IRW (Ile–Arg–Trp) Alleviates DSS–Induced Intestinal Injury by Remodeling Gut Microbiota and Regulating Fecal SCFA Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Histopathological Analysis
2.3. Determination of SCFAs in Feces
2.4. 16S rDNA Pyrophosphate Sequencing
2.5. Statistical Analysis
3. Results
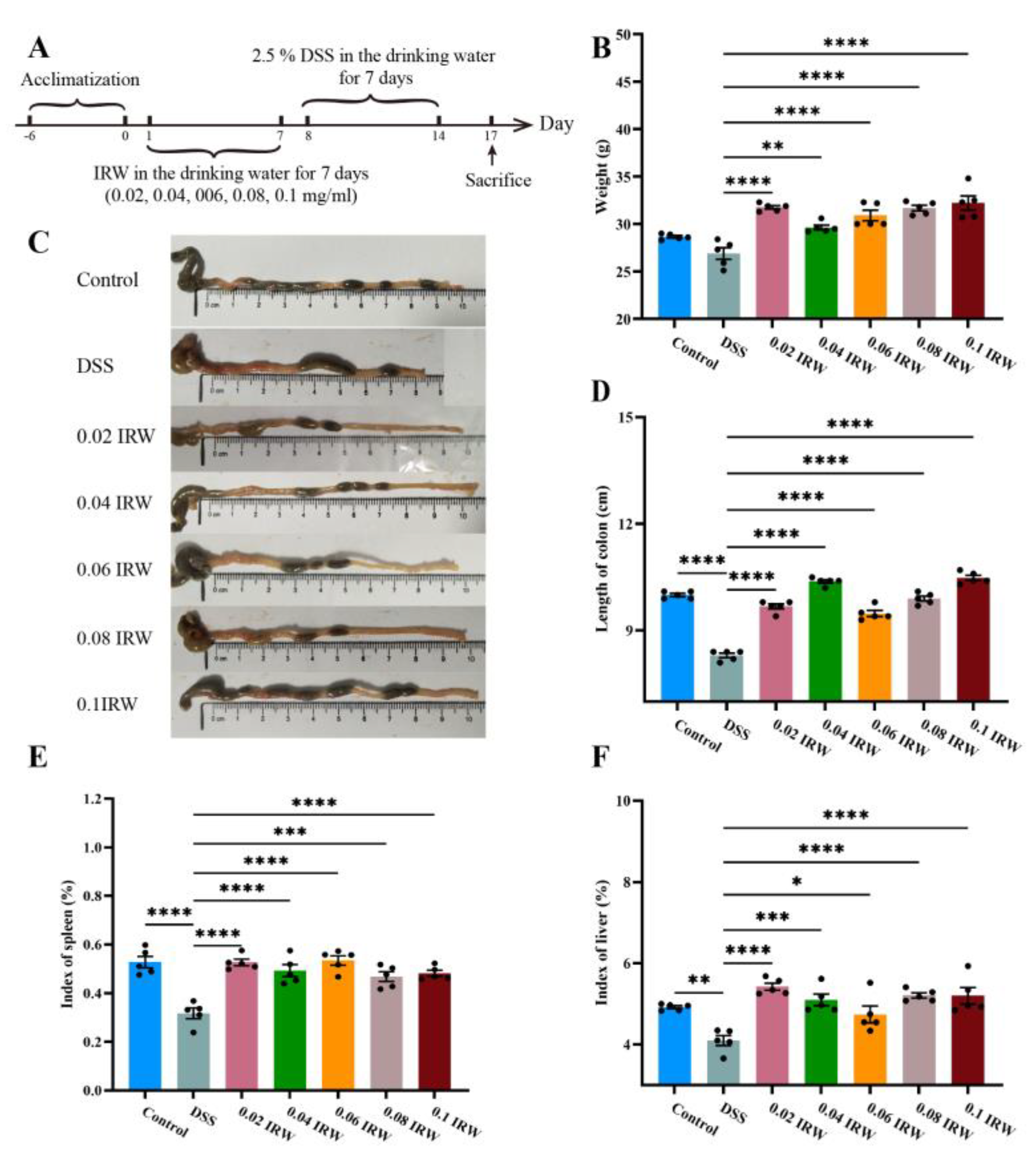
3.1. Effect of Different Concentration Gradients of IRW on Body Weight and Immune Organ Index in Mice with Colitis
3.2. Protective Effect of Different Concentration Gradients of IRW on Intestinal Barrier in Mice with Colitis
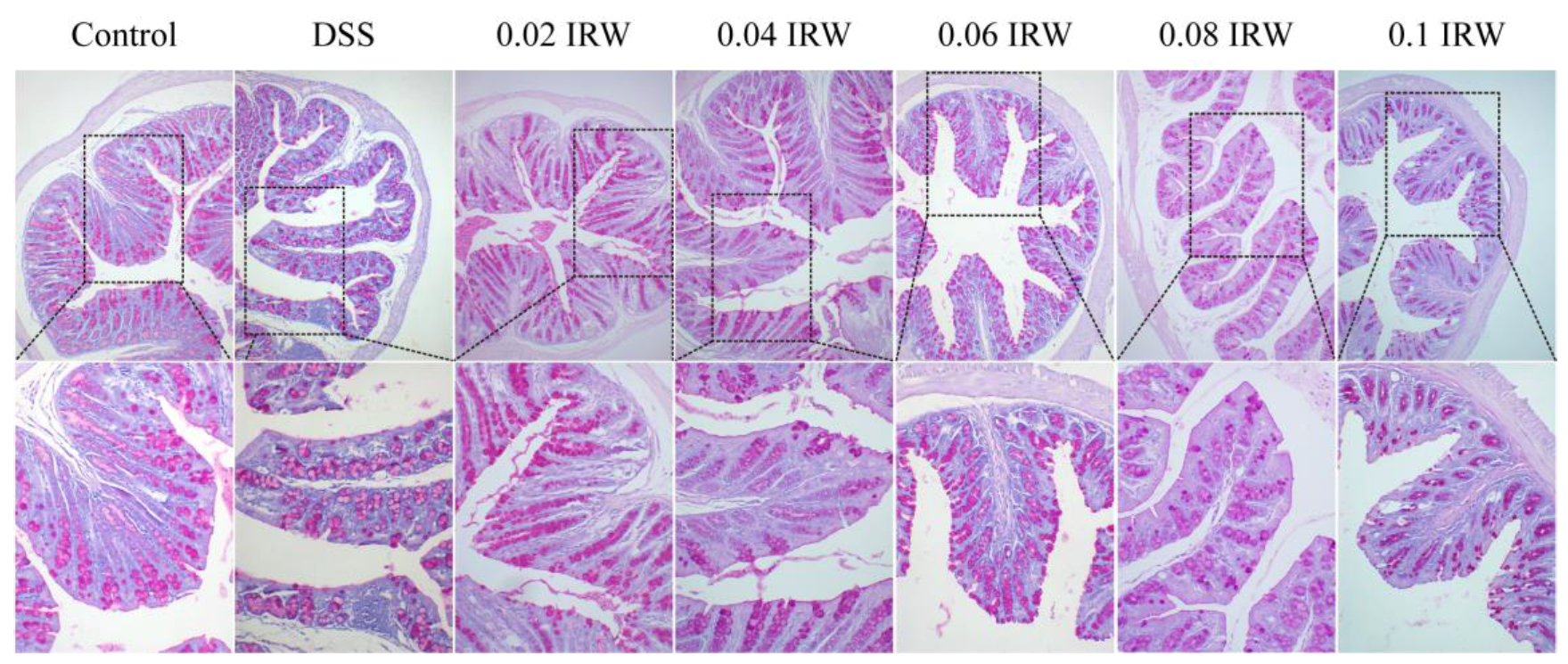
3.3. Inhibitory Effects of Different Concentration Gradients of IRW on Intestinal Mucosal Destruction and Goblet Cell Exhaustion in Mice with Colitis
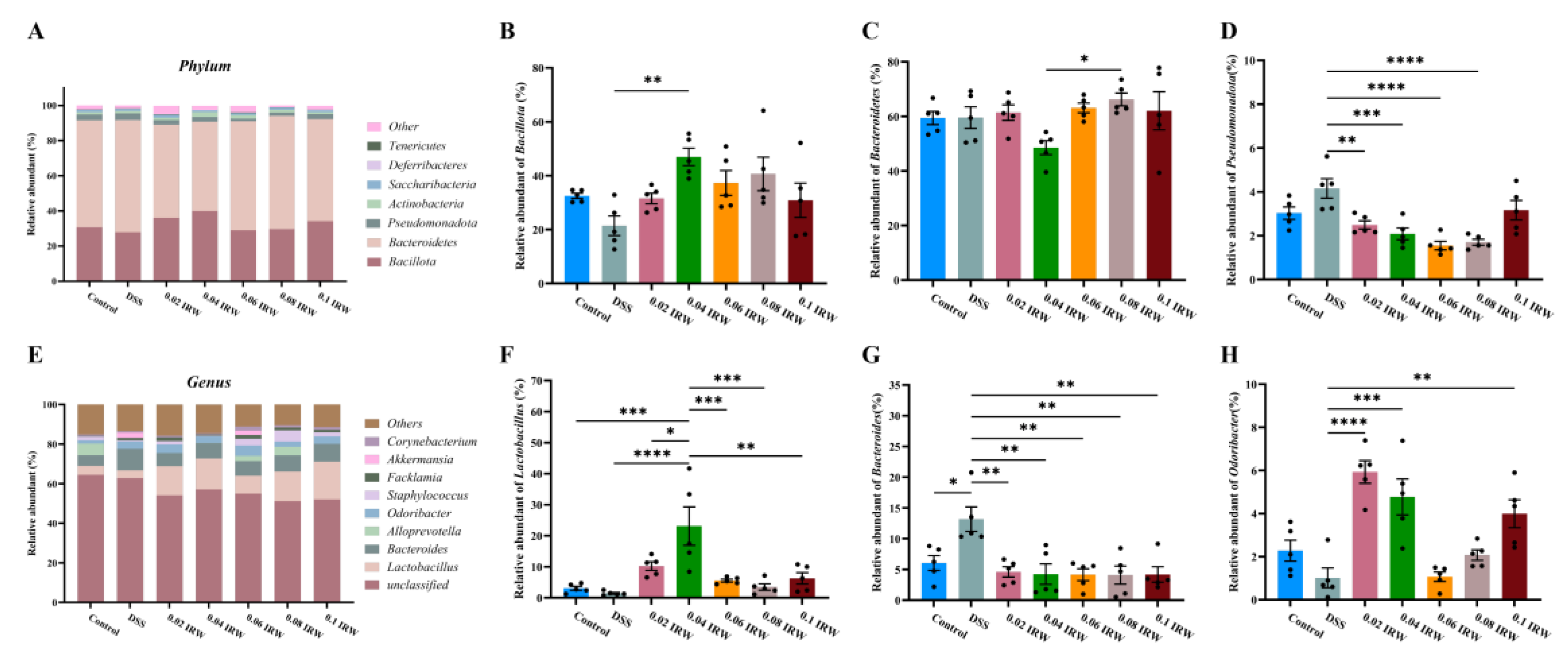
3.4. Different Concentration Gradients of IRW Regulate Gut Microbiota in Mice with Colitis
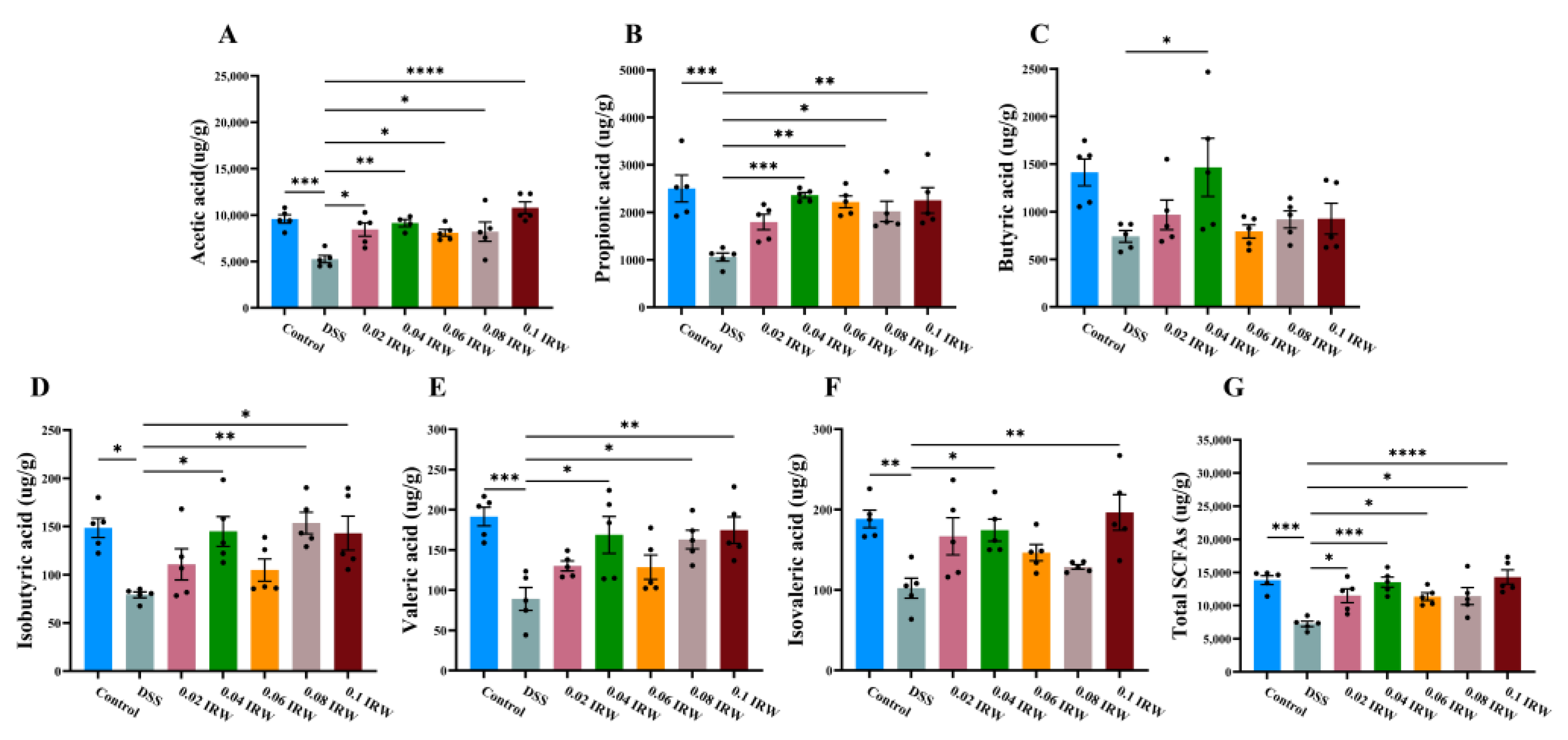
3.5. Effect of Different Concentration Gradients of IRW on the Concentration of SCFAs in the Colon of Mice with Colitis
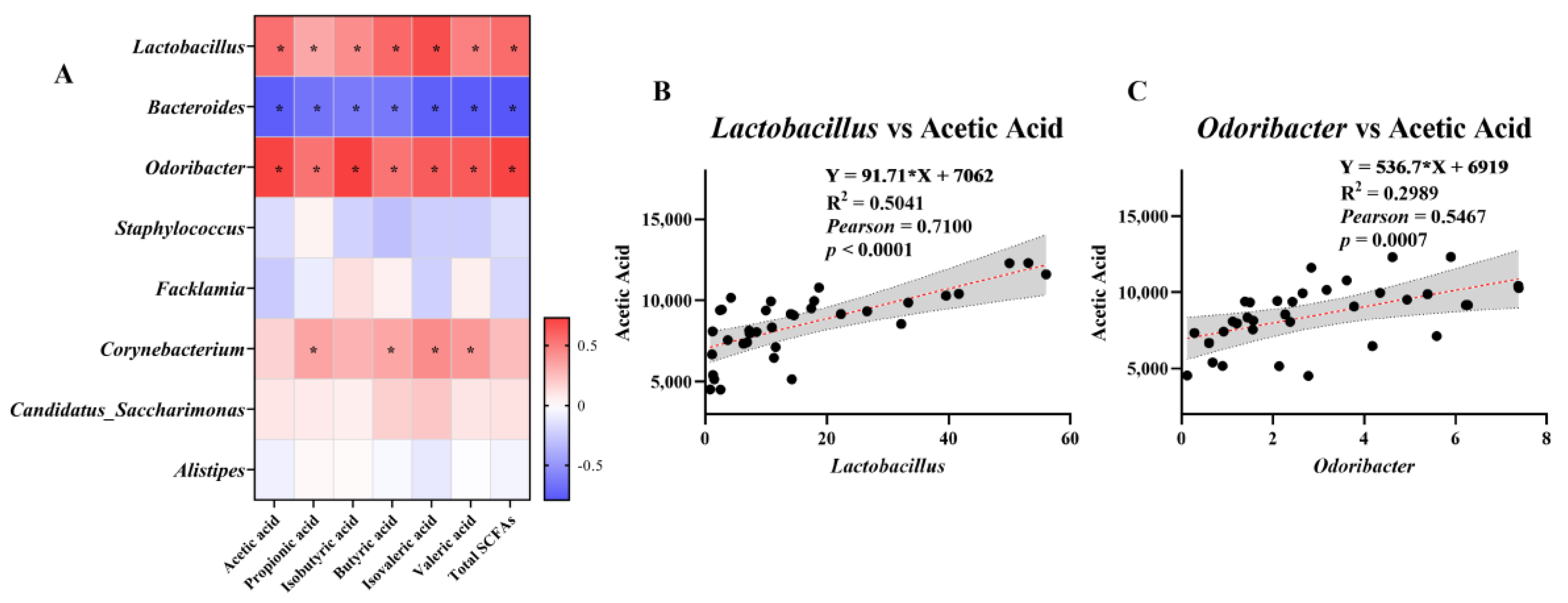
3.6. Analysis of the Association between SCFAs and Microorganisms at the Genus Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-del Rio, L.; Diaz-Rodriguez, P.; Landin, M. New tools to design smart thermosensitive hydrogels for protein rectal delivery in IBD. Mater. Sci. Eng. C 2020, 106, 110252. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Prim. 2020, 6, 74. [Google Scholar] [CrossRef]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Prim. 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A. Is there a possible correlation between antibodies against lipopolysaccharide, intestinal and blood-brain barrier proteins in IBD subjects? Autoimmun. Rev. 2019, 18, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, Y.; Xu, M.; Wang, S.; Wang, X.; Tu, Y. Simulated gastrointestinal digest from preserved egg white exerts anti-inflammatory effects on Caco-2 cells and a mouse model of DSS–induced colitis. J. Funct. Foods 2017, 35, 655–665. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Tang, R.; Lu, H. Resveratrol and its derivates improve inflammatory bowel disease by targeting gut microbiota and inflammatory signaling pathways. Food Sci. Hum. Wellness 2022, 11, 22–31. [Google Scholar] [CrossRef]
- Wu, D.; Chen, S.; Ye, X.; Ahmadi, S.; Hu, W.; Yu, C.; Zhu, K.; Cheng, H.; Linhardt, R.J.; He, Q. Protective effects of six different pectic polysaccharides on DSS–induced IBD in mice. Food Hydrocoll. 2022, 127, 107209. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, C.; Chen, D.; Zeng, X.; Cao, Y. Prebiotic effects in vitro of anthocyanins from the fruits of Lycium ruthenicum Murray on gut microbiota compositions of feces from healthy human and patients with inflammatory bowel disease. LWT 2021, 149, 111829. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J. Egg white ovomucin hydrolysate inhibits intestinal integrity damage in LPS-treated Caco-2 cells. J. Funct. Foods 2021, 87, 104822. [Google Scholar] [CrossRef]
- Hamley, I.W. Small Bioactive Peptides for Biomaterials Design and Therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef]
- Jiang, Q.; Charoensiddhi, S.; Xue, X.; Sun, B.; Liu, Y.; El-Seedi, H.R.; Wang, K. A review on the gastrointestinal protective effects of tropical fruit polyphenols. Crit. Rev. Food Sci. Nutr. 2022, 17, 1–27. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. Purification and characterisation of angiotensin I converting enzyme (ACE) inhibitory peptides derived from enzymatic hydrolysate of ovotransferrin. Food Chem. 2011, 126, 1614–1619. [Google Scholar] [CrossRef]
- Okagu, I.U.; Ezeorba, T.P.C.; Aham, E.C.; Aguchem, R.N.; Nechi, R.N. Recent findings on the cellular and molecular mechanisms of action of novel food-derived antihypertensive peptides. Food Chem. Mol. Sci. 2022, 4, 100078. [Google Scholar] [CrossRef]
- Yi, J.; Zhao, J.; Wu, J. Egg ovotransferrin derived IRW exerts protective effect against H2O2-induced oxidative stress in Caco-2 cells. J. Funct. Foods 2017, 39, 160–167. [Google Scholar] [CrossRef]
- Blachman, A.; Funez, F.; Birocco, A.M.; Saavedra, S.L.; Lazaro-Martinez, J.M.; Camperi, S.A.; Glisoni, R.; Sosnik, A.; Calabrese, G.C. Targeted anti-inflammatory peptide delivery in injured endothelial cells using dermatan sulfate/chitosan nanomaterials. Carbohydr. Polym. 2020, 230, 115610. [Google Scholar] [CrossRef]
- Majumder, K.; Liang, G.; Chen, Y.; Guan, L.; Davidge, S.T.; Wu, J. Egg ovotransferrin-derived ACE inhibitory peptide IRW increases ACE2 but decreases proinflammatory genes expression in mesenteric artery of spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015, 59, 1735–1744. [Google Scholar] [CrossRef]
- Liu, G.; Yan, W.; Ding, S.; Jiang, H.; Ma, Y.; Wang, H.; Fang, J. Effects of IRW and IQW on Oxidative Stress and Gut Microbiota in Dextran Sodium Sulfate-Induced Colitis. Cell. Physiol. Biochem. 2018, 51, 441–451. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Liu, G.; Fang, J.; Yen, W.; Duraipandiyan, V.; Al-Dhabi, N.A.; Esmail, G.A.; Jiang, H. Egg Protein Transferrin-Derived Peptides IRW and IQW Regulate Citrobacter rodentium-Induced, Inflammation-Related Microbial and Metabolomic Profiles. Front. Microbiol. 2019, 10, 643. [Google Scholar] [CrossRef]
- Liao, W.; Fan, H.; Davidge, S.T.; Wu, J. Egg White-Derived Antihypertensive Peptide IRW (Ile–Arg–Trp) Reduces Blood Pressure in Spontaneously Hypertensive Rats via the ACE2/Ang (1-7)/Mas Receptor Axis. Mol. Nutr. Food Res. 2019, 63, e1900063. [Google Scholar] [CrossRef]
- Plaisancié, P.; Claustre, J.; Estienne, M.; Henry, G.; Boutrou, R.; Paquet, A.; Léonil, J. A novel bioactive peptide from yoghurts modulates expression of the gel-forming MUC2 mucin as well as population of goblet cells and Paneth cells along the small intestine. J. Nutr. Biochem. 2013, 24, 213–221. [Google Scholar] [CrossRef]
- Murphy, E.C.; Mörgelin, M.; Cooney, J.C.; Frick, I.M. Interaction of Bacteroides fragilis and Bacteroides thetaiotaomicron with the kallikrein-kinin system. Microbiology 2011, 157, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Calabuig, M.; Sanz, Y. Differences between the fecal microbiota of coeliac infants and healthy controls. Curr. Issues Intest. Microbiol. 2007, 8, 9–14. [Google Scholar] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Reviews. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-E.; Jeong, J.-J.; Kim, J.-K.; Han, M.J.; Kim, D.-H. Simultaneous Amelioratation of Colitis and Liver Injury in Mice by Bifidobacterium longum LC67 and Lactobacillus plantarum LC27. Sci. Rep. 2018, 8, 7500. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef]
- Lima, S.F.; Gogokhia, L.; Viladomiu, M.; Chou, L.; Putzel, G.; Jin, W.-B.; Pires, S.; Guo, C.-J.; Gerardin, Y.; Crawford, C.V.; et al. Transferable Immunoglobulin A–Coated Odoribacter splanchnicus in Responders to Fecal Microbiota Transplantation for Ulcerative Colitis Limits Colonic Inflammation. Gastroenterology 2022, 162, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Suutarinen, M.; Kainulainen, V.; Bowers, J.R.; Lemmer, D.; Engelthaler, D.M.; Eklund, K.K.; et al. Novel Odoribacter splanchnicus Strain and Its Outer Membrane Vesicles Exert Immunoregulatory Effects in vitro. Front. Microbiol. 2020, 11, 575455. [Google Scholar] [CrossRef]
- Xing, C.; Wang, M.; Ajibade, A.A.; Tan, P.; Fu, C.; Chen, L.; Zhu, M.; Hao, Z.Z.; Chu, J.; Yu, X.; et al. Microbiota regulate innate immune signaling and protective immunity against cancer. Cell Host Microbe 2021, 29, 959–974.e957. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y.; et al. Promotion of Intestinal Epithelial Cell Turnover by Commensal Bacteria: Role of Short-Chain Fatty Acids. PLoS ONE 2016, 11, e0156334. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Wolter, M.; Grant, E.T.; Boudaud, M.; Steimle, A.; Pereira, G.V.; Martens, E.C.; Desai, M.S. Leveraging diet to engineer the gut microbiome. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Deutz, N.E. Biomarkers of arginine and lysine excess. J. Nutr. 2007, 137, 1662s–1668s. [Google Scholar] [CrossRef] [PubMed]
- Block, K.P.; Harper, A.E. Valine metabolism in vivo: Effects of high dietary levels of leucine and isoleucine. Metab. Clin. Exp. 1984, 33, 559–566. [Google Scholar] [CrossRef]
- Aviram, M.; Cogan, U.; Mokady, S. Excessive dietary tryptophan enhances plasma lipid peroxidation in rats. Atherosclerosis 1991, 88, 29–34. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, Y.; Li, S.; Wang, Z.; Ma, Y.; Fang, J.; Liu, G. IRW (Ile–Arg–Trp) Alleviates DSS–Induced Intestinal Injury by Remodeling Gut Microbiota and Regulating Fecal SCFA Levels. Nutrients 2023, 15, 953. https://doi.org/10.3390/nu15040953
Fei Y, Li S, Wang Z, Ma Y, Fang J, Liu G. IRW (Ile–Arg–Trp) Alleviates DSS–Induced Intestinal Injury by Remodeling Gut Microbiota and Regulating Fecal SCFA Levels. Nutrients. 2023; 15(4):953. https://doi.org/10.3390/nu15040953
Chicago/Turabian StyleFei, Yanquan, Siying Li, Zaoyi Wang, Yong Ma, Jun Fang, and Gang Liu. 2023. "IRW (Ile–Arg–Trp) Alleviates DSS–Induced Intestinal Injury by Remodeling Gut Microbiota and Regulating Fecal SCFA Levels" Nutrients 15, no. 4: 953. https://doi.org/10.3390/nu15040953
APA StyleFei, Y., Li, S., Wang, Z., Ma, Y., Fang, J., & Liu, G. (2023). IRW (Ile–Arg–Trp) Alleviates DSS–Induced Intestinal Injury by Remodeling Gut Microbiota and Regulating Fecal SCFA Levels. Nutrients, 15(4), 953. https://doi.org/10.3390/nu15040953







