Marine Chitosan-Oligosaccharide Ameliorated Plasma Cholesterol in Hypercholesterolemic Hamsters by Modifying the Gut Microflora, Bile Acids, and Short-Chain Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Diets
2.3. Hamsters
2.4. Plasma Lipid Measurements
2.5. BAs Measurements
2.6. Measurement of Fecal’s SCFAs and pH Values
2.7. Measurement of Intestinal Microflora
2.8. Data Analysis
3. Results
3.1. Food Intake, Energy Intake, and Body and Organ Weights
3.2. Plasma Lipid Profile
3.3. Fecal BAs
3.4. Fecal SCFA Contents and pH Value
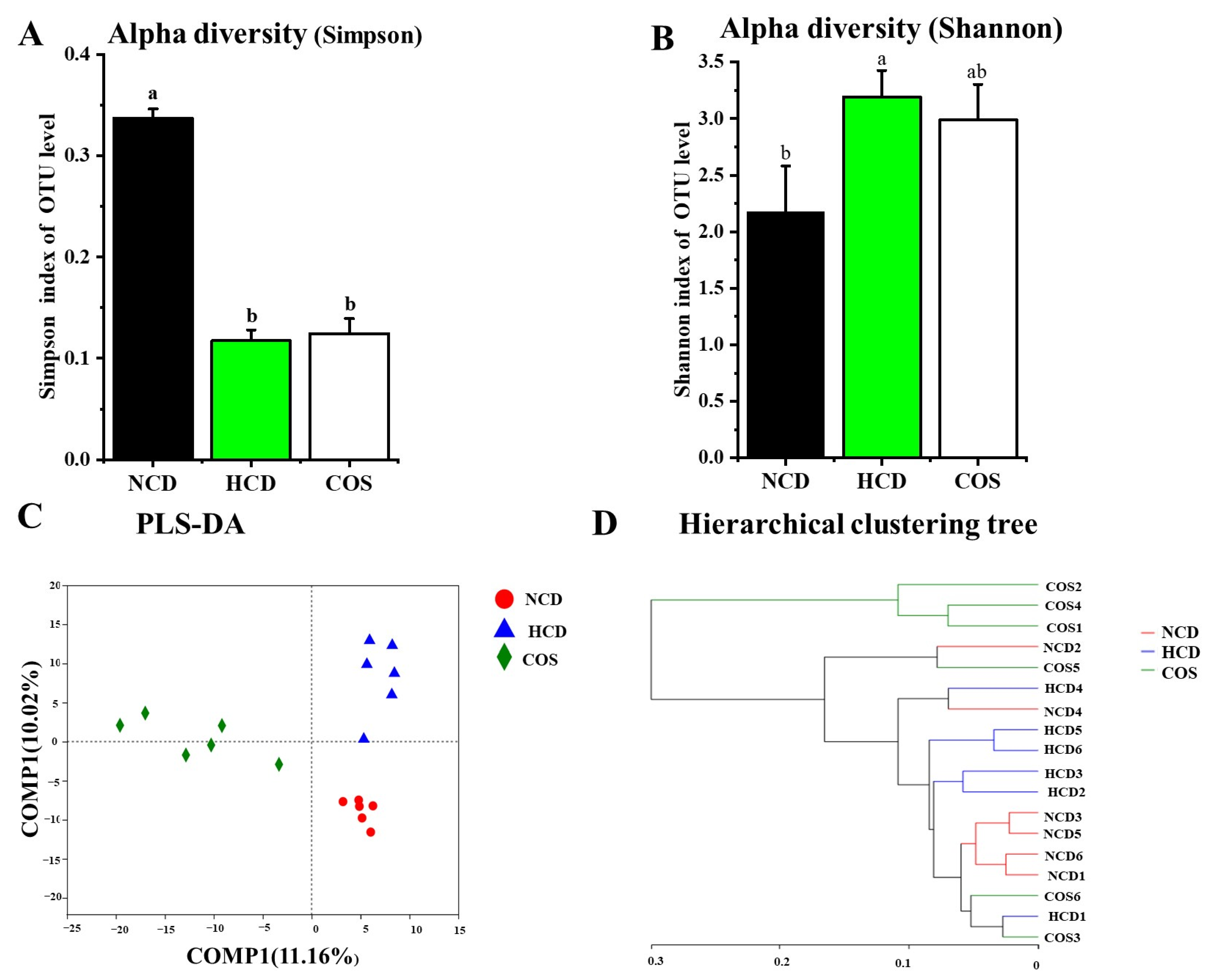
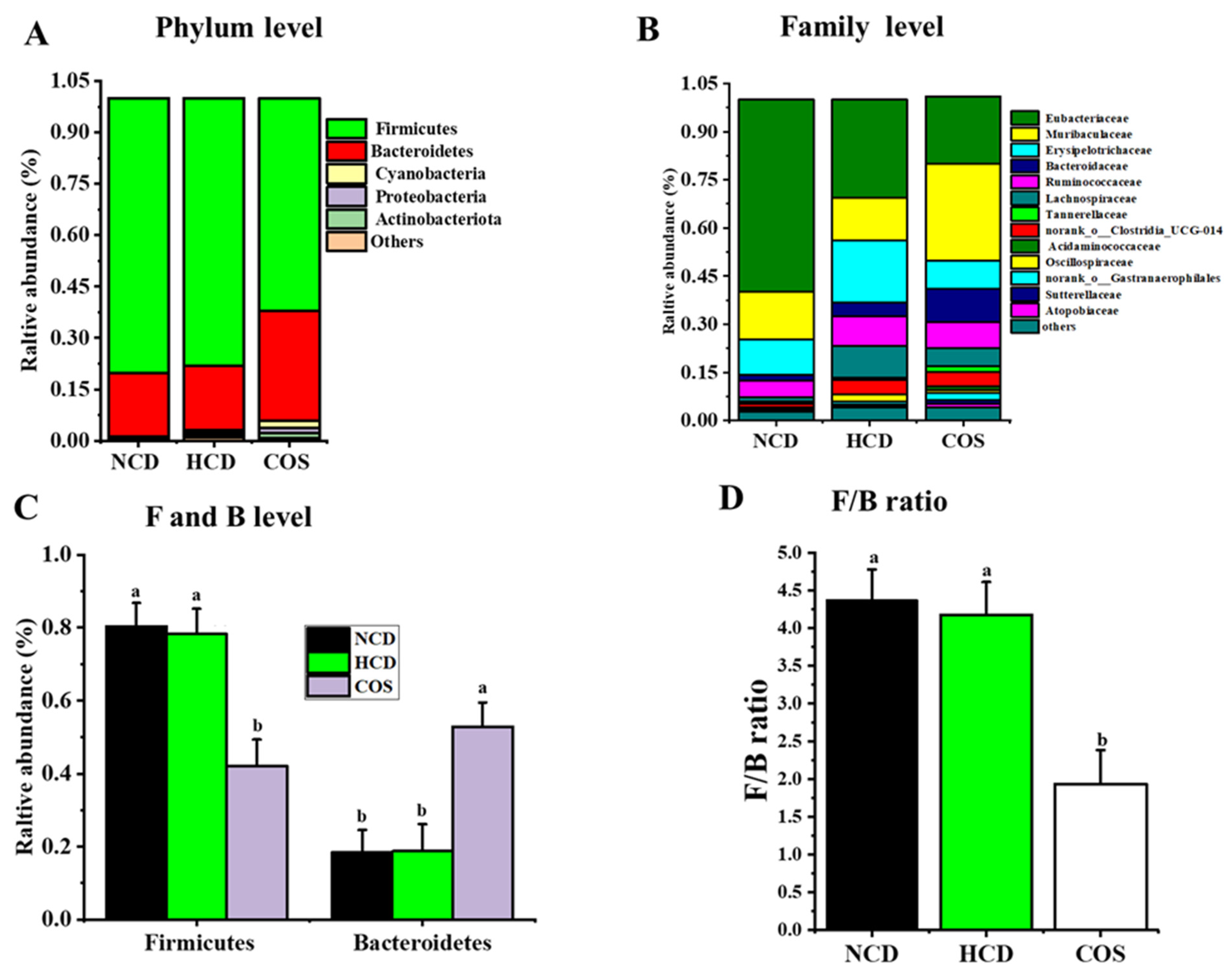
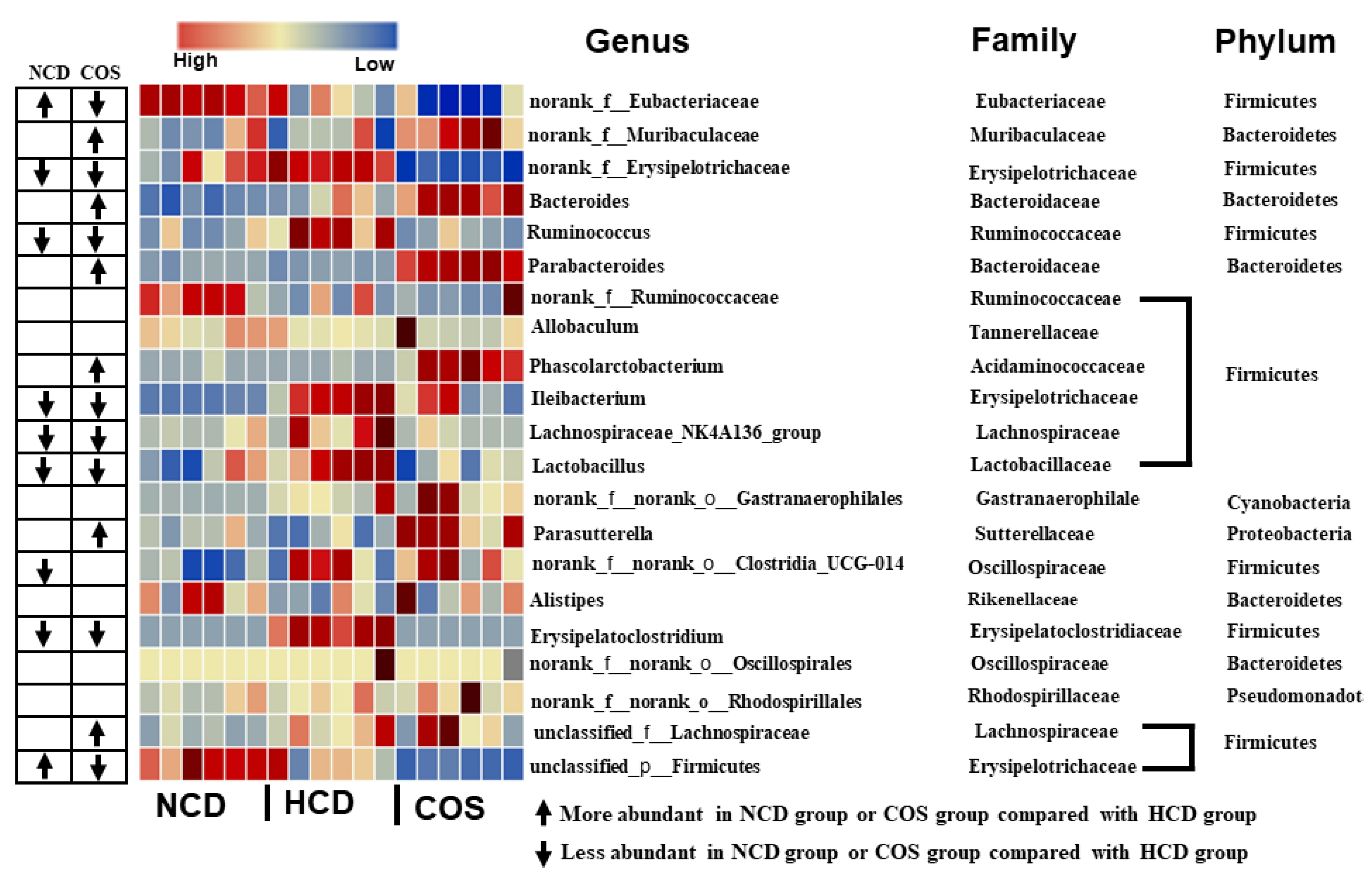
3.5. Overall Structure and Composition of GM
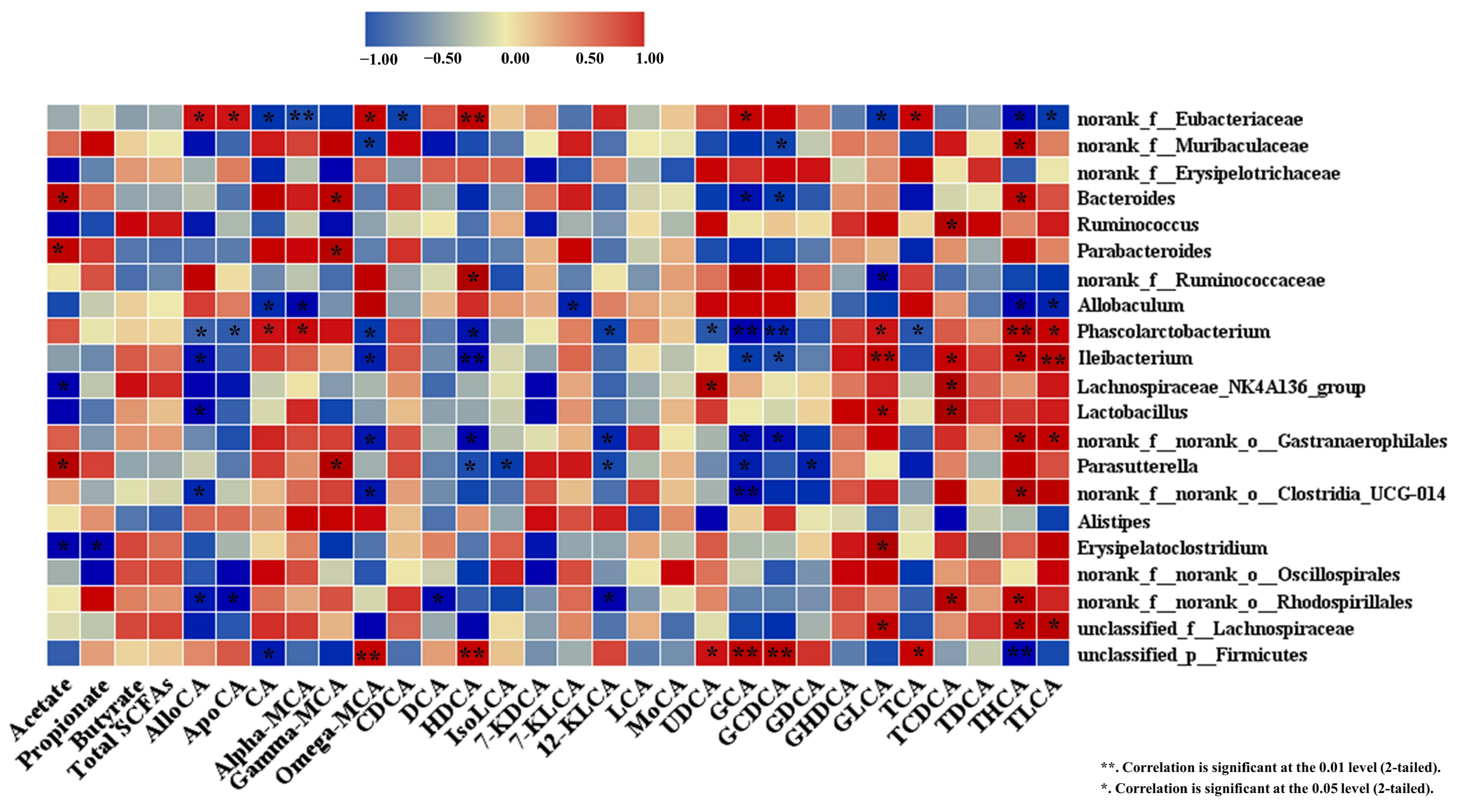
3.6. Spearman Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, T.; Trevisan, M.; Genco, R.J.; Falkner, K.L.; Dorn, J.P.; Sempos, C.T. Examination of the Relation between Periodontal Health Status and Cardiovascular Risk Factors: Serum Total and High Density Lipoprotein Cholesterol, C-Reactive Protein, and Plasma Fibrinogen. Am. J. Epidemiol. 2000, 151, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; He, Z.; Zhu, H.; Liu, J.; Kwek, E.; Zhao, Y.; Ma, K.Y.; He, W.S.; Chen, Z.Y. Sea Buckthorn Seed Oil Reduces Blood Cholesterol and Modulates Gut Microbiota. Food Funct. 2019, 10, 5669–5681. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Fu, C.; Liu, G.; Guo, J.; Su, Z. Cholesterol-Lowering Effects and Potential Mechanisms of Chitooligosaccharide Capsules in Hyperlipidemic Rats. Food Nutr. Res. 2018, 62, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Campins, L.; Camps, M.; Riera, A.; Pleguezuelos, E.; Yebenes, J.C.; Serra-Prat, M. Oral Drugs Related with Muscle Wasting and Sarcopenia. A Review. Pharmacology 2017, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, J.K.; Van Golen, R.F.; Verheij, J.; Dekker, A.M.; Van Gulik, T.M.; Heger, M. Atorvastatin Does Not Protect against Ischemia-Reperfusion Damage in Cholestatic Rat Livers. BMC Surg. 2017, 17, 35. [Google Scholar] [CrossRef]
- Deng, X.; Ye, Z.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Chitosan Oligosaccharide Ameliorated Obesity by Reducing Endoplasmic Reticulum Stress in Diet-Induced Obese Rats. Food Funct. 2020, 11, 6285–6296. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Yen, T.E.; Liu, S.H.; Chiang, M.T. Comparative Effects and Mechanisms of Chitosan and Its Derivatives on Hypercholesterolemia in High-Fat Diet-Fed Rats. Int. J. Mol. Sci. 2020, 21, 92. [Google Scholar] [CrossRef]
- Zhen, H.; Yan, Q.; Liu, Y.; Li, Y.; Yang, S.; Jiang, Z. Food Science and Human Wellness Chitin Oligosaccharides Alleviate Atherosclerosis Progress in ApoE-/-Mice by Regulating Lipid Metabolism and Inhibiting Infl Ammation. Food Sci. Hum. Wellness 2022, 11, 999–1009. [Google Scholar] [CrossRef]
- Bahijri, S.M.; Alsheikh, L.; Ajabnoor, G.; Borai, A. Effect of Supplementation with Chitosan on Weight, Cardiometabolic, and Other Risk Indices in Wistar Rats Fed Normal and High-Fat/High-Cholesterol Diets Ad Libitum. Nutr. Metab. Insights 2017, 10, 1178638817710666. [Google Scholar] [CrossRef]
- Metso, S.; Ylitalo, R.; Nikkilä, M.; Wuolijoki, E.; Ylitalo, P.; Lehtimäki, T. The Effect of Long-Term Microcrystalline Chitosan Therapy on Plasma Lipids and Glucose Concentrations in Subjects with Increased Plasma Total Cholesterol: A Randomised Placebo-Controlled Double-Blind Crossover Trial in Healthy Men and Women. Eur. J. Clin. Pharmacol. 2003, 59, 741–746. [Google Scholar] [CrossRef]
- Ghaffarzadegan, T.; Essén, S.; Verbrugghe, P.; Marungruang, N.; Hållenius, F.F.; Nyman, M.; Sandahl, M. Determination of Free and Conjugated Bile Acids in Serum of Apoe(−/−) Mice Fed Different Lingonberry Fractions by UHPLC-MS. Sci. Rep. 2019, 9, 3800. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Hao, W.; Zhu, H.; Liang, N.; He, Z.; Ma, K.Y.; Chen, Z.Y. Structure-Specific Effects of Short-Chain Fatty Acids on Plasma Cholesterol Concentration in Male Syrian Hamsters. J. Agric. Food Chem. 2017, 65, 10984–10992. [Google Scholar] [CrossRef]
- Zwicker, B.L.; Agellon, L.B. Transport and Biological Activities of Bile Acids. Int. J. Biochem. Cell Biol. 2013, 45, 1389–1398. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Tang, D.; Dong, R.; Feng, Q. Chitosan Oligosaccharide Ameliorates Metabolic Syndrome Induced by Overnutrition via Altering Intestinal Microbiota. Front. Nutr. 2021, 8, 743492. [Google Scholar] [CrossRef]
- Wu, M.; Li, J.; An, Y.; Li, P.; Xiong, W.; Li, J.; Yan, D.; Wang, M.; Zhong, G. Chitooligosaccharides Prevents the Development of Colitis-Associated Colorectal Cancer by Modulating the Intestinal Microbiota and Mycobiota. Front. Microbiol. 2019, 10, 2101. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Jiao, R.; Peng, C.; Wong, Y.M.; Yeung, V.S.Y.; Huang, Y.; Chen, Z. Choosing Hamsters but Not Rats as a Model for Studying Plasma Cholesterol-lowering Activity of Functional Foods. Mol. Nutr. Food Res. 2009, 53, 921–930. [Google Scholar] [CrossRef]
- Schaafsma, G.; Slavin, J.L. Significance of Inulin Fructans in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 37–47. [Google Scholar] [CrossRef]
- Abdo, A.A.A.; Zhang, C.; Lin, Y.; Liang, X.; Kaddour, B.; Wu, Q.; Li, X.; Fan, G.; Yang, R.; Teng, C. Xylo-Oligosaccharides Ameliorate High Cholesterol Diet Induced Hypercholesterolemia and Modulate Sterol Excretion and Gut Microbiota in Hamsters. J. Funct. Foods 2021, 77, 104334. [Google Scholar] [CrossRef]
- Zhang, C.; Abdulaziz Abbod Abdo, A.; Kaddour, B.; Wu, Q.; Xin, L.; Li, X.; Fan, G.; Teng, C. Xylan-Oligosaccharides Ameliorate High Fat Diet Induced Obesity and Glucose Intolerance and Modulate Plasma Lipid Profile and Gut Microbiota in Mice. J. Funct. Foods 2020, 64, 103622. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, Cholesterol Control, and Cardiovascular Disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Choi, S.; Kim, K.; Kim, S.M.; Lee, G.; Park, S.Y.; Kim, Y.; Son, J.S.; Yun, J.; Park, S.M. Effect of Change in Total Cholesterol Levels on Cardiovascular Disease among Young Adults. J. Am. Heart Assoc. 2018, 7, e008819. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, W.M.M.; Jacobs, D.R.; Bloemberg, B.P.M.; Kromhout, D.; Menotti, A.; Aravanis, C.; Blackburn, H.; Buzina, R.; Dontas, A.S.; Fidanza, F. Serum Total Cholesterol and Long-Term Coronary Heart Disease Mortality in Different Cultures: Twenty-Five—Year Follow-up of the Seven Countries Study. JAMA 1995, 274, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, Y.; Kang, W.; Zhou, J.; Dong, R.; Feng, Q. Chitosan Attenuates Obesity by Modifying the Intestinal Microbiota and Increasing Serum Leptin Levels in Mice. J. Funct. Foods 2020, 64, 103659. [Google Scholar] [CrossRef]
- Fatahi, S.; Sayyari, A.A.; Salehi, M.; Safa, M.; Sohouli, M.; Shidfar, F.; Santos, H.O. The Effects of Chitosan Supplementation on Anthropometric Indicators of Obesity, Lipid and Glycemic Profiles, and Appetite-Regulated Hormones in Adolescents with Overweight or Obesity: A Randomized, Double-Blind Clinical Trial. BMC Pediatr. 2022, 22, 527. [Google Scholar] [CrossRef]
- Groen, A.K.; Bloks, V.W.; Verkade, H.; Kuipers, F. Cross-Talk between Liver and Intestine in Control of Cholesterol and Energy Homeostasis. Mol. Aspects Med. 2014, 37, 77–88. [Google Scholar] [CrossRef]
- Hao, W.; Zhu, H.; Chen, J.; Kwek, E.; He, Z.; Liu, J.; Ma, N.; Ma, K.Y.; Chen, Z.Y. Wild Melon Seed Oil Reduces Plasma Cholesterol and Modulates Gut Microbiota in Hypercholesterolemic Hamsters. J. Agric. Food Chem. 2020, 68, 2071–2081. [Google Scholar] [CrossRef]
- Caliceti, C.; Punzo, A.; Silla, A.; Simoni, P.; Roda, G.; Hrelia, S. New Insights into Bile Acids Related Signaling Pathways in the Onset of Colorectal Cancer. Nutrients 2022, 14, 2964. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial Transformations of Human Bile Acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Jiao, A.R.; Diao, H.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Huang, Z.Q.; Luo, Y.H.; Luo, J.Q.; Mao, X.B.; et al. Oral Administration of Short Chain Fatty Acids Could Attenuate Fat Deposition of Pigs. PLoS ONE 2018, 13, e0196867. [Google Scholar] [CrossRef]
- Pushpass, R.A.G.; Alzoufairi, S.; Jackson, K.G.; Lovegrove, J.A. Circulating Bile Acids as a Link between the Gut Microbiota and Cardiovascular Health: Impact of Prebiotics, Probiotics and Polyphenol-Rich Foods. Nutr. Res. Rev. 2022, 35, 161–180. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Ling, T.; Zhao, C.; Li, Y.; Geng, M.; Gai, S.; Qi, W.; Luo, X.; Chen, L.; et al. Chitosan Oligosaccharides Alleviate Colitis by Regulating Intestinal Microbiota and PPARγ/SIRT1-Mediated NF-ΚB Pathway. Mar. Drugs 2022, 20, 96. [Google Scholar] [CrossRef]
- Hao, W.; Kwek, E.; He, Z.; Zhu, H.; Liu, J.; Zhao, Y.; Ma, K.Y.; He, W.S.; Chen, Z.Y. Ursolic Acid Alleviates Hypercholesterolemia and Modulates the Gut Microbiota in Hamsters. Food Funct. 2020, 11, 6091–6103. [Google Scholar] [CrossRef]
- Hu, W.; Lu, W.; Li, L.; Zhang, H. Both Living and Dead Faecalibacterium prausnitzii Alleviate House Dust Mite-Induced Allergic Asthma through the Modulation of Gut Microbiota and Short-Chain Fatty Acid Production. J. Sci. Food Agric. 2021, 101, 5563–5573. [Google Scholar] [CrossRef]
- Opeyemi, O.M.; Rogers, M.B.; Firek, B.A.; Janesko-Feldman, K.; Vagni, V.; Mullett, S.J.; Wendell, S.G.; Nelson, B.P.; New, L.A.; Mariño, E.; et al. Sustained Dysbiosis and Decreased Fecal Short-Chain Fatty Acids after Traumatic Brain Injury and Impact on Neurologic Outcome. J. Neurotrauma 2021, 38, 2610–2621. [Google Scholar] [CrossRef]
- Sun, N.X.; Tong, L.T.; Liang, T.T.; Wang, L.L.; Liu, L.Y.; Zhou, X.R.; Zhou, S.M. Effect of Oat and Tartary Buckwheat—Based Food on Cholesterol—Lowering and Gut Microbiota in Hypercholesterolemic Hamsters. J. Oleo Sci. 2019, 68, 251–259. [Google Scholar] [CrossRef]
- Xiong, Y.; Ji, L.; Zhao, Y.; Liu, A.; Wu, D.; Qian, J. Sodium Butyrate Attenuates Taurocholate-Induced Acute Pancreatitis by Maintaining Colonic Barrier and Regulating Gut Microorganisms in Mice. Front. Physiol. 2022, 13, 813735. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Zhao, Z.; Pi, X.; Meng, Y.; Fei, D.; Liu, D.; Wang, X. Effect of Chitooligosaccharides on Human Gut Microbiota and Antiglycation. Carbohydr. Polym. 2020, 242, 116413. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, S.; Wang, Z.A.; Du, Y. Exploring Effects of Chitosan Oligosaccharides on Mice Gut Microbiota in in Vitro Fermentation and Animal Model. Front. Microbiol. 2018, 9, 2388. [Google Scholar] [CrossRef]
- Kwek, E.; Zhu, H.; Ding, H.; He, Z.; Hao, W.; Liu, J.; Ma, K.Y.; Chen, Z.Y. Peony Seed Oil Decreases Plasma Cholesterol and Favorably Modulates Gut Microbiota in Hypercholesterolemic Hamsters. Eur. J. Nutr. 2022, 61, 2341–2356. [Google Scholar] [CrossRef]
| NCD | HCD | COS | |
|---|---|---|---|
| Ingredients | |||
| Corn starch | 50.9 | 50.9 | 50.9 |
| Sucrose | 11.9 | 11.9 | 11.9 |
| Lard | 5 | 5 | 5 |
| AIN 93 M Mineral Mix | 4 | 4 | 4 |
| AIN 93 Vitamin Mix | 2 | 2 | 2 |
| Gelatin | 2 | 2 | 2 |
| Cholesterol | 0 | 0.2 | 0.2 |
| Casein | 24.2 | 24.2 | 24.2 |
| Chitosan-oligosaccharide | 0 | 0 | 5 |
| Total (g) | 100 | 100.2 | 105.2 |
| Energy % | |||
| Protein | 26 | 26 | 26 |
| Carbohydrates | 63 | 63 | 63 |
| Fat | 11 | 11 | 11 |
| Total (kcal) | 401 | 401 | 401 |
| NCD | HCD | COS | p Value | |
|---|---|---|---|---|
| Food intake (g/hamster/day) | 10.481 ± 0.174 c | 10.924 ± 0.145 a | 10.451 ± 0.254 bc | 0.031 |
| Energy intake | 40.877 ± 0.681 b | 43.698 ± 0.581 a | 39.714 ± 0.965 b | 0.001 |
| (kcal/hamster/day) | ||||
| Body weights (g) | ||||
| week 0 | 142.712 ± 10.302 | 137.887 ± 12.922 | 141.375 ± 7.448 | 0.771 |
| week 6 | 147.725 ± 18.378 | 149.5 ± 13.318 | 139.425 ± 14.41 | 0.233 |
| Organ weights (g) | ||||
| Liver | 5.5453 ± 1.030 b | 7.707 ± 1.189 a | 7.336 ± 1.973 a | 0.005 |
| Heart | 0.65 ± 0.068 | 0.66 ± 0.064 | 0.635 ± 0.076 | 0.932 |
| Kidney | 1.216 ± 0.244 | 1.27 ± 0.097 | 1.37 ± 0.09 | 0.343 |
| Testis | 4.046 ± 0.595 | 4.511 ± 0.686 | 4.136 ± 0.434 | 0.472 |
| Epididymal fat | 2.882 ± 0.713 ab | 3.807 ± 0.806 a | 2.158 ± 1.192 b | 0.006 |
| Perirenal fat | 1.205 ± 0.485 | 1.43 ± 0.386 | 1.001 ± 0.513 | 0.12 |
| NCD | HCD | COS | p Value | |
|---|---|---|---|---|
| Week 0 | ||||
| TC (mmol/L) | 4.047 ± 1.816 | 4.142 ± 0.749 | 4.103 ± 0.443 | 0.992 |
| HDL-C (mmol/L) | 3.081 ± 0.448 | 3.057 ± 0.549 | 3.007 ± 0.365 | 0.192 |
| Non-HDL-C (mmol/L) | 0.966 ± 0.120 | 1.582 ± 0.328 | 1.598 ± 0.175 | 0.597 |
| TG (mmol/L) | 1.476 ± 0.734 | 1.598 ± 0.593 | 1.512 ± 0.709 | 0.192 |
| Week 6 | ||||
| TC (mmol/L) | 5.076 ± 0.396 c | 7.897 ± 0.910 a | 6.901 ± 0.601 b | <0.001 |
| HDL-C (mmol/L) | 3.430 ± 0.258 a | 2.503 ± 0.733 b | 3.245 ± 0.735 a | <0.001 |
| Non-HDL-C (mmol/L) | 1.646 ± 0.405 c | 5.393 ± 0.607 a | 3.656 ± 1.063 b | <0.001 |
| TG (mmol/L) | 1.578 ±0.257 b | 2.602 ± 0.421 a | 1.793 ± 0.594 b | <0.001 |
| Bile Acids (µg/Day/Hamster) | NCD | HCD | COS | p Value |
|---|---|---|---|---|
| Unconjugated | ||||
| Allocholic Acid | 85.915 ± 7.709 a | 0.605 ± 0.092 b | 72.168 ± 2.520 a | <0.001 |
| Apocholic acid | 140.871 ± 23.196 | 89.621 ± 1.84 | 110.233 ± 8.352 | 0.126 |
| Cholic acid | 20.578 ± 1.550 a | 5.892 ± 2.461 b | 18.372 ± 3.245 a | <0.001 |
| Alpha-muricholic acid | 1.838 ±0.085 b | 2.765 ± 0.567 a | 8.238 ± 0.698 a | <0.001 |
| Gamma -muricholic Acid | 3.094 ± 0.748 b | 7.269 ± 2.367 b | 21.244 ± 4.853 a | <0.001 |
| Omega -muricholic acid | 496.639 ±24.622 a | 282.820 ± 8.018 b | 384.075 ± 16.634 ab | <0.001 |
| Chenodeoxycholic acid | 97.371 ± 4.579 | 121.613 ± 8.430 | 157.724 ± 21.487 | 0.465 |
| Deoxycholic acid | 1200.654 ± 89.140 a | 651.065 ± 80.756 b | 916.169 ± 75.202 a | <0.001 |
| Hyodeoxycholic acid | 27.534 ± 1.795 a | 9.880 ± 0.606 b | 19.203 ± 1.705 a | <0.001 |
| Isoalblithocholic acid | 683.351 ± 31.117 | 453.915 ± 25.199 | 683.726 ± 20.337 | 0.136 |
| 7-ketodeoxycholic acid | 33.260 ± 5.170 | 32.864 ±3.13 | 47.268 ± 9.721 | 0.159 |
| 7-ketolithocholic acid | 7.972 ± 1.827 | 11.809 ± 2.694 | 14.670 ± 4.548 | 0.179 |
| 12-ketolithocholic acid | 428. 92 ± 40.582 | 255.830 ± 60.334 | 318.718 ± 17.268 | 0.064 |
| Lithocholic acid | 1158.628 ± 107.725 | 1081.634 ± 101.04 | 1333.827 ± 103.588 | 0.133 |
| Murocholic acid | 13.782 ± 4.008 | 12.644 ± 1.145 | 36.296 ± 4.898 | 0.095 |
| Ursodeoxycholic acid | 56.366 ± 5.591 | 47.326 ± 5.895 | 61.695 ± 3.170 | 0.269 |
| Glycine-conjugated | ||||
| Glycocholic acid | 2.741 ± 0.534 a | 0.29 ± 0.0351 b | 0.393 ± 0.042 b | <0.001 |
| Glycochenodeoxycholic acid | 2.217 ± 0.740 a | 0.1571 ± 0.0359 b | 0.190 ± 0.064 b | <0.001 |
| Glycodeoxycholic acid | 4.089 ± 0.726 a | 1.882 ± 0.346 b | 2.109 ± 0.662 b | 0.019 |
| Glycohyodeoxycholic acid | 0.296 ± 0.034 b | 0.782 ± 0.140 a | 0.277 ± 0.060 b | <0.001 |
| Glycolithocholic acid | 3.336 ± 0.776 b | 7.927 ± 1.55 a | 4.1414 ± 1.050 b | <0.001 |
| Taurine-conjugated | ||||
| Taurocholic acid | 0.903 ± 0.010 a | 0.340 ± 0.0186 b | 0.435 ± 0.097 b | <0.001 |
| Taurochenodeoxycholic acid | 0.276 ± 0.056 ab | 0.351 ± 0.055 a | 0.227 ± 0.010 ab | 0.107 |
| Taurodeoxycholic acid | 0.358 ± 0.017 ab | 0.412 ± 0.053 a | 0.287 ± 0.053 b | <0.001 |
| Taurohyodeoxycholic acid | 0.254 ± 0.011 c | 1.244 ± 0.025 a | 1.211 ± 0.019 a | <0.001 |
| Taurolithocholic acid | 0.129 ± 0.012 b | 0.320 ± 0.073 a | 0.124 ± 0.025 b | <0.001 |
| NCD | HCD | COS | p Value | |
|---|---|---|---|---|
| SCFAs (mg/day/hamster) | ||||
| Acetate | 16.731 ±2.750 ab | 10.283 ± 1.776 c | 32.554 ± 7.933 a | <0.001 |
| Propionate | 2.715 ± 0.271 ab | 1.215 ± 0.271 b | 2.179 ± 2.179 ab | 0.07 |
| Butyrate | 347.207 ± 20.788 | 327.517 ± 33.893 | 343.712 ± 23.534 | 0.785 |
| Total SCFAs | 366.654 ± 21.094 | 339.016 ± 34.119 | 378.446 ± 20.860 | 0.928 |
| PH | ||||
| Fecal PH value | 5.740 ± 0.176 c | 6.427 ± 0.056 a | 6.185 ± 0.190 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdo, A.; Zhang, C.; Al-Dalali, S.; Hou, Y.; Gao, J.; Yahya, M.A.; Saleh, A.; Aleryani, H.; Al-Zamani, Z.; Sang, Y. Marine Chitosan-Oligosaccharide Ameliorated Plasma Cholesterol in Hypercholesterolemic Hamsters by Modifying the Gut Microflora, Bile Acids, and Short-Chain Fatty Acids. Nutrients 2023, 15, 2923. https://doi.org/10.3390/nu15132923
Abdo A, Zhang C, Al-Dalali S, Hou Y, Gao J, Yahya MA, Saleh A, Aleryani H, Al-Zamani Z, Sang Y. Marine Chitosan-Oligosaccharide Ameliorated Plasma Cholesterol in Hypercholesterolemic Hamsters by Modifying the Gut Microflora, Bile Acids, and Short-Chain Fatty Acids. Nutrients. 2023; 15(13):2923. https://doi.org/10.3390/nu15132923
Chicago/Turabian StyleAbdo, Abdullah, Chengnan Zhang, Sam Al-Dalali, Yakun Hou, Jie Gao, Mohammed Abdo Yahya, Ali Saleh, Hamzah Aleryani, Zakarya Al-Zamani, and Yaxin Sang. 2023. "Marine Chitosan-Oligosaccharide Ameliorated Plasma Cholesterol in Hypercholesterolemic Hamsters by Modifying the Gut Microflora, Bile Acids, and Short-Chain Fatty Acids" Nutrients 15, no. 13: 2923. https://doi.org/10.3390/nu15132923
APA StyleAbdo, A., Zhang, C., Al-Dalali, S., Hou, Y., Gao, J., Yahya, M. A., Saleh, A., Aleryani, H., Al-Zamani, Z., & Sang, Y. (2023). Marine Chitosan-Oligosaccharide Ameliorated Plasma Cholesterol in Hypercholesterolemic Hamsters by Modifying the Gut Microflora, Bile Acids, and Short-Chain Fatty Acids. Nutrients, 15(13), 2923. https://doi.org/10.3390/nu15132923







