The Mediterranean Lifestyle to Contrast Low-Grade Inflammation Behavior in Cancer
Abstract
1. Introduction
2. Physical Activity and Inflammation
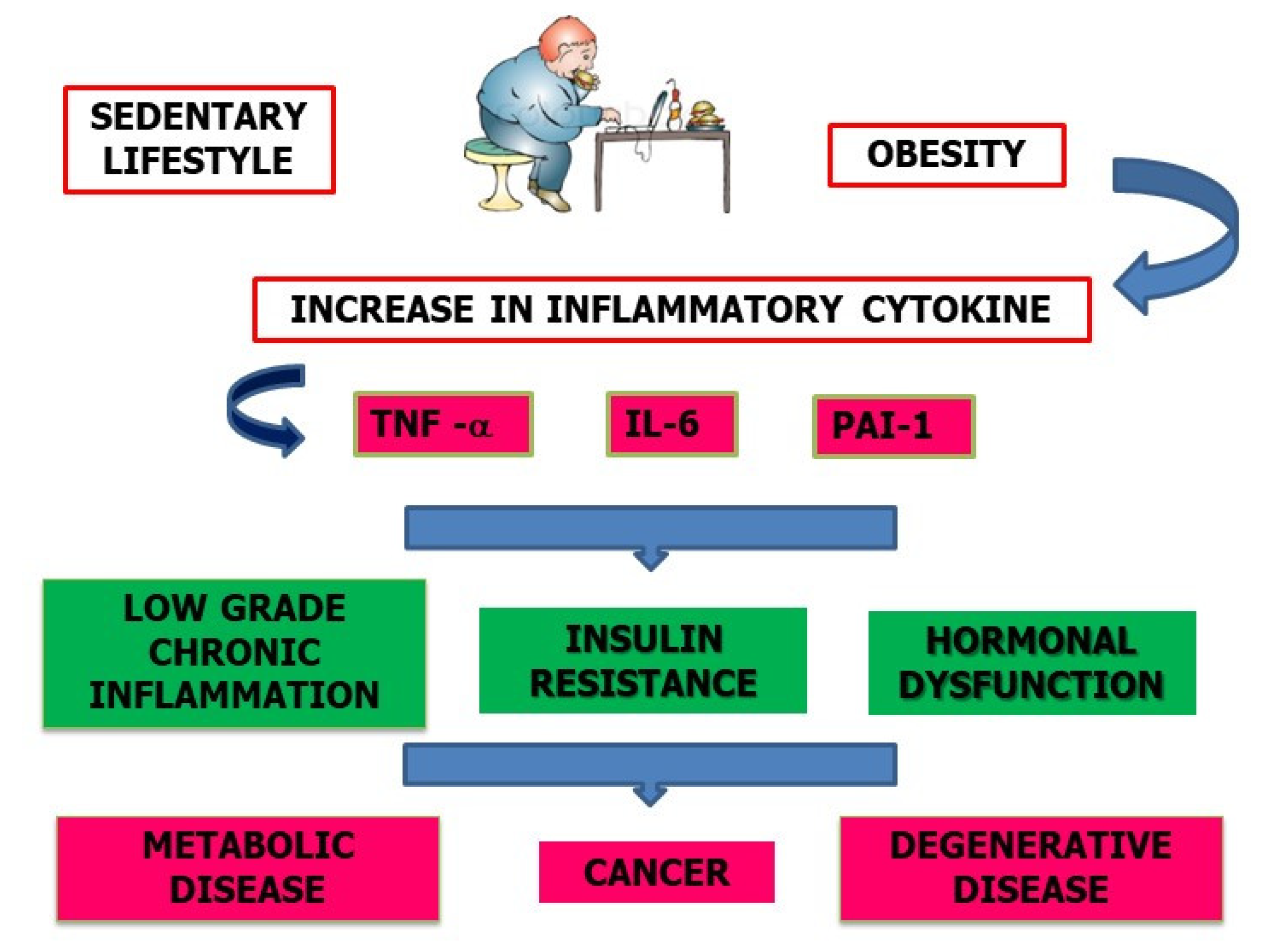
3. From Sedentary Lifestyle to Chronic Inflammation Status
4. Physical Activity and Cancer
5. Diet and Chronic Inflammation
6. The Mediterranean Diet (MD)
7. Role of an Anti-Inflammatory and Calorie-Restricted Diet in Cancer Prevention
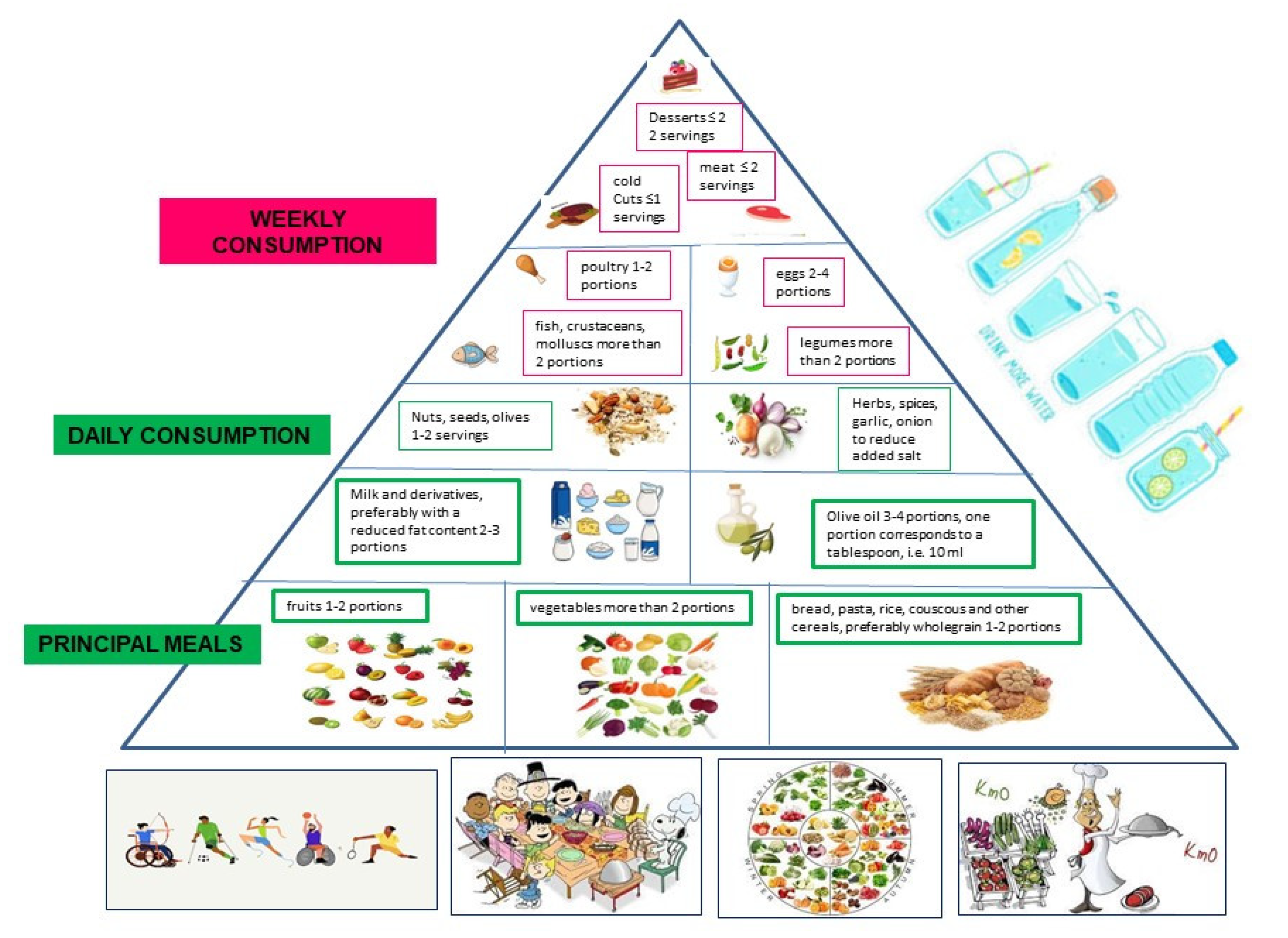
The Typical Mediterranean Diet Recommendations to Follow
- (1)
- Consume whole grains every day, three portions a day of vegetables, two portions a day of fruit, and legumes 3–4 times a week.
- (2)
- Limit the consumption of red meat, avoid processed meats such as preserved meats, cold cuts, and frankfurters.
- (3)
- Stay away from items high on the glycemic index, such as white bread, white rice, and simple sweets. Dinner carbs are acidifying and may raise blood sugar and insulin levels, so it is best to avoid them.
- (4)
- Fruit is best eaten on an empty stomach, either first thing in the morning or late in the afternoon and never toward the conclusion of a meal, especially one that is high in carbs. Do not eat fruit salad since it contains ingredients with wildly varying pH levels, many of which might lead to stomach and bowel issues.
- (5)
- Foods high in acidity and polyamines should be avoided (vegetables of the nightshade family, oranges, tangerines, mandarin oranges).
- (6)
- Stay away from packaged foods with hydrogenated polyunsaturated fatty acids (trans), which cause high cholesterol and inflammation throughout the body, which damages cells. Trans fats can be found in bakery products such as industrial bread, cookies, and pastries, as well as in ready-to-eat meals and French fries.
- (7)
- Food supplements should not be taken unless they have been properly tested in clinical trials.
- (8)
- Observe a moderate calorie restriction while making sure that all essential nutrients are in the diet. This is to make sure that the nutritional status is not affected (for example 1–2 days a week).
- (9)
- Weight should be managed in a healthy way; one should “stay trim” and monitor one’s waist circumference (waist size), which should be no more than 80–88 cm in women and 94–102 cm in males.
- (10)
- Even more so as you get older, it is important to get your body moving every day; try going for a brisk 30 min walk, walking 10,000 steps, or spending an hour at the gym.
- Modulates insulin levels, reduces insulin resistance and insulin-like growth factor (IGF-1).
- Fights against weight gain and obesity.
- Decreases the production of inflammatory adipokines and leptin (mitogenic factor) while increasing the production of adiponectin (pro-apoptotic factor) by adipocytes with anti-inflammatory and antitumor action.
- Reduces plasma levels of estrogens, which are involved in the growth of breast and endometrial cancer cells.
- Contributes to the anti-inflammatory action with the muscular release of anti-inflammatory myokines whose protective effect in many tumors has been demonstrated.
- Increases intestinal motility, thereby reducing the contact time of the intestinal mucosa with carcinogenic compounds.
- Modifies the composition and metabolic profile of the microbiota, exerting a protective action in inflammatory bowel diseases.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, R.; Sutradhar, R.; Yao, Z.; Wodchis, W.P.; Rosella, L.C. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int. J. Epidemiol. 2020, 49, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Beaden, P.; Durrani, H.; Tang, K.; Mogilevskii, R.; Bhutta, Z. The role of the private sector in noncommunicable disease prevention and management in low- and middle-income countries: A series of systematic reviews and thematic syntheses. Int. J. Qual. Stud. Health Well-Being 2023, 18, 2156099. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.K.; Lim, K.K.; Ismail, H.; Mohd Yusoff, M.F.; Kee, C.C. Can the association between hypertension and physical activity be moderated by age? J. Taibah Univ. Med. Sci. 2023, 18, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, A.; Arhire, L.I.; Niță, O.; Popa, A.D.; Graur, M.; Mihalache, L. The relationship between lifestyle components and dietary patterns. Proc. Nutr. Soc. 2020, 79, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Vandoni, M.; Rossi, V.; Fiore, G.; Massini, G.; Berardo, C.; Gatti, A.; Baldassarre, P.; Bianchi, A.; et al. The Effect of Healthy Lifestyle Strategies on the Management of Insulin Resistance in Children and Adolescents with Obesity: A Narrative Review. Nutrients 2022, 14, 4692. [Google Scholar] [CrossRef]
- Sood, S.; Feehan, J.; Itsiopoulos, C.; Wilson, K.; Plebanski, M.; Scott, D.; Hebert, J.R.; Shivappa, N.; Mousa, A.; George, E.S.; et al. Higher Adherence to a Mediterranean Diet Is Associated with Improved Insulin Sensitivity and Selected Markers of Inflammation in Individuals Who Are Overweight and Obese without Diabetes. Nutrients 2022, 14, 4437. [Google Scholar] [CrossRef]
- United Nations Educational, Scientific, and Cultural Organization (UNESCO). Representative List of the Intangible Cultural Heritage of Humanity. Available online: http://www.unesco.org/culture/ich/index.php (accessed on 8 October 2015).
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean diet in the management and prevention of obesity. Exp. Gerontol. 2023, 174, 112121. [Google Scholar] [CrossRef]
- Iolascon, G.; Di Pietro, G.; Gimigliano, F.; Mauro, G.L.; Moretti, A.; Giamattei, M.T.; Ortolani, S.; Tarantino, U.; Brandi, M.L. Physical exercise and sarcopenia in older people: Position paper of the Italian Society of Orthopaedics and Medicine (OrtoMed). Clin. Cases Miner. Bone Metab. 2014, 11, 215–221. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Zhao, R.R.; O’Sullivan, A.J.; Fiatarone Singh, M.A. Exercise or physical activity and cognitive function in adults with type 2 diabetes, insulin resistance or impaired glucose tolerance: A systematic review. Eur. Rev. Aging Phys. Act. 2018, 15, 1. [Google Scholar] [CrossRef]
- García-Muñoz, C.; González-García, P.; Casuso-Holgado, M.J.; Martínez-Calderón, J.; Heredia-Rizo, A.M. Are movement-based mindful exercises (QIGONG, TAI CHI, AND YOGA) beneficial for stroke and Parkinson’s disease? A scoping review. Complement. Ther. Med. 2023, 72, 102912. [Google Scholar] [CrossRef]
- Mills, R.C., 3rd. Breast Cancer Survivors, Common Markers of Inflammation, and Exercise: A Narrative Review. Breast Cancer 2017, 11, 1178223417743976. [Google Scholar] [CrossRef]
- Dimitrov, S.; Hulteng, E.; Hong, S. Inflammation and exercise: Inhibition of monocytic intracellular TNF production by acute exercise via β2-adrenergic activation. Brain Behav. Immun. 2017, 61, 60–68. [Google Scholar] [CrossRef]
- Caprara, G. Mediterranean-Type Dietary Pattern and Physical Activity: The Winning Combination to Counteract the Rising Burden of Non-Communicable Diseases (NCDs). Nutrients 2021, 13, 429. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 2020, 41, 365–373. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. The Importance of Energy Balance. Eur. Endocrinol. 2013, 9, 111–115. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Potrykus, M.; Kaska, Ł.; Malgorzewicz, S. Pro-Inflammatory Profile of Adipokines in Obesity. Encyclopedia. Available online: https://encyclopedia.pub/entry/21914 (accessed on 7 February 2023).
- Ragino, Y.I.; Stakhneva, E.M.; Polonskaya, Y.V.; Kashtanova, E.V. The Role of Secretory Activity Molecules of Visceral Adipocytes in Abdominal Obesity in the Development of Cardiovascular Disease: A Review. Biomolecules 2020, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Rodriguez, R.M.; Segura-Sampedro, J.J.; Ochogavía-Seguí, A.; Romaguera, D.; Barceló-Coblijn, G. Insights behind the Relationship between Colorectal Cancer and Obesity: Is Visceral Adipose Tissue the Missing Link? Int. J. Mol. Sci. 2022, 23, 13128. [Google Scholar] [CrossRef] [PubMed]
- Sidorkiewicz, I.; Jóźwik, M.; Niemira, M.; Krętowski, A. Insulin Resistance and Endometrial Cancer: Emerging Role for microRNA. Cancers 2020, 12, 2559. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.H.; LeRoith, D. Obesity, type 2 diabetes, and cancer: The insulin and IGF connection. Endocr.-Relat. Cancer 2023, 19, F27–F45. [Google Scholar] [CrossRef]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef]
- Vallis, J.; Wang, P.P. The Role of Diet and Lifestyle in Colorectal Cancer Incidence and Survival. In Gastrointestinal Cancers; Morgado-Diaz, J.A., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 2. [Google Scholar]
- Shahid, R.; Haq, I.U.; Mahnoor; Awan, K.A.; Iqbal, M.J.; Munir, H.; Saeed, I. Diet and lifestyle modifications for effective management of polycystic ovarian syndrome (PCOS). J. Food Biochem. 2022, 46, e14117. [Google Scholar] [CrossRef]
- Hoedjes, M.; Nijman, I.; Hinnen, C. Psychosocial Determinants of Lifestyle Change after a Cancer Diagnosis: A Systematic Review of the Literature. Cancers 2022, 14, 2026. [Google Scholar] [CrossRef]
- Thomas, R.J.; Kenfield, S.A.; Jimenez, A. Exercise-induced biochemical changes and their potential influence on cancer: A scientific review. Br. J. Sports Med. 2017, 51, 640–644. [Google Scholar] [CrossRef]
- Torregrosa, C.; Chorin, F.; Beltran, E.E.M.; Neuzillet, C.; Cardot-Ruffino, V. Physical Activity as the Best Supportive Care in Cancer: The Clinician’s and the Researcher’s Perspectives. Cancers 2022, 14, 5402. [Google Scholar] [CrossRef]
- García-Chico, C.; López-Ortiz, S.; Peñín-Grandes, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Emanuele, E.; Ceci, C.; Graziani, G.; Fiuza-Luces, C.; Lista, S.; et al. Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review. Cancers 2023, 15, 324. [Google Scholar] [CrossRef]
- Razzak, Z.A.; Khan, A.A.; Farooqui, S.I. Effect of aerobic and anaerobic exercise on estrogen level, fat mass, and muscle mass among postmenopausal osteoporotic females. Int. J. Health Sci. 2019, 13, 10–16. [Google Scholar]
- Elliott, C.G.; Vidal-Almela, S.; Harvey, P.; O’Donnell, E.; Scheid, J.L.; Visintini, S.; Reed, J.L. Examining the Role of Physical Activity Interventions in Modulating Androgens and Cardiovascular Health in Postmenopausal Women: A Narrative Review. CJC Open. 2022, 5, 54–71. [Google Scholar] [CrossRef]
- Ennour-Idrissi, K.; Maunsell, E.; Diorio, C. Effect of physical activity on sex hormones in women: A systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015, 17, 139. [Google Scholar] [CrossRef]
- Golbidi, S.; Laher, I. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res. 2014, 2014, 726861. [Google Scholar] [CrossRef]
- Schmidt, S.; Monk, J.M.; Robinson, L.E.; Mourtzakis, M. The integrative role of leptin, estrogen and the insulin family in obesity-associated breast cancer: Potential effects of exercise. Obes. Rev. 2015, 16, 473–487. [Google Scholar] [CrossRef]
- Fortunati, N.; Catalano, M.G.; Boccuzzi, G.; Frairia, R. Sex Hormone-Binding Globulin (SHBG), estradiol and breast cancer. Mol. Cell. Endocrinol. 2010, 316, 86–92. [Google Scholar] [CrossRef]
- Del Mar Grasa, M.; Gulfo, J.; Camps, N.; Alcalá, R.; Monserrat, L.; Moreno-Navarrete, J.M.; Ortega, F.J.; Esteve, M.; Remesar, X.; Fernández-López, J.A.; et al. Modulation of SHBG binding to testosterone and estradiol by sex and morbid obesity. Eur. J. Endocrinol. 2017, 176, 393–404. [Google Scholar] [CrossRef]
- Knight, M.G.; Anekwe, C.; Washington, K.; Akam, E.Y.; Wang, E.; Stanford, F.C. Weight regulation in menopause. Menopause 2021, 28, 960–965. [Google Scholar] [CrossRef]
- Geczik, A.M.; Falk, R.T.; Xu, X.; Ansong, D.; Yarney, J.; Wiafe-Addai, B.; Edusei, L.; Dedey, F.; Vanderpuye, V.; Titiloye, N.; et al. Measured body size and serum estrogen metabolism in postmenopausal women: The Ghana Breast Health Study. Breast Cancer Res. 2022, 24, 9. [Google Scholar] [CrossRef]
- Abildgaard, J.; Ploug, T.; Al-Saoudi, E.; Wagner, T.; Thomsen, C.; Ewertsen, C.; Bzorek, M.; Pedersen, B.K.; Pedersen, A.T.; Lindegaard, B. Changes in abdominal subcutaneous adipose tissue phenotype following menopause is associated with increased visceral fat mass. Sci. Rep. 2021, 11, 14750. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Au, C.C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Moges, R.; Brown, K.A. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wong, C.; Hong, Y.; Chen, S. Growth factor signaling enhances aromatase activity of breast cancer cells via post-transcriptional mechanisms. J. Steroid Biochem. Mol. Biol. 2011, 123, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bleach, R.; Sherlock, M.; O’Reilly, M.W.; McIlroy, M. Growth Hormone/Insulin Growth Factor Axis in Sex Steroid Associated Disorders and Related Cancers. Front. Cell Dev. Biol. 2021, 9, 630503. [Google Scholar] [CrossRef]
- Mustian, K.M.; Sprod, L.K.; Palesh, O.G.; Peppone, L.J.; Janelsins, M.C.; Mohile, S.G.; Carroll, J. Exercise for the management of side effects and quality of life among cancer survivors. Curr. Sport. Med. Rep. 2009, 8, 325–330. [Google Scholar] [CrossRef]
- Piraux, E.; Caty, G.; Aboubakar Nana, F.; Reychler, G. Effects of exercise therapy in cancer patients undergoing radiotherapy treatment: A narrative review. SAGE Open Med. 2020, 8, 2050312120922657. [Google Scholar] [CrossRef]
- Zimmerman, A.; Planek, M.I.C.; Chu, C.; Oyenusi, O.; Paner, A.; Reding, K.; Skeete, J.; Clark, B.; Okwuosa, T.M. Exercise, cancer and cardiovascular disease: What should clinicians advise? Cardiovasc. Endocrinol. Metab. 2020, 10, 62–71. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Rubio-Zarapuz, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional and Exercise Interventions in Cancer-Related Cachexia: An Extensive Narrative Review. Int. J. Environ. Res. Public Health. 2022, 19, 4604. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Brown, J.C.; Winters-Stone, K.; Lee, A.; Schmitz, K.H. Cancer, physical activity, and exercise. Compr. Physiol. 2012, 2, 2775–2809. [Google Scholar]
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Newton, R.U. Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prostatic Dis. 2007, 10, 340–346. [Google Scholar] [CrossRef]
- Baumann, F.T.; Reike, A.; Hallek, M.; Wiskemann, J.; Reimer, V. Does Exercise Have a Preventive Effect on Secondary Lymphedema in Breast Cancer Patients Following Local Treatment?—A Systematic Review. Breast Care 2018, 13, 380–385. [Google Scholar] [CrossRef]
- Światowy, W.J.; Drzewiecka, H.; Kliber, M.; Sąsiadek, M.; Karpiński, P.; Pławski, A.; Jagodziński, P.P. Physical Activity and DNA Methylation in Humans. Int. J. Mol. Sci. 2021, 22, 12989. [Google Scholar] [CrossRef]
- Barrón-Cabrera, E.; Ramos-Lopez, O.; González-Becerra, K.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Epigenetic Modifications as Outcomes of Exercise Interventions Related to Specific Metabolic Alterations: A Systematic Review. Lifestyle Genom. 2019, 12, 25–44. [Google Scholar] [CrossRef]
- Nakajima, K.; Takeoka, M.; Mori, M.; Hashimoto, S.; Sakurai, A.; Nose, H.; Higuchi, K.; Itano, N.; Shiohara, M.; Oh, T.; et al. Exercise effects on methylation of ASC gene. Int. J. Sports Med. 2010, 31, 671–675. [Google Scholar] [CrossRef]
- Drummond, M.J.; McCarthy, J.J.; Sinha, M.; Spratt, H.M.; Volpi, E.; Esser, K.A.; Rasmussen, B.B. Aging and microRNA expression in human skeletal muscle: A microarray and bioinformatics analysis. Physiol. Genom. 2011, 43, 595–603. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, C.; Wu, R.; Hu, W. Tumor suppressor p53 meets microRNAs. J. Mol. Cell Biol. 2011, 3, 44–50. [Google Scholar] [CrossRef]
- Zacharewicz, E.; Lamon, S.; Russell, A.P. MicroRNAs in skeletal muscle and their regulation with exercise, ageing, and disease. Front. Physiol. 2013, 4, 266. [Google Scholar] [CrossRef]
- Hong, J.; Park, J. Systematic Review: Recommendations of Levels of Physical Activity among Colorectal Cancer Patients (2010–2019). Int. J. Environ. Res. Public Health 2021, 18, 2896. [Google Scholar] [CrossRef]
- Oruç, Z.; Kaplan, M.A. Effect of exercise on colorectal cancer prevention and treatment. World J. Gastrointest. Oncol. 2019, 11, 348–366. [Google Scholar] [CrossRef]
- Catsburg, C.; Miller, A.B.; Rohan, T.E. Adherence to cancer prevention guidelines and risk of breast cancer. Int. J. Cancer 2014, 135, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, M.; Zauli, G.; Martelli, A.M.; Vitale, M.; McCubrey, J.A.; Ultimo, S.; Capitani, S.; Neri, L.M. Impact of physical exercise in cancer survivors during and after antineoplastic treatments. Oncotarget 2018, 9, 14005–14034. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Booth, C.M. Exercise as cancer treatment: A clinical oncology framework for exercise oncology research. Front. Oncol. 2022, 12, 957135. [Google Scholar] [CrossRef] [PubMed]
- Witlox, L.; Hiensch, A.E.; Velthuis, M.J.; Steins Bisscho, C.N.; Los, M.; Erdkamp, F.L.G.; Bloemendal, H.J.; Verhaar, M.; Ten Bokkel Huinink, D.; Van der Wall, E.; et al. Four-year effects of exercise on fatigue and physical activity in patients with cancer. BMC Med. 2018, 16, 86. [Google Scholar] [CrossRef]
- Burini, R.C.; Anderson, E.; Durstine, J.L.; Carson, J.A. Inflammation, physical activity, and chronic disease: An evolutionary perspective. Sports Med. Health Sci. 2020, 2, 1–6. [Google Scholar] [CrossRef]
- Imierska, M.; Kurianiuk, A.; Błachnio-Zabielska, A. The Influence of Physical Activity on the Bioactive Lipids Metabolism in Obesity-Induced Muscle Insulin Resistance. Biomolecules 2020, 10, 1665. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S5–S78. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Divella, R.; Gadaleta Caldarola, G.; Mazzocca, A. Chronic Inflammation in Obesity and Cancer Cachexia. J. Clin. Med. 2022, 11, 2191. [Google Scholar] [CrossRef]
- Ramos-Nino, M.E. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol. 2013, 2013, 697521. [Google Scholar] [CrossRef]
- Zimta, A.A.; Tigu, A.B.; Muntean, M.; Cenariu, D.; Slaby, O.; Berindan-Neagoe, I. Molecular Links between Central Obesity and Breast Cancer. Int. J. Mol. Sci. 2019, 20, 5364. [Google Scholar] [CrossRef]
- Budek, M.; Nuszkiewicz, J.; Piórkowska, A.; Czuczejko, J.; Szewczyk-Golec, K. Inflammation Related to Obesity in the Etiopathogenesis of Gastroenteropancreatic Neuroendocrine Neoplasms. Biomedicines 2022, 10, 2660. [Google Scholar] [CrossRef]
- Prescott, S.L.; Logan, A.C. Transforming Life: A Broad View of the Developmental Origins of Health and Disease Concept from an Ecological Justice Perspective. Int. J. Environ. Res. Public Health 2016, 13, 1075. [Google Scholar] [CrossRef]
- Mansour, S.R.; Moustafa, M.A.A.; Saad, B.M.; Hamed, R.; Moustafa, A.A. Impact of diet on human gut microbiome and disease risk. New Microbes New Infect. 2021, 41, 100845. [Google Scholar] [CrossRef]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, m511. [Google Scholar] [CrossRef]
- Zhang, F.F.; Cudhea, F.; Shan, Z.; Michaud, D.S.; Imamura, F.; Eom, H.; Ruan, M.; Rehm, C.D.; Liu, J.; Du, M.; et al. Preventable Cancer Burden Associated With Poor Diet in the United States. JNCI Cancer Spectr. 2019, 3, pkz034. [Google Scholar] [CrossRef]
- Turati, F.; Dalmartello, M.; Bravi, F.; Serraino, D.; Augustin, L.; Giacosa, A.; Negri, E.; Levi, F.; La Vecchia, C. Adherence to the World Cancer Research Fund/American Institute for Cancer Research Recommendations and the Risk of Breast Cancer. Nutrients 2020, 12, 607. [Google Scholar] [CrossRef]
- Ribeiro, G.; Ferri, A.; Clarke, G.; Cryan, J.F. Diet and the microbiota–gut–brain-axis: A primer for clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care. 2022, 25, 443–450. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Itsiopoulos, C.; Mayr, H.L.; Thomas, C.J. The anti-inflammatory effects of a Mediterranean diet: A review. Curr. Opin. Clin. Nutr. Metab. Care. 2022, 25, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today 2017, 52, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalón, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef]
- Janssen, J.A. The Impact of Westernization on the Insulin/IGF-I Signaling Pathway and the Metabolic Syndrome: It Is Time for Change. Int. J. Mol. Sci. 2023, 24, 4551. [Google Scholar] [CrossRef]
- Lécuyer, L.; Laouali, N.; Dossus, L.; Shivappa, N.; Hébert, J.R.; Agudo, A.; Tjonneland, A.; Halkjaer, J.; Overvad, K.; Katzke, V.A.; et al. Inflammatory potential of the diet and association with risk of differentiated thyroid cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Eur. J. Nutr. 2022, 61, 3625–3635. [Google Scholar] [CrossRef]
- Hayati, Z.; Montazeri, V.; Shivappa, N.; Hebert, J.R.; Pirouzpanah, S. The association between the inflammatory potential of diet and the risk of histopathological and molecular subtypes of breast cancer in northwestern Iran: Results from the Breast Cancer Risk and Lifestyle study. Cancer 2022, 128, 2298–2312. [Google Scholar] [CrossRef]
- Bifulco, M.; Pisanti, S. The mystery of longevity in Cilento: A mix of a good dose of genetic predisposition and a balanced diet based on the Mediterranean model. Eur. J. Clin. Nutr. 2017, 71, 1020–1021. [Google Scholar] [CrossRef]
- Naureen, Z.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Foods of the Mediterranean diet: Tomato, olives, chili pepper, wheat flour and wheat germ. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E4–E11. [Google Scholar]
- Naureen, Z.; Bonetti, G.; Medori, M.C.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Foods of the Mediterranean diet: Garlic and Mediterranean legumes. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E12–E20. [Google Scholar]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef]
- Millar, S.R.; Navarro, P.; Harrington, J.M.; Shivappa, N.; Hébert, J.R.; Perry, I.J.; Phillips, C.M. Dietary score associations with markers of chronic low-grade inflammation: A cross-sectional comparative analysis of a middle- to older-aged population. Eur. J. Nutr. 2022, 61, 3377–3390. [Google Scholar] [CrossRef]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef]
- Augimeri, G.; Galluccio, A.; Caparello, G.; Avolio, E.; La Russa, D.; De Rose, D.; Morelli, C.; Barone, I.; Catalano, S.; Andò, S.; et al. Potential Antioxidant and An-ti-Inflammatory Properties of Serum from Healthy Adolescents with Optimal Mediterra-nean Diet Adherence: Findings from DIMENU Cross-Sectional Study. Antioxidants 2021, 10, 1172. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- La Russa, D.; Marrone, A.; Mandalà, M.; Macirella, R.; Pellegrino, D. Antioxidant/Anti-Inflammatory Effects of Caloric Restriction in an Aged and Obese Rat Model: The Role of Adiponectin. Biomedicines 2020, 8, 532. [Google Scholar] [CrossRef]
- Martin, B.; Mattson, M.P.; Maudsley, S. Caloric restriction and intermittent fasting: Two potential diets for successful brain aging. Ageing Res. Rev. 2006, 5, 332–353. [Google Scholar] [CrossRef]
- Colleluori, G.; Villareal, D.T. Weight strategy in older adults with obesity: Calorie restriction or not? Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 17–22. [Google Scholar] [CrossRef]
- Brandhorst, S.; Longo, V.D. Fasting and Caloric Restriction in Cancer Prevention and Treatment. Recent Results Cancer Res. 2016, 207, 241–266. [Google Scholar]
- Kopelovich, L.; Fay, J.R.; Sigman, C.C.; Crowell, J.A. The mammalian target of rapamycin pathway as a potential target for cancer chemoprevention. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, G.; Mirisola, M.G.; Longo, V.D. Intermittent and Periodic Fasting, Hormones, and Cancer Prevention. Cancers 2021, 13, 4587. [Google Scholar] [CrossRef] [PubMed]
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.B.; Hursting, S.D. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 2017, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Peisch, S.F.; Van Blarigan, E.L.; Chan, J.M.; Stampfer, M.J.; Kenfield, S.A. Prostate cancer progression and mortality: A review of diet and lifestyle factors. World J. Urol. 2017, 35, 867–874. [Google Scholar] [CrossRef]
- Nyrop, K.A.; Deal, A.M.; Williams, G.R.; Guerard, E.J.; Pergolotti, M.; Muss, H.B. Physical activity communication between oncology providers and patients with early-stage breast, colon, or prostate cancer. Cancer 2016, 122, 470–476. [Google Scholar] [CrossRef]
- Lynch, B.M.; Neilson, H.K.; Friedenreich, C.M. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011, 186, 13–42. [Google Scholar]
- Mugele, H.; Freitag, N.; Wilhelmi, J.; Yang, Y.; Cheng, S.; Bloch, W.; Schumann, M. High-intensity interval training in the therapy and aftercare of cancer patients: A systematic review with meta-analysis. J. Cancer Surviv. 2019, 13, 205–223. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef]
- Lorenzo, P.M.; Izquierdo, A.G.; Rodriguez-Carnero, G.; Fernández-Pombo, A.; Iglesias, A.; Carreira, M.C.; Tejera, C.; Bellido, D.; Martinez-Olmos, M.A.; Leis, R.; et al. Epigenetic Effects of Healthy Foods and Lifestyle Habits from the Southern European Atlantic Diet Pattern: A Narrative Review. Adv. Nutr. 2022, 13, 1725–1747. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Divella, R.; Marino, G.; Infusino, S.; Lanotte, L.; Gadaleta-Caldarola, G.; Gadaleta-Caldarola, G. The Mediterranean Lifestyle to Contrast Low-Grade Inflammation Behavior in Cancer. Nutrients 2023, 15, 1667. https://doi.org/10.3390/nu15071667
Divella R, Marino G, Infusino S, Lanotte L, Gadaleta-Caldarola G, Gadaleta-Caldarola G. The Mediterranean Lifestyle to Contrast Low-Grade Inflammation Behavior in Cancer. Nutrients. 2023; 15(7):1667. https://doi.org/10.3390/nu15071667
Chicago/Turabian StyleDivella, Rosa, Graziella Marino, Stefania Infusino, Laura Lanotte, Gaia Gadaleta-Caldarola, and Gennaro Gadaleta-Caldarola. 2023. "The Mediterranean Lifestyle to Contrast Low-Grade Inflammation Behavior in Cancer" Nutrients 15, no. 7: 1667. https://doi.org/10.3390/nu15071667
APA StyleDivella, R., Marino, G., Infusino, S., Lanotte, L., Gadaleta-Caldarola, G., & Gadaleta-Caldarola, G. (2023). The Mediterranean Lifestyle to Contrast Low-Grade Inflammation Behavior in Cancer. Nutrients, 15(7), 1667. https://doi.org/10.3390/nu15071667







