The Circadian Regulation of Nutrient Metabolism in Diet-Induced Obesity and Metabolic Disease
Abstract
:1. Introduction
2. Circadian Biology
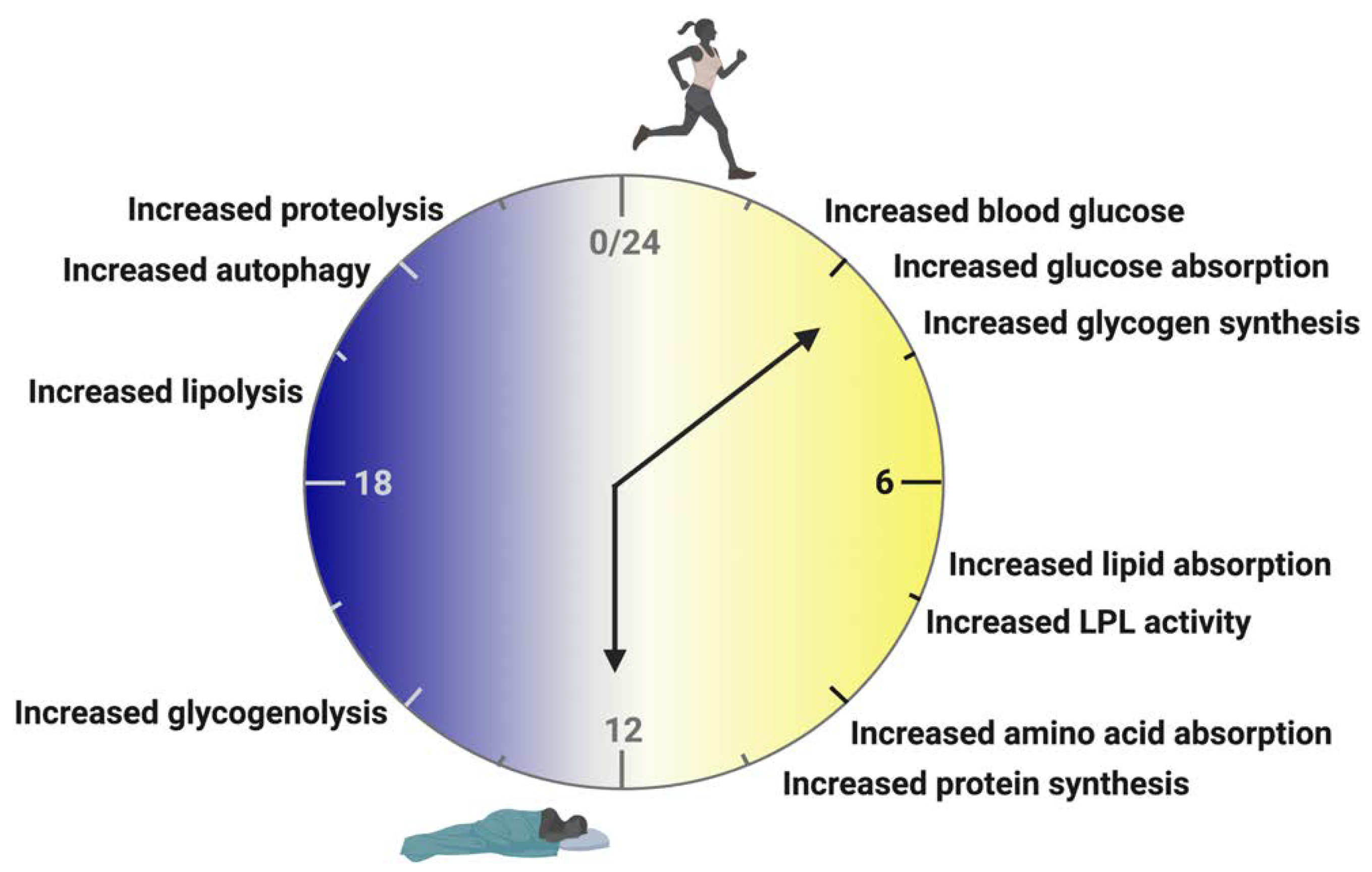
3. Rhythms of Feeding and Fasting
4. Time-Restricted Feeding/Eating
5. Obesity as a Circadian Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in Obesity-Induced T2DM, Dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2016, 13, 36–49. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Graham, S.; Wang, X.; Cai, D.; Huang, M.; Pique-Regi, R.; Dong, X.C.; Chen, Y.E.; Willer, C.; et al. Causal Relationships between NAFLD, T2D and Obesity Have Implications for Disease Subphenotyping. J. Hepatol. 2020, 73, 263–276. [Google Scholar] [CrossRef]
- Twig, G.; Reichman, B.; Afek, A.; Derazne, E.; Hamiel, U.; Furer, A.; Gershovitz, L.; Bader, T.; Cukierman-Yaffe, T.; Kark, J.D.; et al. Severe Obesity and Cardio-Metabolic Comorbidities: A Nationwide Study of 2.8 Million Adolescents. Int. J. Obes. 2018, 437, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Shah, M.; Scherer, P.E. Obesity and Diabetes as Comorbidities for COVID-19: Underlying Mechanisms and the Role of Viral–Bacterial Interactions. Elife 2020, 9, e61330. [Google Scholar] [CrossRef]
- Aghili, S.M.M.; Ebrahimpur, M.; Arjmand, B.; Shadman, Z.; Pejman Sani, M.; Qorbani, M.; Larijani, B.; Payab, M. Obesity in COVID-19 Era, Implications for Mechanisms, Comorbidities, and Prognosis: A Review and Meta-Analysis. Int. J. Obes. 2021, 45, 998–1016. [Google Scholar] [CrossRef] [PubMed]
- Christopher, G.; Fleming, D.; Harris, R.T.; Spencer, T.; Mayfield Gibson, S.; Harris, C.M.; Lakey, D.; Martinez, O., Jr.; Executive Director, F.; Rich, J.A.; et al. TFAH Board Of Directors-19 Response Initiative Resolve to Save Lives; American Heart Association: Chicago, IL, USA, 2021. [Google Scholar]
- Hernández-García, J.; Navas-Carrillo, D.; Orenes-Piñero, E. Alterations of Circadian Rhythms and Their Impact on Obesity, Metabolic Syndrome and Cardiovascular Diseases. Crit. Rev. Food Sci. Nutr. 2019, 60, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Laermans, J.; Depoortere, I. Chronobesity: Role of the Circadian System in the Obesity Epidemic. Obes. Rev. 2016, 17, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Froy, O. Metabolism and Circadian Rhythms—Implications for Obesity. Endocr. Rev. 2010, 31, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Shan, Z.; Li, Y.; Zong, G.; Guo, Y.; Li, J.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Schernhammer, E.S.; Bhupathiraju, S.N. Rotating Night Shift Work and Adherence to Unhealthy Lifestyle in Predicting Risk of Type 2 Diabetes: Results from Two Large US Cohorts of Female Nurses. Br. Med. J. 2018, 363, 4641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, J.S.; Hong, H.-K.; Ko, C.H.; McDearmon, E.L. The Genetics of Mammalian Circadian Order and Disorder: Implications for Physiology and Disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef]
- Guan, D.; Lazar, M.A. Interconnections between Circadian Clocks and Metabolism. J. Clin. Investig. 2021, 131, e148278. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and Peripheral Circadian Clocks in Mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [Green Version]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F.A.J.L. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef]
- Gerstner, J.R.; Yin, J.C.P. Circadian Rhythms and Memory Formation. Nat. Rev. Neurosci. 2010, 11, 577–588. [Google Scholar] [CrossRef]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking in to Immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef]
- Neumann, A.M.; Schmidt, C.X.; Brockmann, R.M.; Oster, H. Circadian Regulation of Endocrine Systems. Auton. Neurosci. 2019, 216, 1–8. [Google Scholar] [CrossRef]
- Tinius, R.A.; Blankenship, M.M.; Furgal, K.E.; Cade, W.T.; Pearson, K.J.; Rowland, N.S.; Pearson, R.C.; Hoover, D.L.; Maples, J.M. Metabolic Flexibility Is Impaired in Women Who Are Pregnant and Overweight/Obese and Related to Insulin Resistance and Inflammation. Metabolism 2020, 104, 154142. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of Metabolic Flexibility in the Failing Heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [Green Version]
- Begaye, B.; Vinales, K.L.; Hollstein, T.; Ando, T.; Walter, M.; Bogardus, C.; Krakoff, J.; Piaggi, P. Impaired Metabolic Flexibility to High-Fat Overfeeding Predicts Future Weight Gain in Healthy Adults. Diabetes 2020, 69, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Logan, R.W.; McClung, C.A. Rhythms of Life: Circadian Disruption and Brain Disorders across the Lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Heyde, I.; Oster, H. Induction of Internal Circadian Desynchrony by Misaligning Zeitgebers. Sci. Reports 2022, 12, 1601. [Google Scholar] [CrossRef] [PubMed]
- Adlanmerini, M.; Krusen, B.M.; Nguyen, H.C.B.; Teng, C.W.; Woodie, L.N.; Tackenberg, M.C.; Geisler, C.E.; Gaisinsky, J.; Peed, L.C.; Carpenter, B.J.; et al. REV-ERB Nuclear Receptors in the Suprachiasmatic Nucleus Control Circadian Period and Restrict Diet-Induced Obesity. Sci. Adv. 2021, 7, eabh2007. [Google Scholar] [CrossRef]
- Kolbe, I.; Leinweber, B.; Brandenburger, M.; Oster, H. Circadian Clock Network Desynchrony Promotes Weight Gain and Alters Glucose Homeostasis in Mice. Mol. Metab. 2019, 30, 140–151. [Google Scholar] [CrossRef] [PubMed]
- van Ee, R.; Van de Cruys, S.; Schlangen, L.J.M.; Vlaskamp, B.N.S. Circadian-Time Sickness: Time-of-Day Cue-Conflicts Directly Affect Health. Trends Neurosci. 2016, 39, 738–749. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.; Mook-Kanamori, D.O.; Donga, E.; van Dijk, M.; van Dijk, J.G.; Lammers, G.-J.; van Kralingen, K.W.; Prehn, C.; Romijn, J.A.; Willems van Dijk, K.; et al. A Single Night of Sleep Curtailment Increases Plasma Acylcarnitines: Novel Insights in the Relationship between Sleep and Insulin Resistance. Arch. Biochem. Biophys. 2016, 589, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Cedernaes, J.; Lampola, L.; Axelsson, E.K.; Liethof, L.; Hassanzadeh, S.; Yeganeh, A.; Broman, J.-E.; Schiöth, H.B.; Benedict, C. A Single Night of Partial Sleep Loss Impairs Fasting Insulin Sensitivity but Does Not Affect Cephalic Phase Insulin Release in Young Men. J. Sleep Res. 2016, 25, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedeltcheva, A.V.; Kessler, L.; Imperial, J.; Penev, P.D. Exposure to Recurrent Sleep Restriction in the Setting of High Caloric Intake and Physical Inactivity Results in Increased Insulin Resistance and Reduced Glucose Tolerance. J. Clin. Endocrinol. Metab. 2009, 94, 3242–3250. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, X.; Winkelman, J.W.; Cespedes, E.M.; Jackson, C.L.; Walters, A.S.; Schernhammer, E.; Redline, S.; Hu, F.B. Association between Sleeping Difficulty and Type 2 Diabetes in Women. Diabetologia 2016, 59, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Tsuneki, H.; Sasaoka, T.; Sakurai, T. Sleep Control, GPCRs, and Glucose Metabolism. Trends Endocrinol. Metab. 2016, 27, 633–642. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef] [Green Version]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of Circadian Rhythms in the Suprachiasmatic Nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Palm, I.F.; La Fleur, S.E.; Scheer, F.A.J.L.; Perreau-Lenz, S.; Ruiter, M.; Kreier, F.; Cailotto, C.; Buijs, R.M. SCN Outputs and the Hypothalamic Balance of Life. J. Biol. Rhythms 2006, 21, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Ralph, M.R.; Foster, R.G.; Davis, F.C.; Menaker, M. Transplanted Suprachiasmatic Nucleus Determines Circadian Period. Science 1990, 247, 975–978. [Google Scholar] [CrossRef] [Green Version]
- Sujino, M.; Masumoto, K.; Yamaguchi, S.; van der Horst, G.T.J.; Okamura, H.; Inouye, S.-I.T. Suprachiasmatic Nucleus Grafts Restore Circadian Behavioral Rhythms of Genetically Arrhythmic Mice. Curr. Biol. 2003, 13, 664–668. [Google Scholar] [CrossRef] [Green Version]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratmann, M.; Schibler, U. REV-ERBs: More Than the Sum of the Individual Parts. Cell Metab. 2012, 15, 791–793. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.; Oster, H.; Lehnert, H.; Schmid, S.M.; Salamat, N.; Barclay, J.L.; Maronde, E.; Inder, W.; Rawashdeh, O. Coupling the Circadian Clock to Homeostasis: The Role of Period in Timing Physiology. Endocr. Rev. 2019, 40, 66–95. [Google Scholar] [CrossRef] [Green Version]
- Schmutz, I.; Ripperger, J.A.; Baeriswyl-Aebischer, S.; Albrecht, U. The Mammalian Clock Component PERIOD2 Coordinates Circadian Output by Interaction with Nuclear Receptors. Genes Dev. 2010, 24, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Bartness, T.J.; Song, C.K.; Demas, G.E. SCN Efferents to Peripheral Tissues: Implications for Biological Rhythms. J. Biol. Rhythm. 2001, 16, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, H.; Liu, Y.; Shao, C. Analysis of Gene Regulatory Networks in the Mammalian Circadian Rhythm. PLoS Comput. Biol. 2008, 4, e1000193. [Google Scholar] [CrossRef] [Green Version]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [Green Version]
- Guan, D.; Xiong, Y.; Trinh, T.M.; Xiao, Y.; Hu, W.; Jiang, C.; Dierickx, P.; Jang, C.; Rabinowitz, J.D.; Lazar, M.A. The Hepatocyte Clock and Feeding Control Chronophysiology of Multiple Liver Cell Types Downloaded From. Science 2020, 369, 1388–1394. [Google Scholar] [CrossRef]
- Woodie, L.N.; Luo, Y.; Wayne, M.J.; Graff, E.C.; Ahmed, B.; O’Neill, A.M.; Greene, M.W. Restricted Feeding for 9 h in the Active Period Partially Abrogates the Detrimental Metabolic Effects of a Western Diet with Liquid Sugar Consumption in Mice. Metabolism 2018, 82, 1–13. [Google Scholar] [CrossRef]
- Woodie, L.N.; Johnson, R.M.; Ahmed, B.; Fowler, S.; Haynes, W.; Carmona, B.; Reed, M.; Suppiramaniam, V.; Greene, M.W. Western Diet-Induced Obesity Disrupts the Diurnal Rhythmicity of Hippocampal Core Clock Gene Expression in a Mouse Model. Brain. Behav. Immun. 2020, 88, 815–825. [Google Scholar] [CrossRef]
- Li, M.D. Clock-Modulated Checkpoints in Time-Restricted Eating. Trends Mol. Med. 2022, 28, 25–35. [Google Scholar] [CrossRef]
- Hussain, M.M.; Pan, X. Circadian Regulation of Macronutrient Absorption. J. Biol. Rhythm. 2015, 30, 459–469. [Google Scholar] [CrossRef]
- Sinturel, F.; Petrenko, V.; Dibner, C. Circadian Clocks Make Metabolism Run. J. Mol. Biol. 2020, 432, 3680–3699. [Google Scholar] [CrossRef] [PubMed]
- Reinke, H.; Asher, G. Crosstalk between Metabolism and Circadian Clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Panda, S. Circadian Physiology of Metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Bass, J. Circadian Topology of Metabolism. Nature 2012, 491, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, S.M.; Udoh, U.S.; Young, M.E. Circadian Regulation of Metabolism. J. Endocrinol. 2014, 222, R75–R96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism and the Circadian Clock Converge. Physiol. Rev. 2013, 93, 107–135. [Google Scholar] [CrossRef]
- Oosterman, J.E.; Foppen, E.; van der Spek, R.; Fliers, E.; Kalsbeek, A.; la Fleur, S.E. Timing of Fat and Liquid Sugar Intake Alters Substrate Oxidation and Food Efficiency in Male Wistar Rats. Chronobiol. Int. 2015, 32, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hutchison, A.T.; Heilbronn, L.K. Carbohydrate Intake and Circadian Synchronicity in the Regulation of Glucose Homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 342–348. [Google Scholar] [CrossRef]
- Kumar Jha, P.; Challet, E.; Kalsbeek, A. Circadian Rhythms in Glucose and Lipid Metabolism in Nocturnal and Diurnal Mammals. Mol. Cell. Endocrinol. 2015, 418, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Vollmers, C.; Gill, S.; Ditacchio, L.; Pulivarthy, S.R.; Le, H.D.; Panda, S. Time of Feeding and the Intrinsic Circadian Clock Drive Rhythms in Hepatic Gene Expression. Proc. Natl. Acad. Sci. 2009, 106, 21453–21458. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Wang, J.; Klein, P.S.; Lazar, M.A. Nuclear Receptor Rev-Erbα Is a Critical Lithium-Sensitive Component of the Circadian Clock. Science 2006, 311, 1002–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gooley, J.J.; Chua, E.C.P. Diurnal Regulation of Lipid Metabolism and Applications of Circadian Lipidomics. J. Genet. Genom. 2014, 41, 231–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, D.; Liu, T.; Sun, Z.; Bugge, A.; Mullican, S.E.; Alenghat, T.; Liu, X.S.; Lazar, M.A. A Circadian Rhythm Orchestrated by Histone Deacetylase 3 Controls Hepatic Lipid Metabolism. Science 2011, 331, 1315–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gooley, J.J. Circadian Regulation of Lipid Metabolism. Proc. Nutr. Soc. 2016, 75, 440–450. [Google Scholar] [CrossRef]
- Dyar, K.A.; Hubert, J.; Mir, A.A.; Ciciliot, S.; Lutter, D.; Greulich, F.; Quagliarini, F.; Kleinert, M.; Fischer, K.; Eichmann, T.O.; et al. Transcriptional Programming of Lipid and Amino Acid Metabolism by the Skeletal Muscle Circadian Clock. PLoS Biol. 2005, 16, e3000035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atger, F.; Gobeta, C.; Marquis, J.; Martin, E.; Wang, J.; Weger, B.; Lefebvre, G.; Descombes, P.; Naef, F.; Gachon, F. Circadian and Feeding Rhythms Differentially Affect Rhythmic MRNA Transcription and Translation in Mouse Liver. Proc. Natl. Acad. Sci. USA 2015, 112, E6579–E6588. [Google Scholar] [CrossRef] [Green Version]
- Jeyaraj, D.; Scheer, F.A.J.L.; Ripperger, J.A.; Haldar, S.M.; Lu, Y.; Prosdocimo, D.A.; Eapen, S.J.; Eapen, B.L.; Cui, Y.; Mahabeleshwar, G.H.; et al. Klf15 Orchestrates Circadian Nitrogen Homeostasis. Cell Metab. 2012, 15, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalfalah, F.; Janke, L.; Schiavi, A.; Tigges, J.; Ix, A.; Ventura, N.; Boege, F.; Reinke, H. Crosstalk of Clock Gene Expression and Autophagy in Aging. Aging 2016, 8, 1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeld-Cohen, A.; Robles, M.S.; Aviram, R.; Manella, G.; Adamovich, Y.; Ladeuix, B.; Nir, D.; Rousso-Noori, L.; Kuperman, Y.; Golik, M.; et al. Circadian Control of Oscillations in Mitochondrial Rate-Limiting Enzymes and Nutrient Utilization by PERIOD Proteins. Proc. Natl. Acad. Sci. USA 2016, 113, E1673–E1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Li, S.; Molusky, M.M.; Lin, J.D. Circadian Autophagy Rhythm: A Link between Clock and Metabolism? Trends Endocrinol. Metab. 2012, 23, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almon, R.R.; Yang, E.; Lai, W.; Androulakis, I.P.; Ghimbovschi, S.; Hoffman, E.P.; Jusko, W.J.; DuBois, D.C. Relationships between Circadian Rhythms and Modulation of Gene Expression by Glucocorticoids in Skeletal Muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1031–R1047. [Google Scholar] [CrossRef] [Green Version]
- Oosterman, J.E.; Kalsbeek, A.; La Fleur, S.E.; Belsham, D.D. Impact of Nutrients on Circadian Rhythmicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R337–R350. [Google Scholar] [CrossRef] [Green Version]
- Mukherji, A.; Kobiita, A.; Damara, M.; Misra, N.; Meziane, H.; Champy, M.-F.; Chambon, P. Shifting Eating to the Circadian Rest Phase Misaligns the Peripheral Clocks with the Master SCN Clock and Leads to a Metabolic Syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, E6691–E6698. [Google Scholar] [CrossRef] [Green Version]
- Marchant, E.G.; Mistlberger, R.E. Anticipation and Entrainment to Feeding Time in Intact and SCN-Ablated C57BL/6j Mice. Brain Res. 1997, 765, 273–282. [Google Scholar] [CrossRef]
- Patton, D.F.; Mistlberger, R.E. Circadian Adaptations to Meal Timing: Neuroendocrine Mechanisms. Front. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 Is an Essential Component of the Master Circadian Pacemaker in Mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early Aging and Age-Related Pathologies in Mice Deficient in BMAL1, the Core Componentof the Circadian Clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yang, Z.; Niu, Z.; Wang, W.; Peng, J.; Li, Q.; Ma, M.Y.; Zhao, Y. The Mortality of MOP3 Deficient Mice with a Systemic Functional Failure. J. Biomed. Sci. 2006, 13, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Kinouchi, K.; Welz, P.-S.; Smith, J.G.; Zinna, V.M.; Shi, J.; Samad, M.; Chen, S.; Magnan, C.N.; Kinchen, J.M.; et al. Defining the Independence of the Liver Circadian Clock. Cell 2019, 177, 1448–1462.e14. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Koronowski, K.B.; Smith, J.G.; Shi, J.; Kunderfranco, P.; Carriero, R.; Chen, S.; Samad, M.; Welz, P.S.; Zinna, V.M.; et al. Integration of Feeding Behavior by the Liver Circadian Clock Reveals Network Dependency of Metabolic Rhythms. Sci. Adv. 2021, 7, eabi7828. [Google Scholar] [CrossRef] [PubMed]
- Lamia, K.A.; Papp, S.J.; Yu, R.T.; Barish, G.D.; Uhlenhaut, N.H.; Jonker, J.W.; Downes, M.; Evans, R.M. Cryptochromes Mediate Rhythmic Repression of the Glucocorticoid Receptor. Nature 2011, 480, 552–556. [Google Scholar] [CrossRef]
- Jordan, S.D.; Kriebs, A.; Vaughan, M.; Duglan, D.; Fan, W.; Henriksson, E.; Huber, A.-L.; Papp, S.J.; Nguyen, M.; Afetian, M.; et al. CRY1/2 Selectively Repress PPARδ and Limit Exercise Capacity. Cell Metab. 2017, 26, 243–255.e6. [Google Scholar] [CrossRef] [Green Version]
- Bugge, A.; Feng, D.; Everett, L.J.; Briggs, E.R.; Mullican, S.E.; Wang, F.; Jager, J.; Lazar, M.A. Rev-Erbα and Rev-Erbβ Coordinately Protect the Circadian Clock and Normal Metabolic Function. Genes Dev. 2012, 26, 657–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preitner, N.; Damiola, F.; Luis-Lopez-Molina; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The Orphan Nuclear Receptor REV-ERBα Controls Circadian Transcription within the Positive Limb of the Mammalian Circadian Oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Raspé, E.; Duez, H.; Gervois, P.; Fiévet, C.; Fruchart, J.C.; Besnard, S.; Mariani, J.; Tedgui, A.; Staels, B. Transcriptional Regulation of Apolipoprotein C-III Gene Expression by the Orphan Nuclear Receptor RORα. J. Biol. Chem. 2001, 276, 2865–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspé, E.; Duez, H.; Mansén, A.; Fontaine, C.; Fiévet, C.; Fruchart, J.-C.; Vennström, B.; Staels, B. Identification of Rev-Erbalpha as a Physiological Repressor of ApoC-III Gene Transcription. J. Lipid Res. 2002, 43, 2172–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; Ditacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of Circadian Behavior and Metabolism by Rev-Erbα and Rev-Erbβ. Nature 2012, 485, 123. [Google Scholar] [CrossRef] [Green Version]
- Bray, M.S.; Tsai, J.-Y.; Villegas-Montoya, C.; Boland, B.B.; Blasier, Z.; Egbejimi, O.; Kueht, M.; Young, M.E. Time-of-Day-Dependent Dietary Fat Consumption Influences Multiple Cardiometabolic Syndrome Parameters in Mice. Int. J. Obes. 2010, 34, 1589–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans Who Are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319.e4. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D.; Wright, K.P.; Wright, J.; et al. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef] [Green Version]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [Green Version]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Wakamatsu, H.; Yoshinobu, Y.; Aida, R.; Moriya, T.; Akiyama, M.; Shibata, S. Restricted-Feeding-Induced Anticipatory Activity Rhythm Is Associated with a Phase-Shift of the Expression of MPer1 and MPer2 MRNA in the Cerebral Cortex and Hippocampus but Not in the Suprachiasmatic Nucleus of Mice. Eur. J. Neurosci. 2001, 13, 1190–1196. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, V.; Rijo-Ferreira, F.; Izumo, M.; Xu, P.; Wight-Carter, M.; Green, C.B.; Takahashi, J.S. Circadian Alignment of Early Onset Caloric Restriction Promotes Longevity in Male C57BL/6J Mice. Science 2022, 376, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Barrea, L.; Laudisio, D.; Salzano, C.; Aprano, S.; Colao, A.; Savastano, S.; Muscogiuri, G. Sleep Apnea, Obesity, and Disturbed Glucose Homeostasis: Epidemiologic Evidence, Biologic Insights, and Therapeutic Strategies. Curr. Obes. Rep. 2020, 9, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.C.B.; Dantas Filho, F.F.; Schnorr, C.C.; Bertoletti, O.A.; Bottega, G.B.; Da Costa Rodrigues, T. Night Shift Work, Short Sleep and Obesity. Diabetol. Metab. Syndr. 2020, 12, 13. [Google Scholar] [CrossRef] [Green Version]
- Mullington, J.M.; Cunningham, T.J.; Haack, M.; Yang, H. Causes and Consequences of Chronic Sleep Deficiency and the Role of Orexin. Front. Neurol. Neurosci. 2021, 45, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Rosado, I.; Ruiz, R.; Figueroa, A.; Zen, N.; Fernández-Vázquez, G. Melatonin Improves Glucose Homeostasis in Young Zucker Diabetic Fatty Rats. J. Pineal Res. 2012, 52, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Du Toit, E.F.; Blackhurst, D.; Marais, D.; Lochner, A. Chronic Melatonin Consumption Prevents Obesity-Related Metabolic Abnormalities and Protects the Heart against Myocardial Ischemia and Reperfusion Injury in a Prediabetic Model of Diet-Induced Obesity. J. Pineal Res. 2010, 50, 171–182. [Google Scholar] [CrossRef]
- Peschke, E.; Frese, T.; Chankiewitz, E.; Peschke, D.; Preiss, U.; Schneyer, U.; Spessert, R.; Muhlbauer, E. Diabetic Goto Kakizaki Rats as Well as Type 2 Diabetic Patients Show a Decreased Diurnal Serum Melatonin Level and an Increased Pancreatic Melatonin-Receptor Status. J. Pineal Res. 2006, 40, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Lugo, M.J.; Cano, P.; Jiménez-Ortega, V.; Fernández-Mateos, M.P.; Scacchi, P.A.; Cardinali, D.P.; Esquifino, A.I. Melatonin Effect on Plasma Adiponectin, Leptin, Insulin, Glucose, Triglycerides and Cholesterol in Normal and High Fat-Fed Rats. J. Pineal Res. 2010, 49, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhart-Hines, Z.; Lazar, M.A. Circadian Metabolism in the Light of Evolution. Endocr. Rev. 2015, 36, 289. [Google Scholar] [CrossRef]
- Salgado-Delgado, R.; Angeles-Castellanos, M.; Saderi, N.; Buijs, R.M.; Escobar, C. Food Intake during the Normal Activity Phase Prevents Obesity and Circadian Desynchrony in a Rat Model of Night Work. Endocrinology 2010, 151, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, Y.; Hashimoto, C.; Nakao, R.; Yamazaki, H.; Hiroyama, H.; Nemoto, T.; Yamamoto, S.; Sakurai, M.; Oike, H.; Wada, N.; et al. Short-Term Feeding at the Wrong Time Is Sufficient to Desynchronize Peripheral Clocks and Induce Obesity with Hyperphagia, Physical Inactivity and Metabolic Disorders in Mice. Metabolism 2016, 65, 714–727. [Google Scholar] [CrossRef]
- Ding, G.; Li, X.; Hou, X.; Zhou, W.; Gong, Y.; Liu, F.; He, Y.; Song, J.; Wang, J.; Basil, P.; et al. Corrections & Amendments Author Correction: REV-ERB in GABAergic Neurons Controls Diurnal Hepatic Insulin Sensitivity Check for Updates. Nature 2021, 595, E2. [Google Scholar] [CrossRef]
- Reitz, C.J.; Alibhai, F.J.; De Lima-Seolin, B.G.; Nemec-Bakk, A.; Khaper, N.; Martino, T.A. Circadian Mutant Mice with Obesity and Metabolic Syndrome Are Resilient to Cardiovascular Disease. Am. J. Physiol. Hear. Circ. Physiol. 2020, 319, H1097–H1111. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kon, K.; Tsuneki, H.; Ito, H.; Takemura, Y.; Sato, K.; Yamazaki, M.; Ishii, Y.; Sasahara, M.; Rudich, A.; Maeda, T.; et al. Chronotherapeutic Effect of Orexin Antagonists on Glucose Metabolism in Diabetic Mice. J. Endocrinol. 2019, 243, 59–72. [Google Scholar] [CrossRef]


| Study | Organism | Timing of TRF/TRE | Length of TRF/TRE | Effects on Metabolism and Health |
|---|---|---|---|---|
| Bray et al. International Journal of Obesity 2010 [88] | FVB/N mouse | 8 h | 12 weeks | No protection against HFD-induced weight gain, but improved glucose and lipid metabolism |
| Hatori et al. Cell Metabolism 2012 | C57Bl/6J mouse | 8 h | 17 weeks | Protected against HFD-induced disruptions in glucose and lipid metabolism |
| Chaix et al. Cell Metabolism 2014 [44] | C57Bl/6J mouse | 8–12 h range | 12–36 weeks | 8–9 h range protected against diet-induced weight gain, inflammation, hyperglycemia, hyperinsulinemia, and disruption in metabolite flux |
| Wehrens et al. Current Biology 2017 [93] | Healthy human males | 5 h in late active phase | 6 days | Shift in adipose Per2 expression, preserved behavioral activity, no added metabolic benefit of TRE for healthy human males |
| Woodie et al. Metabolism 2018 [46] | C57Bl/6N mouse | 8 h | 4 and 10 weeks | No protection against HFD-induced weight gain, but metabolic flexibility, insulin and glucose tolerance, and hepatic steatosis |
| Sutton et al. Cell Metabolism 2018 [91] | Pre-diabetic human males | 6–7 h in early active phase | 5 days | Improved insulin sensitivity, pancreatic beta cell responsivity, blood pressure, and markers of oxidative stress |
| Jamshed et al. Nutrients 2019 [94] | Pre-diabetic human males and females | 7 h in early active phase | 4 days | Improved glycemic excursions and increased markers of autophagy and anti-aging |
| Wilkinson et al. Cell Metabolism 2020 [89] | Human males and females with metabolic syndrome | 10 h | 12 weeks | Decreased body weight, blood pressure, cholesterol and A1C while improving sleep quality |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodie, L.N.; Oral, K.T.; Krusen, B.M.; Lazar, M.A. The Circadian Regulation of Nutrient Metabolism in Diet-Induced Obesity and Metabolic Disease. Nutrients 2022, 14, 3136. https://doi.org/10.3390/nu14153136
Woodie LN, Oral KT, Krusen BM, Lazar MA. The Circadian Regulation of Nutrient Metabolism in Diet-Induced Obesity and Metabolic Disease. Nutrients. 2022; 14(15):3136. https://doi.org/10.3390/nu14153136
Chicago/Turabian StyleWoodie, Lauren N., Kaan T. Oral, Brianna M. Krusen, and Mitchell A. Lazar. 2022. "The Circadian Regulation of Nutrient Metabolism in Diet-Induced Obesity and Metabolic Disease" Nutrients 14, no. 15: 3136. https://doi.org/10.3390/nu14153136
APA StyleWoodie, L. N., Oral, K. T., Krusen, B. M., & Lazar, M. A. (2022). The Circadian Regulation of Nutrient Metabolism in Diet-Induced Obesity and Metabolic Disease. Nutrients, 14(15), 3136. https://doi.org/10.3390/nu14153136






