Oral Nutritional Supplements Reduce Body Weight Loss after Gastrectomy in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Literature Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Assessment of Potential Publication Bias
2.6. Quality Assessment
3. Results
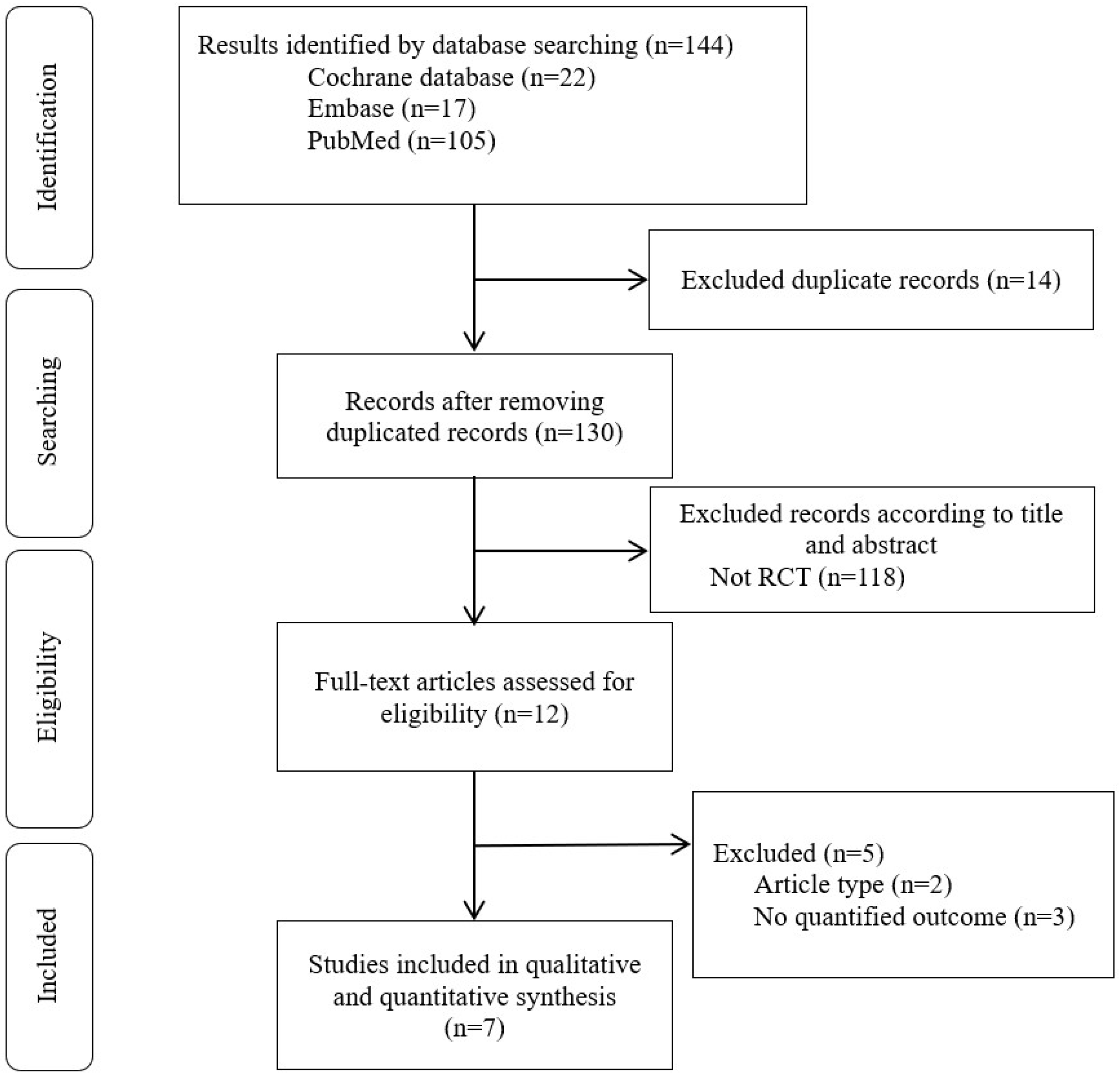
3.1. Study Selection
3.2. Outcome Findings
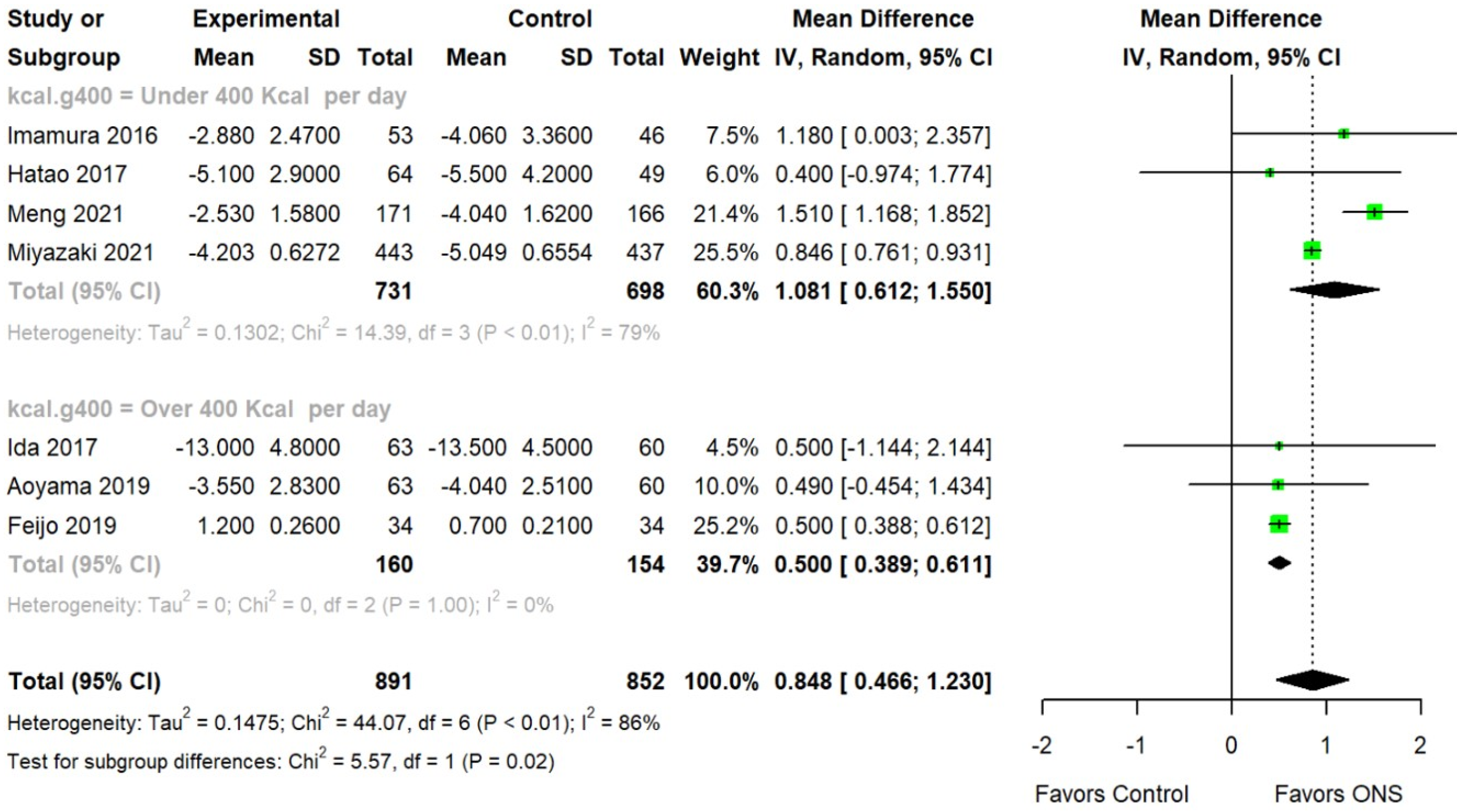
3.3. Moderator Analysis
3.4. Publication Bias
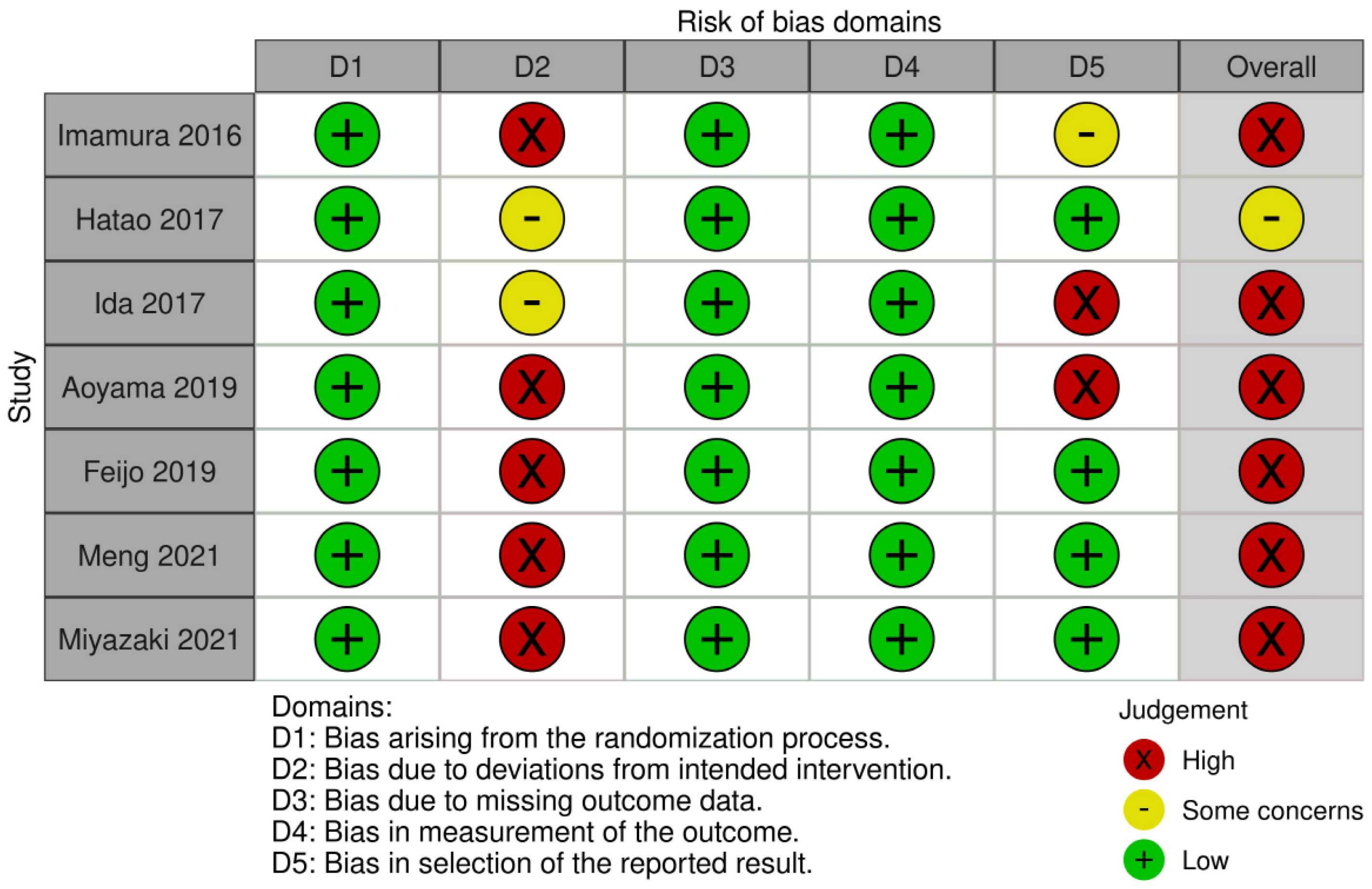
3.5. Quality Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef]
- Proserpio, I.; Rausei, S.; Barzaghi, S.; Frattini, F.; Galli, F.; Iovino, D.; Rovera, F.; Boni, L.; Dionigi, G.; Pinotti, G. Multimodal treatment of gastric cancer. World J. Gastrointest. Surg. 2014, 6, 55–58. [Google Scholar] [CrossRef]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef]
- Oh, S.E.; Choi, M.G.; Seo, J.M.; An, J.Y.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin. Nutr. 2019, 38, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Fein, M.; Fuchs, K.H.; Thalheimer, A.; Freys, S.M.; Heimbucher, J.; Thiede, A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: A randomized trial. Ann. Surg. 2008, 247, 759–765. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, P.; Song, Y.; Sun, J.; Chen, X.; Zhao, J.; Ma, B.; Wang, Z. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Chen, X.; Yang, K.; Zhang, X.; Li, K. Meta-analysis of preoperative oral nutritional supplements for patients with gastric cancer: East Asian experience. Eur. J. Clin. Nutr. 2020, 74, 991–1000. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Effects of nutritional interventions on nutritional status in patients with gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Tan, S.; Jiang, Y.; Han, J.; Xi, Q.; Zhuang, Q.; Wu, G. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin. Nutr. 2021, 40, 40–46. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Omori, T.; Fujitani, K.; Fujita, J.; Kawabata, R.; Imamura, H.; Okada, K.; Moon, J.H.; Hirao, M.; Matsuyama, J.; et al. Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: A phase 3, multicenter, open-label randomized controlled trial. Gastric Cancer 2021, 24, 1150–1159. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.R.; Kim, S.J. Intervention meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019008. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Yoshikawa, T.; Ida, S.; Cho, H.; Sakamaki, K.; Ito, Y.; Fujitani, K.; Takiguchi, N.; Kawashima, Y.; Nishikawa, K.; et al. Effects of perioperative Eicosapentaenoic acid-enriched oral nutritional supplement on lean body mass after total gastrectomy for gastric cancer. J. Cancer 2019, 10, 1070–1076. [Google Scholar] [CrossRef]
- Feijó, P.M.; Rodrigues, V.D.; Viana, M.S.; Dos Santos, M.P.; Abdelhay, E.; Viola, J.P.; de Pinho, N.B.; Martucci, R.B. Effects of ω-3 supplementation on the nutritional status, immune, and inflammatory profiles of gastric cancer patients: A randomized controlled trial. Nutrition 2019, 61, 125–131. [Google Scholar] [CrossRef]
- Hatao, F.; Chen, K.Y.; Wu, J.M.; Wang, M.Y.; Aikou, S.; Onoyama, H.; Shimizu, N.; Fukatsu, K.; Seto, Y.; Lin, M.T. Randomized controlled clinical trial assessing the effects of oral nutritional supplements in postoperative gastric cancer patients. Langenbecks Arch. Surg. 2017, 402, 203–211. [Google Scholar] [CrossRef]
- Ida, S.; Hiki, N.; Cho, H.; Sakamaki, K.; Ito, S.; Fujitani, K.; Takiguchi, N.; Kawashima, Y.; Nishikawa, K.; Sasako, M.; et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br. J. Surg. 2017, 104, 377–383. [Google Scholar] [CrossRef]
- Imamura, H.; Nishikawa, K.; Kishi, K.; Inoue, K.; Matsuyama, J.; Akamaru, Y.; Kimura, Y.; Tamura, S.; Kawabata, R.; Kawada, J.; et al. Effects of an Oral Elemental Nutritional Supplement on Post-gastrectomy Body Weight Loss in Gastric Cancer Patients: A Randomized Controlled Clinical Trial. Ann. Surg. Oncol. 2016, 23, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Wobith, M.; Weimann, A. Oral Nutritional Supplements and Enteral Nutrition in Patients with Gastrointestinal Surgery. Nutrients 2021, 13, 2655. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Na, J.R.; Suh, Y.S.; Kong, S.H.; Lim, J.H.; Ju, D.L.; Yang, H.K.; Lee, H.J. A Prospective Observational Study Evaluating the Change of Nutritional Status and the Incidence of Dumping Syndrome after Gastrectomy. J. Clin. Nutr. 2014, 6, 59–70. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, W.G.; Cho, Y.G.; Lee, Y.H.; Kim, J.P. A study of nutritional assessment and dietary intake after gastrectomy of gastric cancer patients. Korean J. Nutr. 1994, 27, 844–855. [Google Scholar]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Wu, G. Expert consensus on oral nutritional supplements for adults. Chin. J. Gastrointest. Surg. 2017, 20, 361–365. [Google Scholar]
- Berg, P.; McCallum, R. Dumping Syndrome: A Review of the Current Concepts of Pathophysiology, Diagnosis, and Treatment. Dig. Dis. Sci. 2016, 61, 11–18. [Google Scholar] [CrossRef]
- Eom, B.W.; Kim, J.; Kim, D.H.; Kim, Y.I.; Yoon, H.M.; Cho, S.J.; Lee, J.Y.; Kim, C.G.; Choi, I.J.; Kim, Y.W.; et al. Recovery of Food Intake after Gastrectomy for Gastric Cancer: Based on a Large-Scale Gastric Cancer Cohort. Dig. Surg. 2018, 35, 220–229. [Google Scholar] [CrossRef]
- Park, Y.O.; Yoon, S.Y.; Kang, S.S.; Han, S.M.; Kang, E.H. Nutritional Status and Dietary Change after Gastrectomy of Gastric Cancer Patients. Korean J. Commun. Nutr. 2012, 17, 101–108. [Google Scholar] [CrossRef][Green Version]
- Seo, B.Y.; Jung, H.Y.; Yoo, W.S. Relation between body weight changes and prognosis in patients with gastric cancer. Korean J. Food Cook Sci. 2000, 36, 607–613. [Google Scholar]
- Yoon, K.Y.; Ahn, S.M.; Lee, K.S.; Choi, K.H. Nutritional Assessment in Gastric Carcinoma. Ann. Surg. Treat. Res. 2005, 68, 185–193. [Google Scholar]
- Stein, T.P.; Buzby, G.P. Protein metabolism in surgical patients. Surg. Clin. N. Am. 1981, 61, 519–527. [Google Scholar] [CrossRef]
- Rey-Ferro, M.; Castaño, R.; Orozco, O.; Serna, A.; Moreno, A. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition 1997, 13, 878–881. [Google Scholar] [CrossRef]
- Kimura, Y.; Nishikawa, K.; Kishi, K.; Inoue, K.; Matsuyama, J.; Akamaru, Y.; Tamura, S.; Kawada, J.; Kawase, T.; Kawabata, R.; et al. Long-term effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients (KSES002). Ann. Gastroenterol. Surg. 2019, 3, 648–656. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Study Design | Disease | Average Age (Years) | No. of Patients (% Female) | Kcal Consumption Per Day | ONSs Nutrient | Controls | Duration (Months) | TNM Stage (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Imamura 2016 [20] | Japan | RCT | Gastric Cancer | 66.15 | 99 (29.75) | 300 | ONSs | Regular diet | 2 | I (60.3), II (21.6), III (17.2), IV (0.9) |
| Hatao 2017 [18] | Japan, Taiwan | RCT | Gastric Cancer | 64.7 | 113 (38.9) | 400 | ONSs | Usual postoperative diet | 3 | I (53.1), II (22.1), III (24.8), IV (0) |
| Ida 2017 [19] | Japan | RCT | Gastric Cancer | 65.35 | 123 (27.6) | 600 | ONS plus EPA (2.2 g/d) | Standard diet | 3 | I (40), II (32), III (29), IV (0) |
| Aoyama 2019 [16] | Japan | RCT | Gastric Cancer | 65.35 | 123 (27.6) | 600 | ONSs plus EPA (2.2 g/d) | Standard diet | 3 | I (22.8), II (18.7), III (18.7), IV (39.8) |
| Feijo 2019 [17] | Brazil | RCT | Gastric Cancer | 55.9 | 68 (35.3) | 600 | ONSs plus EPA (3.2 g/d) | Standard formula | 1 | I (4.4), II (25), III (45.6), IV (7.4) |
| Meng 2021 [10] | China | RCT | Gastric Cancer | 59.91 | 337 (32.3) | 100 | ONSs | Dietary advice alone | 3 | I (25.8), II (28.8), III (38.6), IV (6.8) |
| Miyazaki 2021 [11] | Japan | RCT | Gastric Cancer | 66.4 | 880 (35.6) | 400 | ONSs | Regular diet | 3, 6, 12 | I (61.4), II (22.9), III (15.4), IV (0.4) |
| Variables | k | β | MD | 95% CIL | 95% CIH | p |
|---|---|---|---|---|---|---|
| No. of total patients | 7 | 0.000 | −0.001 | 0.002 | 0.620 | |
| Age | 7 | 0.003 | −0.098 | 0.104 | 0.956 | |
| Female rate | 7 | −2.505 | −14.767 | 9.757 | 0.689 | |
| Duration (month) | 7 | 0.232 | −0.150 | 0.614 | 0.233 | |
| TNM stage | 0.019 | |||||
| ≥3 | 2 | 0.500 | 0.388 | 0.611 | ||
| <3 | 5 | 1.046 | 0.603 | 1.488 | ||
| Kcal consumption | 0.018 | |||||
| Over 400 Kcal | 3 | 0.500 | 0.389 | 0.611 | ||
| Under 400 Kcal | 4 | 1.081 | 0.612 | 1.550 | ||
| Country | <0.001 | |||||
| Brazil | 1 | 0.500 | 0.388 | 0.612 | ||
| Japan | 6 | 0.842 | 0.758 | 0.926 | ||
| China | 1 | 1.510 | 1.168 | 1.852 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.; Kim, J.-Y.; Kang, H.-H.; Park, E.; Shim, S.R. Oral Nutritional Supplements Reduce Body Weight Loss after Gastrectomy in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2023, 15, 3924. https://doi.org/10.3390/nu15183924
Choi M, Kim J-Y, Kang H-H, Park E, Shim SR. Oral Nutritional Supplements Reduce Body Weight Loss after Gastrectomy in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2023; 15(18):3924. https://doi.org/10.3390/nu15183924
Chicago/Turabian StyleChoi, Mijoo, Jong-Yeup Kim, Hyun-Hi Kang, Eunju Park, and Sung Ryul Shim. 2023. "Oral Nutritional Supplements Reduce Body Weight Loss after Gastrectomy in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 15, no. 18: 3924. https://doi.org/10.3390/nu15183924
APA StyleChoi, M., Kim, J.-Y., Kang, H.-H., Park, E., & Shim, S. R. (2023). Oral Nutritional Supplements Reduce Body Weight Loss after Gastrectomy in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 15(18), 3924. https://doi.org/10.3390/nu15183924






