A Novel Symbiotic Formulation Reduces Obesity and Concomitant Metabolic Syndrome in Rats by Raising the Relative Abundance of Blautia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Symbiotic Treatments and Groups
2.3. Body Weight and Biomarkers
2.4. Histopathological Analysis
2.5. The 16S rRNA Sequencing of Fecal Samples and Bioinformatics Analysis
2.6. Broadly Targeted Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Measurement of Metabolites in Plasma Samples
2.7. Formatting of Mathematical Components
2.8. Statistical Analysis
3. Results
3.1. Symbiotic Treatment Reduced Body Weight and Body Fat in Obese Rats
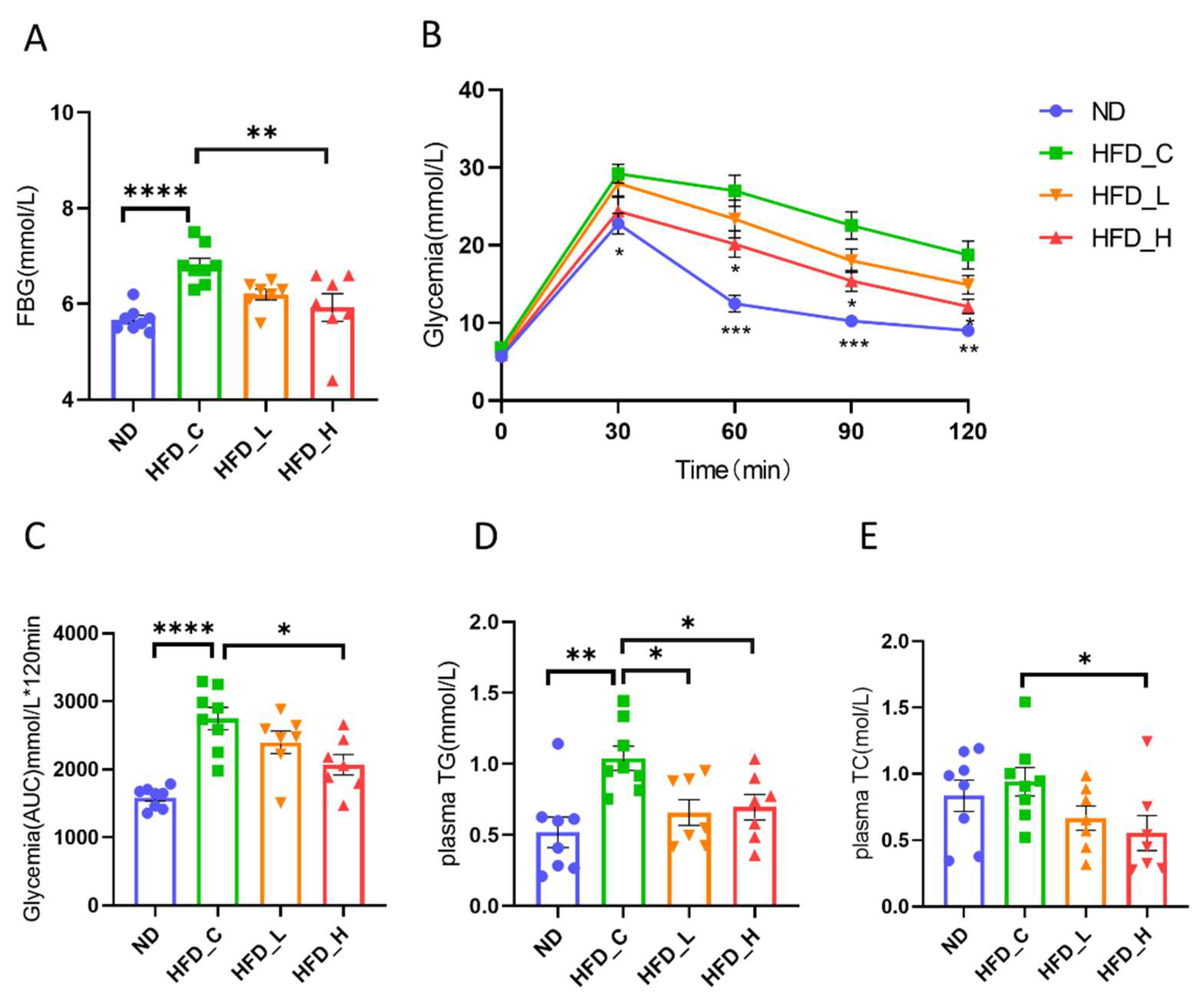
3.2. Symbiotic Treatment Improved Dysglycemia and Dyslipidemia in Obese Rats
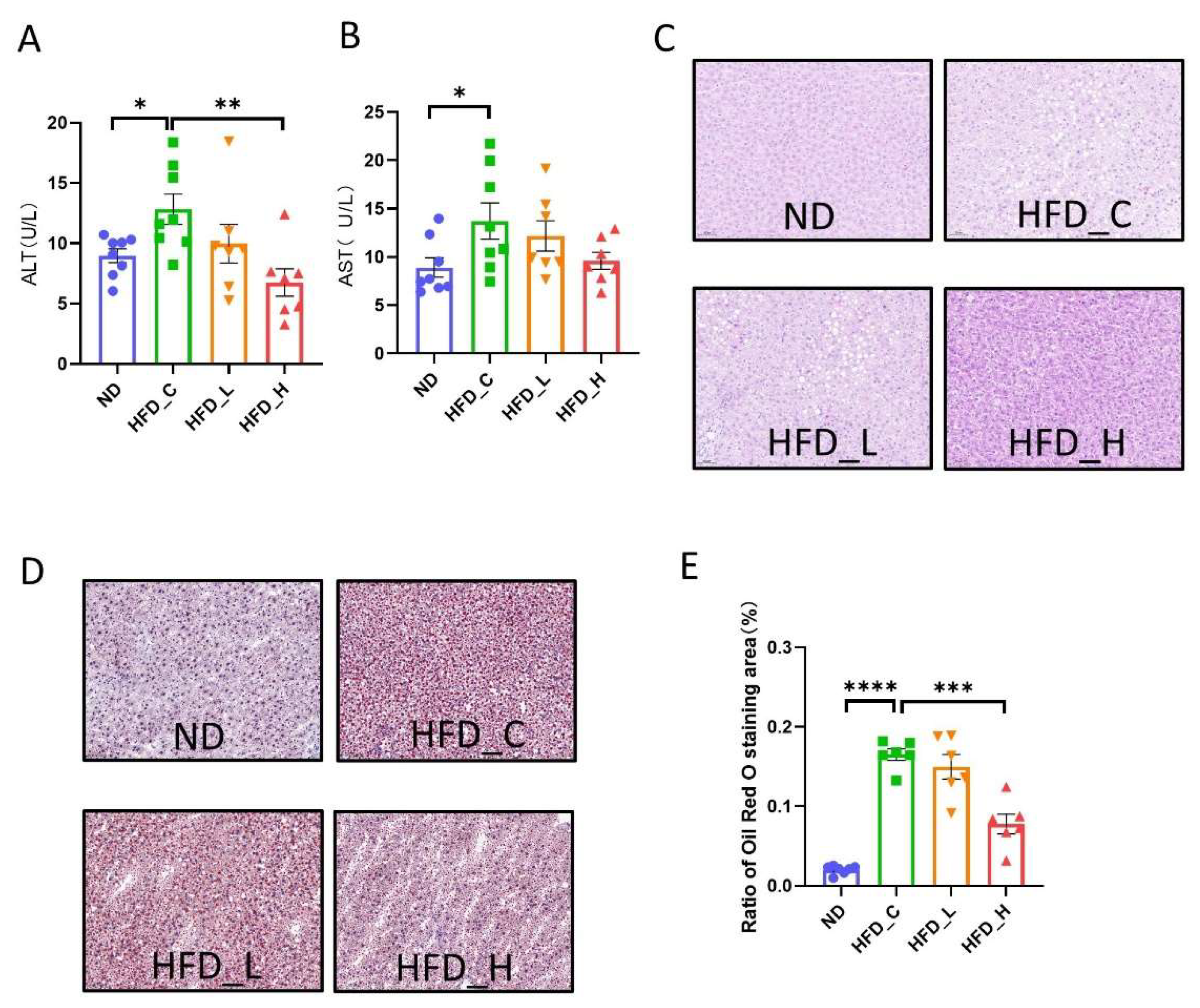
3.3. Symbiotic Treatment Inhibited Liver Lesions and Restored Liver Function in Obese Rats
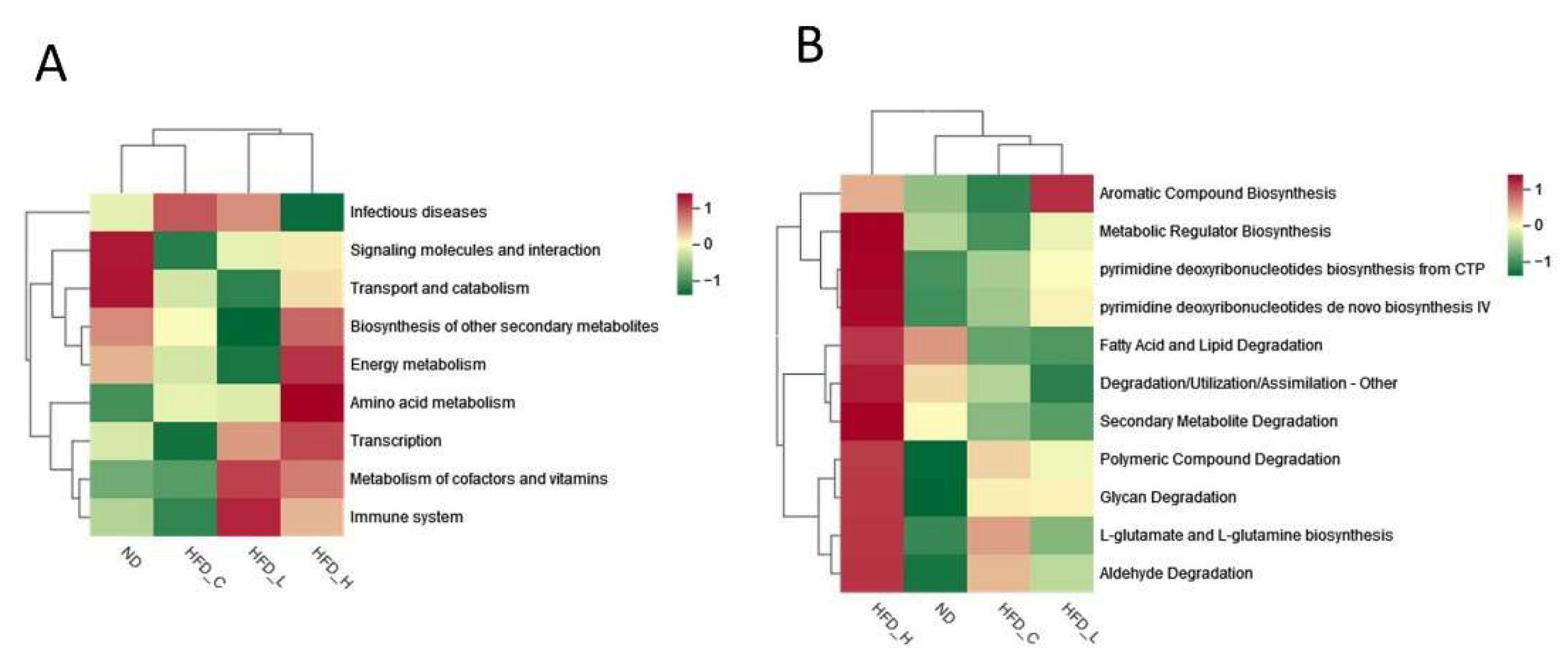
3.4. Symbiotic Treatment Altered the Composition and Function of the Gut Microbiota in Obese Rats
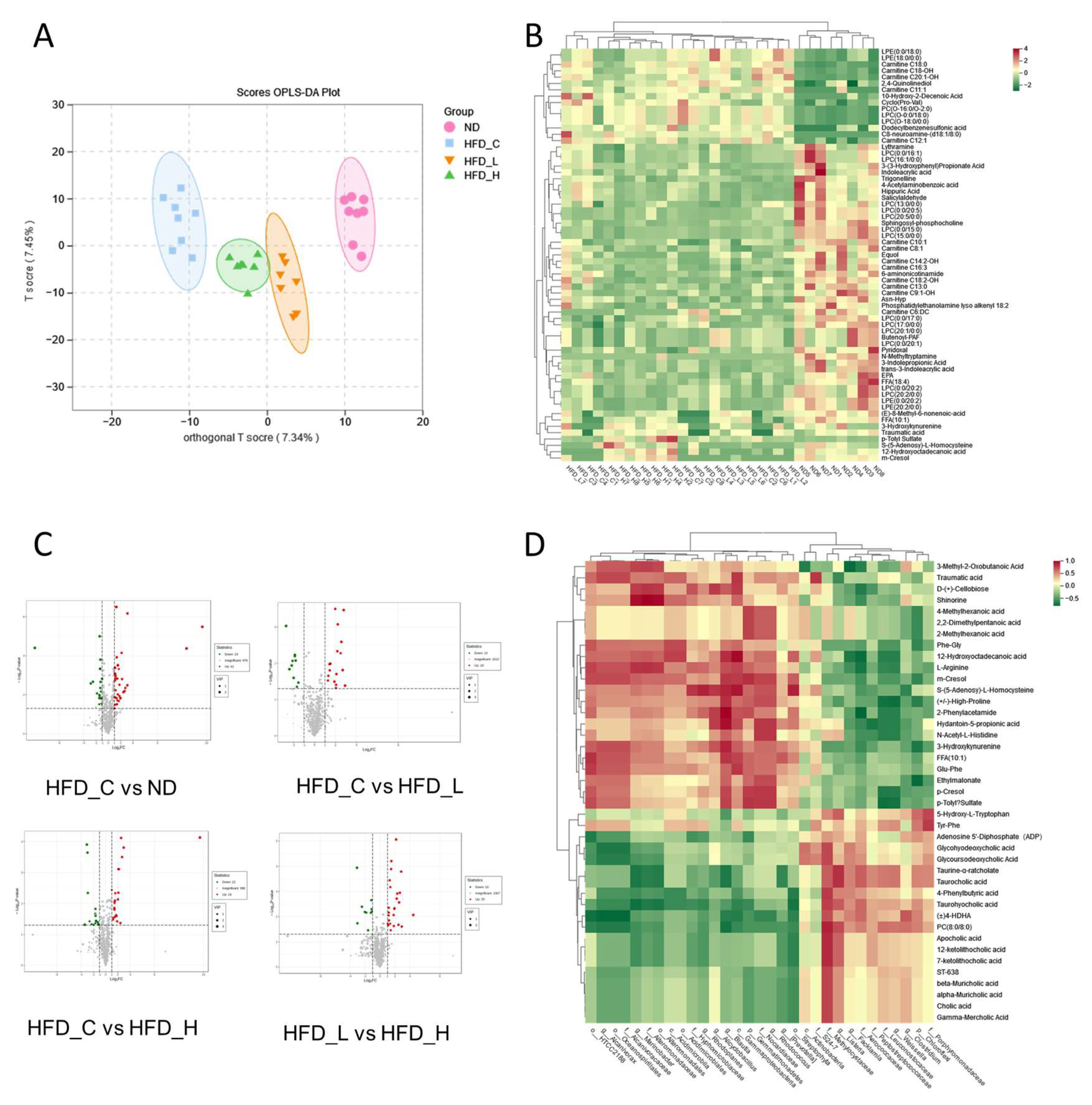
3.5. Impact of Symbiotic Treatment on the Metabolic Profile in Blood Plasma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 December 2021).
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- van Geel, M.; Vedder, P.; Tanilon, J. Are overweight and obese youths more often bullied by their peers? A meta-analysis on the correlation between weight status and bullying. Int. J. Obes. 2014, 38, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Prim. 2017, 3, 17034. [Google Scholar] [CrossRef]
- Xu, J.; Mahowald, M.A.; Ley, R.E.; Lozupone, C.A.; Hamady, M.; Martens, E.C.; Henrissat, B.; Coutinho, P.M.; Minx, P.; Latreille, P.; et al. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 2007, 5, e156. [Google Scholar] [CrossRef]
- Al-Assal, K.; Martinez, A.C.; Torrinhas, R.S.; Cardinelli, C.; Waitzberg, D. Gut microbiota and obesity. Clin. Nutr. Exp. 2018, 20, 60–64. [Google Scholar] [CrossRef]
- Stenman, L.K.; Waget, A.; Garret, C.; Klopp, P.; Burcelin, R.; Lahtinen, S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef. Microbes 2014, 5, 437–445. [Google Scholar] [CrossRef]
- Kondo, S.; Xiao, J.-Z.; Satoh, T.; Odamaki, T.; Takahashi, S.; Sugahara, H.; Yaeshima, T.; Iwatsuki, K.; Kamei, A.; Abe, K. Antiobesity Effects of Bifidobacterium breve Strain B-3 Supplementation in a Mouse Model with High-Fat Diet-Induced Obesity. Biosci. Biotechnol. Biochem. 2010, 74, 1656–1661. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Lu, J.; Shi, J.; Guan, J.; Yan, F.; Li, B.; Huo, G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Visitchanakun, P.; Saisorn, W.; Kanacharoen, S.; Wongsaroj, L.; Kullapanich, C.; Ngamwongsatit, N.; Settachaimongkon, S.; Somboonna, N.; et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021, 11, 6367. [Google Scholar] [CrossRef]
- Tang, J.; Chen, X.; Shi, H.; Zhang, M.; Zhou, Z.; Zhang, C.; Ke, T.; Kong, D.; Li, C. Prebiotic inulin nanocoating for pancreatic islet surface engineering. Biomater. Sci. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Nair, K.K.; Kharb, S.; Thompkinson, D.K. Inulin Dietary Fiber with Functional and Health AttributesA Review. Food Rev. Int. 2010, 26, 189–203. [Google Scholar] [CrossRef]
- Bomhof, M.R.; Parnell, J.A.; Ramay, H.R.; Crotty, P.; Rioux, K.P.; Probert, C.S.; Jayakumar, S.; Raman, M.; Reimer, R.A. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: A pilot clinical trial. Eur. J. Nutr. 2019, 58, 1735–1745. [Google Scholar] [CrossRef]
- Nakamura, Y.; Natsume, M.; Yasuda, A.; Ishizaka, M.; Kawahata, K.; Koga, J. Fructooligosaccharides suppress high-fat diet-induced fat accumulation in C57BL/6J mice. BioFactors 2017, 43, 145–151. [Google Scholar] [CrossRef]
- Bai, Y.M.; Wu, P.; Wang, K.; Li, C.; Li, E.P.; Gilbert, R.G. Effects of pectin on molecular structural changes in starch during digestion. Food Hydrocoll. 2017, 69, 10–18. [Google Scholar] [CrossRef]
- Hamden, K.; Boujibiha, M.A.; Abdeljelil Nb Njima, M.; Achour, L. Inhibitory Effect of fermented pectin on key metabolic enzymes associated with diabetes, obesity; and Liver-Kidney tissues toxicities. Bioact. Carbohydr. Diet. Fibre 2018, 16, 82–89. [Google Scholar] [CrossRef]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.B.; et al. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Shimizu, K.; Ono, M.; Imoto, A.; Nagayama, H.; Tetsumura, N.; Terada, T.; Tomita, K.; Nishinaka, T. Cranberry Attenuates Progression of Non-alcoholic Fatty Liver Disease Induced by High-Fat Diet in Mice. Biol. Pharm. Bull. 2019, 42, 1295–1302. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Liao, C.-A.; Huang, C.-H.; Ho, H.-H.; Chen, J.-F.; Kuo, Y.-W.; Lin, J.-H.; Tsai, S.-Y.; Tsai, H.-Y.; Yeh, Y.-T. A Combined Supplement of Probiotic Strains AP-32, bv-77, and CP-9 Increased Akkermansia mucinphila and Reduced Non-Esterified Fatty Acids and Energy Metabolism in HFD-Induced Obese Rats. Nutrients 2022, 14, 527. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-R.; Kwon, E.-Y.; Kim, H.-J.; Choi, M.-S. Role of Synbiotics Containing d-Allulose in the Alteration of Body Fat and Hepatic Lipids in Diet-Induced Obese Mice. Nutrients 2018, 10, 1797. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. QIIME 2 Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Prepr. 2018, 6, e27295v27292. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Juárez-Fernández, M.; Porras, D.; Petrov, P.; Román-Sagüillo, S.; García-Mediavilla, M.V.; Soluyanova, P.; Martínez-Flórez, S.; González-Gallego, J.; Nistal, E.; Jover, R.; et al. The Synbiotic Combination of Akkermansia muciniphila and Quercetin Ameliorates Early Obesity and NAFLD through Gut Microbiota Reshaping and Bile Acid Metabolism Modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Mulders, R.J.; de Git, K.C.G.; Schéle, E.; Dickson, S.L.; Sanz, Y.; Adan, R.A.H. Microbiota in obesity: Interactions with enteroendocrine, immune and central nervous systems. Obes. Rev. 2018, 19, 435–451. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Liu, X.; Zhong, W.; Li, Y.; Ran, Y.; Guo, L.; Chen, X.; Zhao, J.; Wang, B.; et al. Bifidobacterium animalis ssp. Lactis 420 Mitigates Autoimmune Hepatitis Through Regulating Intestinal Barrier and Liver Immune Cells. Front. Immunol. 2020, 11, 569104. [Google Scholar] [CrossRef]
- Van Hul, M.; Karnik, K.; Canene-Adams, K.; De Souza, M.; Van den Abbeele, P.; Marzorati, M.; Delzenne, N.M.; Everard, A.; Cani, P.D. Comparison of the effects of soluble corn fiber and fructooligosaccharides on metabolism, inflammation and gut microbiome of high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E779–E791. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef]
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef]
- Adam, C.L.; Gratz, S.W.; Peinado, D.I.; Thomson, L.M.; Garden, K.E.; Williams, P.A.; Richardson, A.J.; Ross, A.W. Effects of Dietary Fibre (Pectin) and/or Increased Protein (Casein or Pea) on Satiety, Body Weight, Adiposity and Caecal Fermentation in High Fat Diet-Induced Obese Rats. PLoS One 2016, 11, e0155871. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Liver enzymes, metabolomics and genome-wide association studies: From systems biology to the personalized medicine. World J. Gastroenterol. 2015, 21, 711–725. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Bai, G.; Ni, K.; Tsuruta, T.; Nishino, N. Dietary Casein and Soy Protein Isolate Modulate the Effects of Raffinose and Fructooligosaccharides on the Composition and Fermentation of Gut Microbiota in Rats. J. Food Sci. 2016, 81, H2093–H2098. [Google Scholar] [CrossRef]
- Yang, J.; Bindels, L.B.; Segura Munoz, R.R.; Martínez, I.; Walter, J.; Ramer-Tait, A.E.; Rose, D.J. Disparate Metabolic Responses in Mice Fed a High-Fat Diet Supplemented with Maize-Derived Non-Digestible Feruloylated Oligo- and Polysaccharides Are Linked to Changes in the Gut Microbiota. PloS One 2016, 11, e0146144. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Gómez Del Pugar, E.M.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y. Depletion of Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. MSystems 2020, 5, e00819–e00857. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20-76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wei, M.; Rajani, C.; Zheng, X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell 2021, 12, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Pushpass, R.-A.G.; Alzoufairi, S.; Jackson, K.G.; Lovegrove, J.A. Circulating bile acids as a link between the gut microbiota and cardiovascular health: Impact of prebiotics, probiotics and polyphenol-rich foods. Nutr. Res. Rev. 2022, 35, 161–180. [Google Scholar] [CrossRef]
- Fiorucci, S.; Mencarelli, A.; Palladino, G.; Cipriani, S. Bile-acid-activated receptors: Targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol. Sci. 2009, 30, 570–580. [Google Scholar] [CrossRef]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4523–4530. [Google Scholar] [CrossRef]
- Oteng, A.-B.; Higuchi, S.; Banks, A.S.; Haeusler, R.A. Cyp2c-deficiency depletes muricholic acids and protects against high-fat diet-induced obesity in male mice but promotes liver damage. Mol. Metab. 2021, 53, 101326. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuñé, J. A randomized clinical trial evaluating a proprietary mixture of Lactobacillus plantarum strains for lowering cholesterol. Mediterr. J. Nutr. Metab. 2016, 9, 125–135. [Google Scholar]
- Lee, Y.G.; Cho, J.-Y.; Hwang, E.J.; Jeon, T.-I.; Moon, J.-H. Glu-Phe from onion (Allium cepa L.) attenuates lipogenesis in hepatocytes. Biosci. Biotechnol. Biochem. 2017, 81, 1409–1416. [Google Scholar] [CrossRef]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.-R.; Chen, Z.-Z.; Dong, X.-L.; Zhao, Q.-P.; Cai, J. A Novel Symbiotic Formulation Reduces Obesity and Concomitant Metabolic Syndrome in Rats by Raising the Relative Abundance of Blautia. Nutrients 2023, 15, 956. https://doi.org/10.3390/nu15040956
Wu X-R, Chen Z-Z, Dong X-L, Zhao Q-P, Cai J. A Novel Symbiotic Formulation Reduces Obesity and Concomitant Metabolic Syndrome in Rats by Raising the Relative Abundance of Blautia. Nutrients. 2023; 15(4):956. https://doi.org/10.3390/nu15040956
Chicago/Turabian StyleWu, Xiu-Rong, Zhen-Zhen Chen, Xi-Lan Dong, Qiu-Ping Zhao, and Jun Cai. 2023. "A Novel Symbiotic Formulation Reduces Obesity and Concomitant Metabolic Syndrome in Rats by Raising the Relative Abundance of Blautia" Nutrients 15, no. 4: 956. https://doi.org/10.3390/nu15040956
APA StyleWu, X.-R., Chen, Z.-Z., Dong, X.-L., Zhao, Q.-P., & Cai, J. (2023). A Novel Symbiotic Formulation Reduces Obesity and Concomitant Metabolic Syndrome in Rats by Raising the Relative Abundance of Blautia. Nutrients, 15(4), 956. https://doi.org/10.3390/nu15040956






