Growth and Duration of Inflammation Determine Short- and Long-Term Outcome in Very-Low-Birth-Weight Infants Requiring Abdominal Surgery
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Long-Term Outcome Measures
2.3. Statistical Analysis
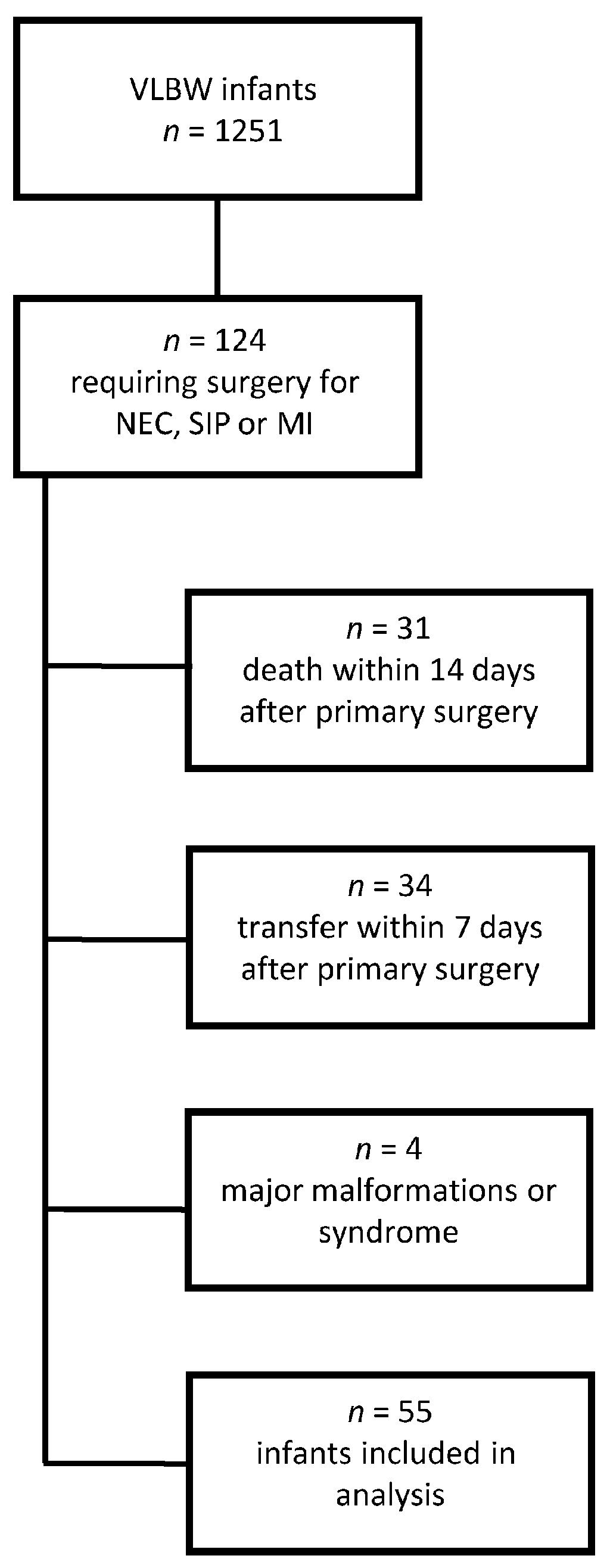
3. Results
3.1. Patient Characteristics
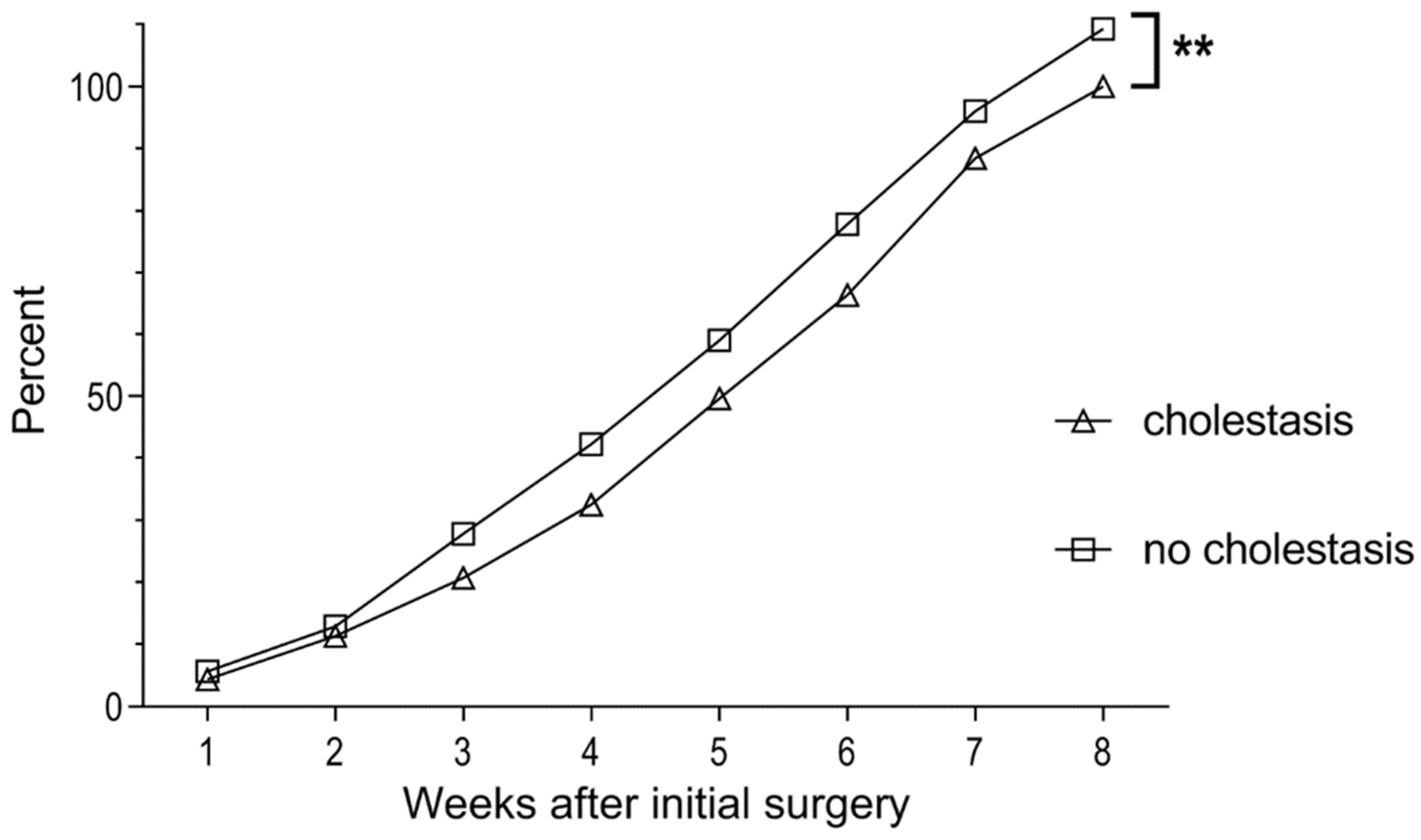
3.2. Cholestasis
3.3. Outcome at 2 Years CA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Patel, R.M. Short- and Long-Term Outcomes for Extremely Preterm Infants. Am. J. Perinatol. 2016, 33, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.H.; Hall, N.J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis—A Systematic Review. J. Pediatr. 2020, 220, 86–92.e3. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, I.; Lee, H.-J.; Oh, H.J.; Ahn, M.K.; Baek, W.I.; Kim, Y.E.; Oh, S.H.; Lee, B.S.; Namgoong, J.-M.; et al. Clinical characteristics of neonatal cholestasis in a tertiary hospital and the development of a novel prediction model for mortality. Ebiomedicine 2022, 77, 103890. [Google Scholar] [CrossRef] [PubMed]
- Satrom, K.; Gourley, G. Cholestasis in Preterm Infants. Clin. Perinatol. 2016, 43, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Niccum, M.; Khan, M.N.; Middleton, J.P.; Vergales, B.D.; Syed, S. Cholestasis affects enteral tolerance and prospective weight gain in the NICU. Clin. Nutr. ESPEN 2019, 30, 119–125. [Google Scholar] [CrossRef] [PubMed]
- De Rose, D.U.; Cota, F.; Gallini, F.; Bottoni, A.; Fabrizio, G.C.; Ricci, D.; Romeo, D.M.; Mercuri, E.; Vento, G.; Maggio, L. Extra-uterine growth restriction in preterm infants: Neurodevelopmental outcomes according to different definitions. Eur. J. Paediatr. Neurol. 2021, 33, 135–145. [Google Scholar] [CrossRef]
- Goepfert, A.R.; Andrews, W.W.; Carlo, W.; Ramsey, P.S.; Cliver, S.P.; Goldenberg, R.L.; Hauth, J.C. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am. J. Obstet. Gynecol. 2004, 191, 1375–1381. [Google Scholar] [CrossRef]
- Rallis, D.; Balomenou, F.; Kappatou, K.; Karantanou, K.; Tzoufi, M.; Giapros, V. C-reactive protein in infants with no evidence of early-onset sepsis. J. Matern. Fetal. Neonatal Med. 2021, 35, 5659–5664. [Google Scholar] [CrossRef]
- Voigt, M.; Rochow, N.; Schneider, K.T.M.; Hagenah, H.-P.; Scholz, R.; Hesse, V.; Wittwer-Backofen, U.; Straube, S.; Olbertz, D. New Percentile Values for the Anthropometric Dimensions of Singleton Neonates: Analysis of Perinatal Survey Data of 2007–2011 from all 16 States of Germany. Z. Geburtshilfe Neonatol. 2014, 218, 210–217. [Google Scholar] [CrossRef]
- Brandt, I. Growth dynamics of low-birth-weight infants with emphasis on the perinatal period. In Human Growth: A Comprehensive Treatise, 2nd ed.; Falkner, F., Tanner, J.M., Eds.; Plenum Press: New York, NY, USA, 1986; Volume 1, pp. 415–475. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant and Toddler Development, 3rd ed.; APA Psyc Tests; APA: Worcester, MA, USA, 2005. [Google Scholar] [CrossRef]
- Griffiths, R.; Huntley, M. Griffiths Mental Developmental Scales—Revised: Birth to 2 Years (GMDS 0-2); APA Psyc Tests; APA: Worcester, MA, USA, 1996. [Google Scholar] [CrossRef]
- Johnson, S.; Moore, T.; Marlow, N. Using the Bayley-III to assess neurodevelopmental delay: Which cut-off should be used? Pediatr. Res. 2014, 75, 670–674. [Google Scholar] [CrossRef]
- Radmacher, P.G.; Looney, S.W.; Rafail, S.T.; Adamkin, D.H. Prediction of Extrauterine Growth Retardation (EUGR) in VVLBW Infants. J. Perinatol. 2003, 23, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Wolf, L.; Gfroerer, S.; Fiegel, H.; Rolle, U. Complications of newborn enterostomies. World J. Clin. Cases 2018, 6, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, M.; Danielson, L.; Brownie, E.; Saba, M.; Natarajan, G.; Klein, M. Enteral nutrition and total parenteral nutrition components in the course of total parenteral nutrition–associated cholestasis in neonatal necrotizing enterocolitis. Surgery 2014, 156, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, M.; Clark, R.H.; Kelleher, A.S.; Flores, C.; White, R.; Chace, D.H.; Spitzer, A.R.; For the Pediatrix Amino-Acid Study Group. Demographic and nutritional factors associated with prolonged cholestatic jaundice in the premature infant. J. Perinatol. 2008, 28, 129–135. [Google Scholar] [CrossRef]
- Salas, A.; Carlo, W.A.; Ambalavanan, N.; Nolen, T.L.; Stoll, B.J.; Das, A.; Higgins, R.D. Gestational age and birthweight for risk assessment of neurodevelopmental impairment or death in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F494–F501. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Kim, E.-K.; Kim, S.-H.; Kim, H.-Y.; Kim, H.-S. Head Growth and Neurodevelopment of Preterm Infants with Surgical Necrotizing Enterocolitis and Spontaneous Intestinal Perforation. Children 2021, 8, 833. [Google Scholar] [CrossRef]
- Humberg, A.; Spiegler, J.; Fortmann, M.I.; Zemlin, M.; Marissen, J.; Swoboda, I.; Rausch, T.K.; Herting, E.; Göpel, W.; Härtel, C.; et al. Surgical necrotizing enterocolitis but not spontaneous intestinal perforation is associated with adverse neurological outcome at school age. Sci. Rep. 2020, 10, 2373. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, R.; Oh, W.; Hintz, S.R.; Blakely, M.L.; Das, A.; Bell, E.F.; Saha, S.; Laptook, A.R.; Shankaran, S.; Stoll, B.J.; et al. Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J. Perinatol. 2014, 34, 64–70. [Google Scholar] [CrossRef]
- Keogh, M.J.; Bennet, L.; Drury, P.P.; Booth, L.C.; Mathai, S.; Naylor, A.S.; Fraser, M.; Gunn, A.J. Subclinical exposure to low-dose endotoxin impairs EEG maturation in preterm fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R270–R278. [Google Scholar] [CrossRef] [PubMed]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Gopel, W.; Hartel, C. German Neonatal Network; German Center for Lung Research and Priming Immunity at the beginning of life (PRIMAL) Consortium. Preterm birth and sustained inflammation: Consequences for the neonate. Semin. Immunopathol. 2020, 42, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Aebischer, M.; Adams, M.; Natalucci, G.; Bonhoeffer, J.; Latzin, P.; Nelle, M.; Bucher, H.U.; Latal, B.; the Swiss Neonatal Network and Follow-Up Group. Impact of Sepsis on Neurodevelopmental Outcome in a Swiss National Cohort of Extremely Premature Infants. Pediatrics 2011, 128, e348–e357. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, E.-K.; Shin, S.H.; Choi, Y.-H.; Jung, Y.H.; Kim, S.Y.; Koh, J.W.; Choi, E.K.; Cheon, J.-E.; Kim, H.-S. Factors associated with neurodevelopment in preterm infants with systematic inflammation. BMC Pediatr. 2021, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Allred, E.N.; Kuban, K.; Dammann, O.; Paneth, N.; Fichorova, R.; Hirtz, D.; Leviton, A. Elevated Concentrations of Inflammation-Related Proteins in Postnatal Blood Predict Severe Developmental Delay at 2 Years of Age in Extremely Preterm Infants. J. Pediatr. 2012, 160, 395–401.e4. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Shah, B.; Allred, E.N.; Fichorova, R.N.; Kuban, K.C.; Dammann, O.; Leviton, A. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav. Immun. 2013, 29, 104–112. [Google Scholar] [CrossRef]
- Nist, M.D.; Pickler, R.H. An Integrative Review of Cytokine/Chemokine Predictors of Neurodevelopment in Preterm Infants. Biol. Res. Nurs. 2019, 21, 366–376. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Median (Range) or n (%) |
|---|---|
| Gestational age, weeks | 25.3 (23.1–32.3) |
| Birth weight, grams | 710 (315–1450) |
| Birth weight percentile | 30 (1–92) |
| HC at birth, cm | 23 (19–29) |
| HC percentile at birth | 30 (1–80) |
| Sex, male | 36 (65.5) |
| Out-born | 21 (33.2) |
| Age at primary surgery, days | 12 (2–50) |
| Age at reversal of enterostomy, days | 75 (37–280) |
| Bronchopulmonary dysplasia, moderate or severe | 8 (14.5) |
| Intraventricular haemorrhage > 2° | 7 (12.7) |
| NEC n = 16 | SIP n = 32 | MI n = 7 | p Value a | |
|---|---|---|---|---|
| Gestational age, weeks | 25.8 (24.3–32.3) | 25.0 (23.1–30.4) | 26.0 (23.3–32.0) | 0.26 |
| Birth weight, grams | 663 (380–1370) | 713 (315–1450) | 790 (645–1375) | 0.69 |
| Head circumference, cm | 23.5 (19–29) | 22.9 (20–28) | 22 (22–28.5) | 0.75 |
| Cholestasis | 9 (56.3) | 10 (31.3) | 1 (14.3) | 0.11 |
| Data on neurodevelopmental outcome available | 12 (75.0) | 26 (81.3) | 5 (71.4) | 0.80 |
| Neurodevelopmental delay at 2 years CA | 8 (66.7) | 15 (57.7) | 2 (40.0) | 0.60 |
| Cholestasis n = 20 | No Cholestasis n = 35 | p Value | |
|---|---|---|---|
| Gestational age, weeks | 25.4 (23.1–31.7) | 25.7 (23.3–32.3) | 0.71 a |
| Birth weight, grams | 760 (315–1370) | 695 (575–1450) | 0.77 a |
| Birth weight percentile | 25 (1–92) | 30 (5–90) | 0.63 a |
| Sex, male | 11 (55.0) | 25 (71.4) | 0.22 b |
| Out-born | 12 (60.0) | 9 (25.7) | 0.01 b |
| Aetiology of intestinal complication | NEC 9 (45.0) SIP 10 (50.0) MI 1 (5.0) | NEC 7 (20.0) SIP 22 (62.9) MI 6 (17.1) | 0.47 b |
| CrP max, mg/L | 145.5 (21–275) | 75 (1.7–401) | 0.03 a |
| Duration of CrP elevation, days | 19 (2–104) | 6 (0–20) | <0.0001 a |
| IL-6 max, ng/L | 2040 (18–15,900) | 153.5 (30–11,000) | 0.07 a |
| Duration of IL-6 elevation, days | 4 (0–10) | 0 (0–7) | 0.0005 a |
| Enteral nutrition prior to initial surgery | 18 (90.0) | 31 (88.6) | 0.87 b |
| Duration of parenteral nutrition after initial surgery, days | 24 (3–96) | 10 (4–59) | 0.001 a |
| Type of enteral nutrition after initial surgery, exclusive formula | 11 (55.0) | 22 (62.9) | 0.57 b |
| Duration between creation and reversal of enterostomy, days | 63.5 (33–251) | 58 (18–121) | 0.50 a |
| Short bowel syndrome prior to reversal of enterostomy c | 4 (20.0) | 2 (5.7) | 0.10 b |
| Number of CLABSIs | 1 (0–4) | 0 (0–2) | 0.04 a |
| Number of blood transfusions | 9 (5–36) | 5 (1–16) | 0.0005 a |
| Number of laparotomies before reversal of enterostomy | 2 (1–9) | 1 (1–7) | 0.03 a |
| Age at initial surgery, days | 15 (7–40) | 10 (2–50) | 0.09 a |
| Outcome Parameter | Median (Range) or n (%) |
|---|---|
| Neurodevelopmental delay, moderate or severe | 25 (58.1) |
| MDI | 90 (50–120) |
| Body weight, grams | 10,800 (8300–15,700) |
| Weight percentile | 20 (1–83) |
| Delta weight percentile a | −6 (−70–+47) |
| HC, cm | 46.8 (38.6–52) |
| HC percentile | 4 (1–80) |
| Delta HC percentile a | −19 (−69–+50) |
| Neurodevelopmental Delay n = 25 | No Neurodevelopmental Delay n = 18 | p Value | |
|---|---|---|---|
| Gestational age, weeks | 25.0 (23.1–32.3) | 25.6 (23.3–32.0) | 0.82 b |
| Aetiology of intestinal complication | NEC 8 (32.0) | NEC 4 (22.2) | 0.60 c |
| SIP 15 (60.0) | SIP 11 (61.1) | ||
| MI 2 (8.0) | MI 3 (16.7) | ||
| Outborn | 7 (28.0) | 5 (27.8) | 0.99 c |
| Sex (male) | 19 (76.0) | 11 (61.1) | 0.29 c |
| Age at initial surgery, days | 9 (2–50) | 12 (2–37) | 0.87 b |
| Number of transfusions | 7 (1–36) | 6.5 (1–32) | 0.93 b |
| Number of CLABSIs | 0 (0–3) | 0.5 (0–4) | 0.45 b |
| Number of laparotomies before reversal of enterostomy | 2 (1–9) | 1 (1–5) | 0.24 b |
| Bronchopulmonary dysplasia, moderate or severe | 5 (20.0) | 1 (5.5) | 0.18 c |
| Intraventricular haemorrhage > 2° | 5 (20.0) | 2 (11.1) | 0.44 c |
| Maximum CrP, mg/L | 114 (7–474) | 121 (1.7–327) | 0.96 b |
| Duration of CrP elevation, days | 9.5 (0–104) | 9.5 (0–38) | 0.62 b |
| Maximum IL-6, ng/L | 688.5 (18–15,900) | 1889 (34–11,000) | 0.99 b |
| Duration of IL-6 elevation, days | 2 (0–10) | 0 (0–5) | 0.048 b |
| Growth percentiles at birth | |||
| Weight, grams | 690 (315–1450) | 788 (510–1375) | 0.38 b |
| Weight percentile | 30 (1–90) | 40 (9–90) | 0.15 b |
| HC, cm | 22.5 (19–28) | 23.3 (21–28.5) | 0.53 b |
| HC percentile | 30 (1–80) | 40 (15–70) | 0.40 b |
| Growth percentiles at initial surgery | |||
| Weight percentile | 15 (1–50) | 20 (1–50) | 0.70 b |
| Delta weight percentile a | −15 (−66–0) | −9 (−45–5) | 0.02 b |
| HC percentile | 8 (1–40) | 9 (1–40) | 0.70 b |
| Delta HC percentile a | −23.5 (−69–0) | −15 (−55–0) | 0.18 b |
| Growth percentiles at discharge | |||
| Weight percentile | 4 (1–85) | 3.5 (1–30) | 0.96 b |
| Delta weight percentile a | −24 (−70–27) | −23.5 (−80–−8) | 0.15 b |
| HC percentile | 1 (1–98) | 1 (1–50) | 0.74 b |
| Delta HC percentile a | −20 (−70–28) | −23 (−69–10) | 0.60 b |
| Growth percentiles at 2 years CA | |||
| Weight percentile | 20 (1–60) | 30 (1–83) | 0.57 b |
| Delta weight percentile a | −18.5 (−70–45) | 6.5 (−69–47) | 0.03 b |
| HC percentile | 2 (1–80) | 4 (1–26) | 0.89 b |
| Delta HC percentile a | −16 (−69–50) | −27.5 (−66–0) | 0.25 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, C.; Abukhris, A.; Brendel, J.; Böhne, C.; Bohnhorst, B.; Pirr, S. Growth and Duration of Inflammation Determine Short- and Long-Term Outcome in Very-Low-Birth-Weight Infants Requiring Abdominal Surgery. Nutrients 2023, 15, 1668. https://doi.org/10.3390/nu15071668
Peter C, Abukhris A, Brendel J, Böhne C, Bohnhorst B, Pirr S. Growth and Duration of Inflammation Determine Short- and Long-Term Outcome in Very-Low-Birth-Weight Infants Requiring Abdominal Surgery. Nutrients. 2023; 15(7):1668. https://doi.org/10.3390/nu15071668
Chicago/Turabian StylePeter, Corinna, Abdulmonem Abukhris, Julia Brendel, Carolin Böhne, Bettina Bohnhorst, and Sabine Pirr. 2023. "Growth and Duration of Inflammation Determine Short- and Long-Term Outcome in Very-Low-Birth-Weight Infants Requiring Abdominal Surgery" Nutrients 15, no. 7: 1668. https://doi.org/10.3390/nu15071668
APA StylePeter, C., Abukhris, A., Brendel, J., Böhne, C., Bohnhorst, B., & Pirr, S. (2023). Growth and Duration of Inflammation Determine Short- and Long-Term Outcome in Very-Low-Birth-Weight Infants Requiring Abdominal Surgery. Nutrients, 15(7), 1668. https://doi.org/10.3390/nu15071668






