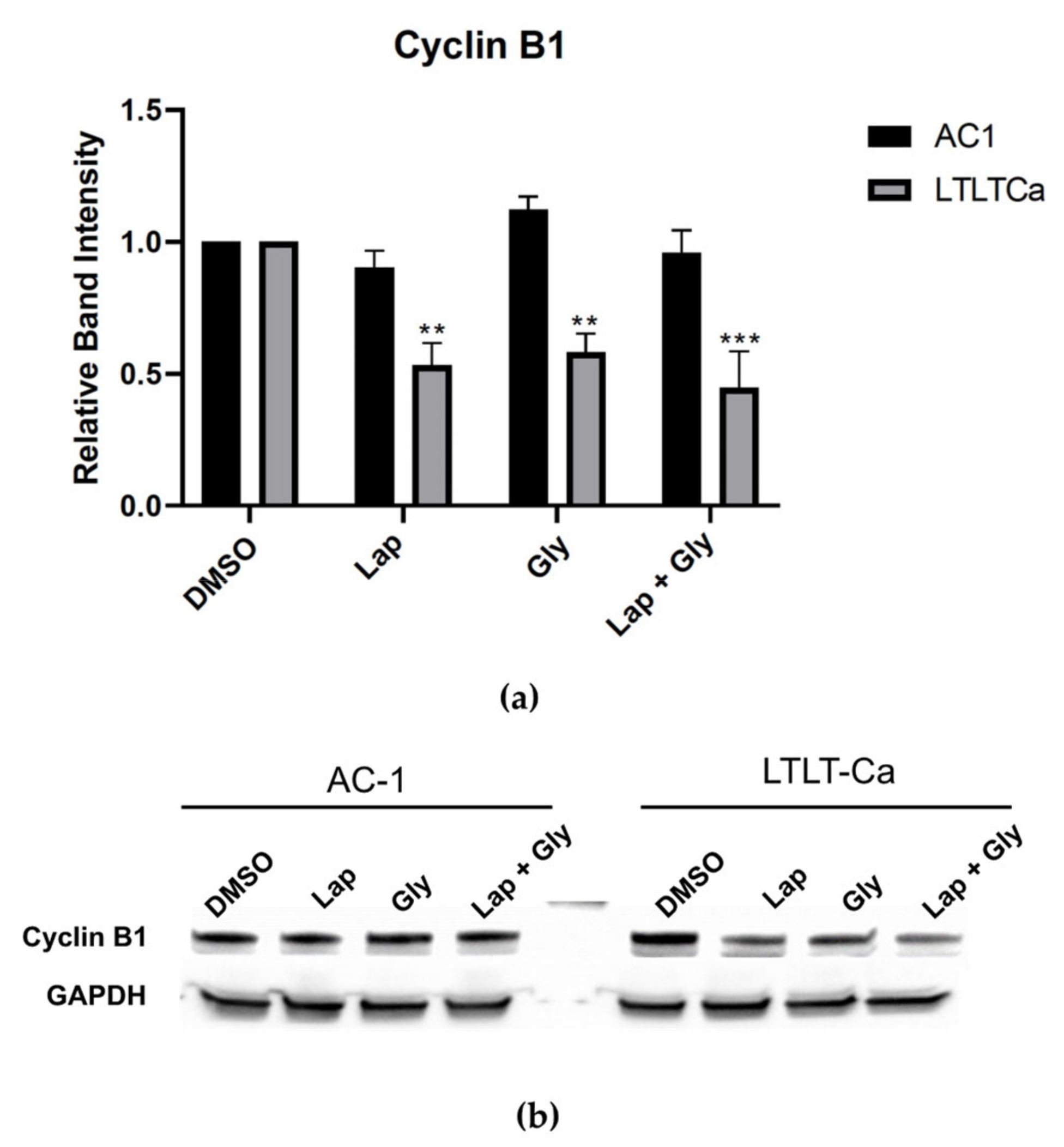
3.4. Glyceollin and Lapatinib Induce S and G2/M Phase Cell-Cycle Arrest in Letrozole-Resistant Breast Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, S.R.; Martin, L.A.; Leary, A.; Head, J.; Dowsett, M. Clinical strategies for rationale combinations of aromatase inhibitors with novel therapies for breast cancer. J. Steroid Biochem. Mol. Biol. 2007, 106, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Larionov, A.A. Understanding the mechanisms of aromatase inhibitor resistance. Breast Cancer Res. 2012, 14, 201. [Google Scholar] [CrossRef]
- Brodie, A.; Jelovac, D.; Sabnis, G.; Long, B.; Macedo, L.; Goloubeva, O. Model systems: Mechanisms involved in the loss of sensitivity to letrozole. J. Steroid Biochem. Mol. Biol. 2005, 95, 41–48. [Google Scholar] [CrossRef]
- Jelovac, D.; Sabnis, G.; Long, B.J.; Macedo, L.; Goloubeva, O.G.; Brodie, A.M. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 2005, 65, 5380–5389. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, G.; Schayowitz, A.; Goloubeva, O.; Macedo, L.; Brodie, A. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009, 69, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Tilghman, S.L.; Townley, I.; Zhong, Q.; Carriere, P.P.; Zou, J.; Llopis, S.D.; Preyan, L.C.; Williams, C.C.; Skripnikova, E.; Bratton, M.R.; et al. Proteomic signatures of acquired letrozole resistance in breast cancer: Suppressed estrogen signaling and increased cell motility and invasiveness. Mol. Cell. Proteom. 2013, 12, 2440–2455. [Google Scholar] [CrossRef]
- Miller, T.W.; Balko, J.M.; Fox, E.M.; Ghazoui, Z.; Dunbier, A.; Anderson, H.; Dowsett, M.; Jiang, A.; Smith, R.A.; Maira, S.M.; et al. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011, 1, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Cheng, J.; Bloomquist, E.; Sanchez, J.; Wedam, S.B.; Singh, H.; Amiri-Kordestani, L.; Ibrahim, A.; Sridhara, R.; Goldberg, K.B.; et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2020, 21, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, M.; Luo, X.; Li, H.; Shan, H.; Du, Q.; Zhai, Q. Pharmacoeconomic evaluations of CDK4/6 inhibitors plus endocrine therapy for advanced hormone receptor-positive (HR+) and human epidermal growth factor receptor-2 negative (HER2-) breast cancer: A systematic review. Ann. Transl. Med. 2022, 10, 233. [Google Scholar] [CrossRef]
- Costa, C.; Wang, Y.; Ly, A.; Hosono, Y.; Murchie, E.; Walmsley, C.S.; Huynh, T.; Healy, C.; Peterson, R.; Yanase, S.; et al. PTEN Loss Mediates Clinical Cross-Resistance to CDK4/6 and PI3Kα Inhibitors in Breast Cancer. Cancer Discov. 2020, 10, 72–85. [Google Scholar] [CrossRef]
- Walker, R.R.; Gallegos, K.M.; Bratton, M.R.; Lemieux, K.P.; Zhang, K.; Wang, G.; Davidson, A.M.; Tilghman, S.L. Acquisition of Letrozole Resistance Through Activation of the p38/MAPK Signaling Cascade. Anticancer Res. 2021, 41, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, G.J.; Goloubeva, O.G.; Kazi, A.A.; Shah, P.; Brodie, A.H. HDAC inhibitor entinostat restores responsiveness of letrozole-resistant MCF-7Ca xenografts to aromatase inhibitors through modulation of Her-2. Mol. Cancer Ther. 2013, 12, 2804–2816. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, G.J.; Macedo, L.F.; Goloubeva, O.; Schayowitz, A.; Brodie, A.M. Stopping treatment can reverse acquired resistance to letrozole. Cancer Res. 2008, 68, 4518–4524. [Google Scholar] [CrossRef]
- Kubo, M.; Kanaya, N.; Petrossian, K.; Ye, J.; Warden, C.; Liu, Z.; Nishimura, R.; Osako, T.; Okido, M.; Shimada, K.; et al. Inhibition of the proliferation of acquired aromatase inhibitor-resistant breast cancer cells by histone deacetylase inhibitor LBH589 (panobinostat). Breast Cancer Res. Treat. 2013, 137, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Serra, F.; Lapidari, P.; Quaquarini, E.; Tagliaferri, B.; Sottotetti, F.; Palumbo, R. Palbociclib in metastatic breast cancer: Current evidence and real-life data. Drugs Context 2019, 8, 212579. [Google Scholar] [CrossRef]
- Al-Qasem, A.J.; Alves, C.L.; Ehmsen, S.; Tuttolomondo, M.; Terp, M.G.; Johansen, L.E.; Vever, H.; Hoeg, L.V.A.; Elias, D.; Bak, M.; et al. Co-targeting CDK2 and CDK4/6 overcomes resistance to aromatase and CDK4/6 inhibitors in ER+ breast cancer. NPJ Precis. Oncol. 2022, 6, 68. [Google Scholar] [CrossRef]
- Pham, T.H.; Lecomte, S.; Efstathiou, T.; Ferriere, F.; Pakdel, F. An Update on the Effects of Glyceollins on Human Health: Possible Anticancer Effects and Underlying Mechanisms. Nutrients 2019, 11, 79. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Burow, M.E.; Boue, S.M.; Collins-Burow, B.M.; Melnik, L.I.; Duong, B.N.; Carter-Wientjes, C.H.; Li, S.; Wiese, T.E.; Cleveland, T.E.; McLachlan, J.A. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J. Clin. Endocrinol. Metab. 2001, 86, 1750–1758. [Google Scholar] [CrossRef]
- Salvo, V.A.; Boue, S.M.; Fonseca, J.P.; Elliott, S.; Corbitt, C.; Collins-Burow, B.M.; Curiel, T.J.; Srivastav, S.K.; Shih, B.Y.; Carter-Wientjes, C.; et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 7159–7164. [Google Scholar] [CrossRef]
- Tilghman, S.L.; Boué, S.M.; Burow, M.E. Glyceollins, A Novel Class of Antiestrogenic Phytoalexins. Mol. Cell. Pharmacol. 2010, 2, 155–160. [Google Scholar] [CrossRef]
- Lecomte, S.; Chalmel, F.; Ferriere, F.; Percevault, F.; Plu, N.; Saligaut, C.; Surel, C.; Lelong, M.; Efstathiou, T.; Pakdel, F. Glyceollins trigger anti-proliferative effects through estradiol-dependent and independent pathways in breast cancer cells. Cell Commun. Signal. 2017, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Carriere, P.P.; Llopis, S.D.; Naiki, A.C.; Nguyen, G.; Phan, T.; Nguyen, M.M.; Preyan, L.C.; Yearby, L.; Pratt, J.; Burks, H.; et al. Glyceollin I Reverses Epithelial to Mesenchymal Transition in Letrozole Resistant Breast Cancer through ZEB1. Int. J. Environ. Res. Public Health 2016, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.V.; Tilghman, S.L.; Boue, S.M.; Wang, S.; Khalili, H.; Muir, S.E.; Bratton, M.R.; Zhang, Q.; Wang, G.; Burow, M.E.; et al. Glyceollins as novel targeted therapeutic for the treatment of triple-negative breast cancer. Oncol. Lett. 2012, 3, 163–171. [Google Scholar] [CrossRef]
- Patel, J.R.; Gallegos, K.M.; Walker, R.R.; Davidson, A.M.; Davenport, I.; Tilghman, S.L. Mammospheres of letrozole-resistant breast cancer cells enhance breast cancer aggressiveness. Oncol. Lett. 2021, 22, 620. [Google Scholar] [CrossRef]
- Walker, R.R.; Patel, J.R.; Gupta, A.; Davidson, A.M.; Williams, C.C.; Payton-Stewart, F.; Boué, S.M.; Burow, M.E.; Khupse, R.; Tilghman, S.L. Glyceollins Trigger Anti-Proliferative Effects in Hormone-Dependent Aromatase-Inhibitor-Resistant Breast Cancer Cells through the Induction of Apoptosis. Int. J. Mol. Sci. 2022, 23, 2887. [Google Scholar] [CrossRef]
- Doostan, I.; Karakas, C.; Kohansal, M.; Low, K.H.; Ellis, M.J.; Olson, J.A., Jr.; Suman, V.J.; Hunt, K.K.; Moulder, S.L.; Keyomarsi, K. Cytoplasmic Cyclin E Mediates Resistance to Aromatase Inhibitors in Breast Cancer. Clin. Cancer Res. 2017, 23, 7288–7300. [Google Scholar] [CrossRef]
- Hunt, K.K.; Karakas, C.; Ha, M.J.; Biernacka, A.; Yi, M.; Sahin, A.A.; Adjapong, O.; Hortobagyi, G.N.; Bondy, M.; Thompson, P.; et al. Cytoplasmic Cyclin E Predicts Recurrence in Patients with Breast Cancer. Clin. Cancer Res. 2017, 23, 2991–3002. [Google Scholar] [CrossRef]
- Karakas, C.; Biernacka, A.; Bui, T.; Sahin, A.A.; Yi, M.; Akli, S.; Schafer, J.; Alexander, A.; Adjapong, O.; Hunt, K.K.; et al. Cytoplasmic Cyclin E and Phospho-Cyclin-Dependent Kinase 2 Are Biomarkers of Aggressive Breast Cancer. Am. J. Pathol. 2016, 186, 1900–1912. [Google Scholar] [CrossRef]
- Payton-Stewart, F.; Schoene, N.W.; Kim, Y.S.; Burow, M.E.; Cleveland, T.E.; Boue, S.M.; Wang, T.T. Molecular effects of soy phytoalexin glyceollins in human prostate cancer cells LNCaP. Mol. Carcinog. 2009, 48, 862–871. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sakamoto, C.; Tachiwana, H.; Kumabe, M.; Matsui, T.; Yamashita, T.; Shinagawa, M.; Ochiai, K.; Saitoh, N.; Nakao, M. Endocrine therapy-resistant breast cancer model cells are inhibited by soybean glyceollin I through Eleanor non-coding RNA. Sci. Rep. 2018, 8, 15202. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Hegg, R.; Im, S.A.; Park, I.H.; Burdaeva, O.; Kurteva, G.; Press, M.F.; Tjulandin, S.; Iwata, H.; Simon, S.D.; et al. Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade With Lapatinib Plus Trastuzumab in Combination With an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor-Positive Metastatic Breast Cancer: Updated Results of ALTERNATIVE. J. Clin. Oncol. 2021, 39, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mehta, R.; Alimirah, F.; Peng, X.; Murillo, G.; Wiehle, R.; Mehta, R.G. Efficacy and mechanism of action of Proellex, an antiprogestin in aromatase overexpressing and Letrozole resistant T47D breast cancer cells. J. Steroid Biochem. Mol. Biol. 2013, 133, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Long, B.J.; Tilghman, S.L.; Yue, W.; Thiantanawat, A.; Grigoryev, D.N.; Brodie, A.M. The steroidal antiestrogen ICI 182,780 is an inhibitor of cellular aromatase activity. J. Steroid Biochem. Mol. Biol. 1998, 67, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Abrahamsson, A.; Dabrosin, C. Fulvestrant inhibits growth of triple negative breast cancer and synergizes with tamoxifen in ERα positive breast cancer by up-regulation of ERβ. Oncotarget 2016, 7, 56876–56888. [Google Scholar] [CrossRef] [PubMed]
- Yamane, N.; Makino, M.; Kaibara, N. S-phase accumulation precedes apoptosis induced by preoperative treatment with 5-fluorouracil in human colorectal carcinoma cells. Cancer 1999, 85, 309–317. [Google Scholar] [CrossRef]
- Androic, I.; Krämer, A.; Yan, R.; Rödel, F.; Gätje, R.; Kaufmann, M.; Strebhardt, K.; Yuan, J. Targeting cyclin B1 inhibits proliferation and sensitizes breast cancer cells to taxol. BMC Cancer 2008, 8, 391. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, J.R.; Banjara, B.; Ohemeng, A.; Davidson, A.M.; Boué, S.M.; Burow, M.E.; Tilghman, S.L. Novel Therapeutic Combination Targets the Growth of Letrozole-Resistant Breast Cancer through Decreased Cyclin B1. Nutrients 2023, 15, 1632. https://doi.org/10.3390/nu15071632
Patel JR, Banjara B, Ohemeng A, Davidson AM, Boué SM, Burow ME, Tilghman SL. Novel Therapeutic Combination Targets the Growth of Letrozole-Resistant Breast Cancer through Decreased Cyclin B1. Nutrients. 2023; 15(7):1632. https://doi.org/10.3390/nu15071632
Chicago/Turabian StylePatel, Jankiben R., Bipika Banjara, Afia Ohemeng, A. Michael Davidson, Stephen M. Boué, Matthew E. Burow, and Syreeta L. Tilghman. 2023. "Novel Therapeutic Combination Targets the Growth of Letrozole-Resistant Breast Cancer through Decreased Cyclin B1" Nutrients 15, no. 7: 1632. https://doi.org/10.3390/nu15071632
APA StylePatel, J. R., Banjara, B., Ohemeng, A., Davidson, A. M., Boué, S. M., Burow, M. E., & Tilghman, S. L. (2023). Novel Therapeutic Combination Targets the Growth of Letrozole-Resistant Breast Cancer through Decreased Cyclin B1. Nutrients, 15(7), 1632. https://doi.org/10.3390/nu15071632