Validation of ELISAs for Isoflavones and Enterolactone for Phytoestrogen Intake Assessment in the French Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Volunteers
2.1.2. Sample Treatments
- Digestion and extraction of the conjugated isoflavones
- ELISA measurements
2.1.3. Creatinine Measurements
2.2. Methods
2.2.1. Dietary Inquiry and Dietary Scores
2.2.2. ELISA Measurements in Body Fluids
2.2.3. Statistical Treatments
3. Results
3.1. Biological Fluid Measurements
3.1.1. Isoflavones (IFs)
3.1.2. Enterolactone (ENL)
3.2. Dietary Inquiry and Scores
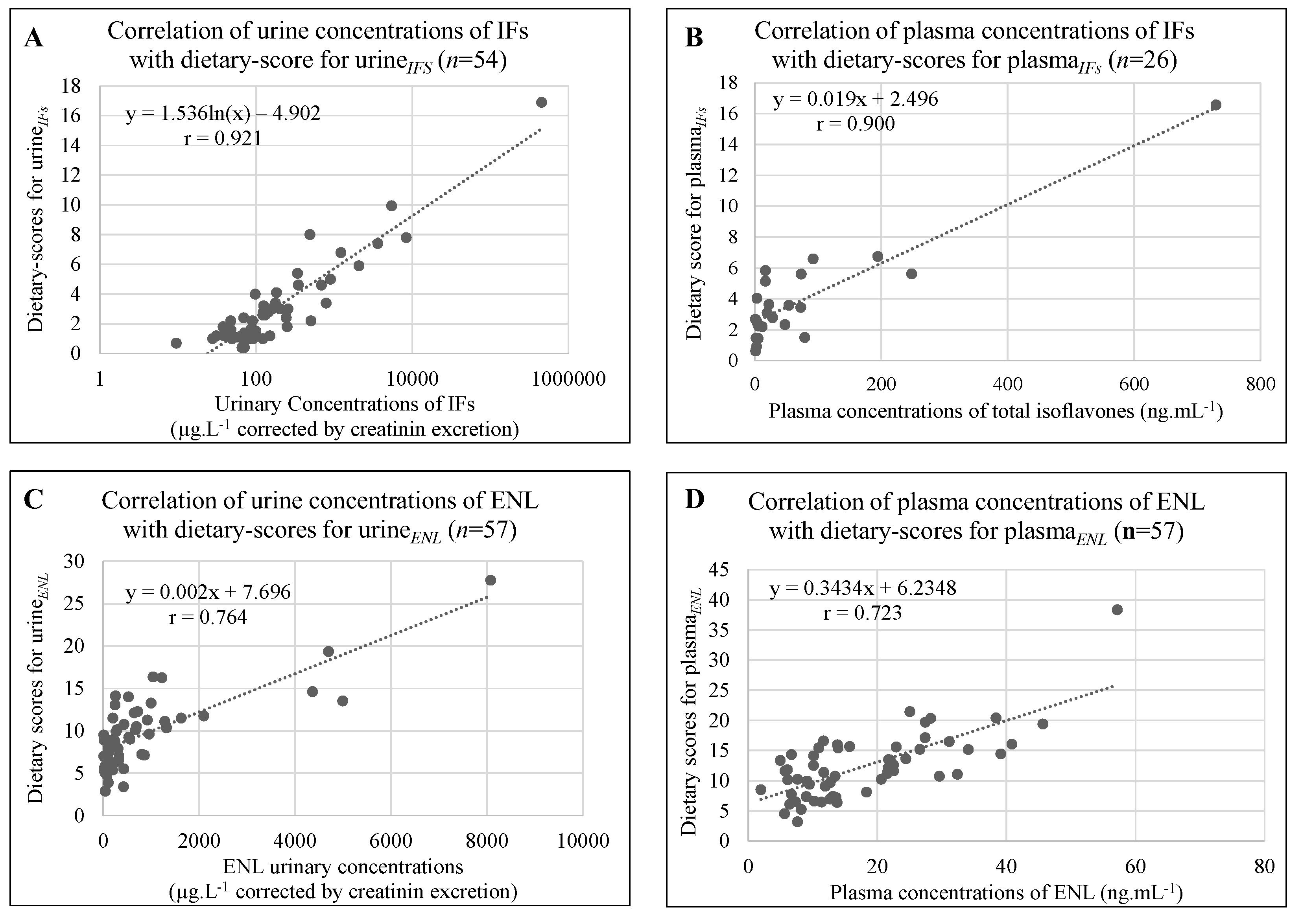
3.3. Correlations between Dietary Scores and Measurements in Biological Fluids
4. Discussion
4.1. Concentrations of PHYTOs in the Body Fluids of French Volunteers
4.1.1. IFs Concentrations
4.1.2. Comparisons between Urine and Plasma IFs Concentrations
4.1.3. Equol Production
4.1.4. The Case of the Heavy Consumer of Soybean
4.1.5. ENL Plasma and Urine Concentrations
4.2. Discussion on Correlations
4.2.1. Calculations of Dietary Scores
4.2.2. Comparisons of Correlation between Dietary Scores and IF in Urine or in Plasma
4.2.3. IFs and ENL as Dietary Biomarkers
4.2.4. Comparisons with Other Published Data
4.3. Limits of the Study
4.3.1. Phytoestrogens Analyzed
4.3.2. Accuracy of the Dietary Scores
4.3.3. Dietary Score Uncertainties
4.3.4. Uncertainties on Correlations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennetau-Pelissero, C. Natural Estrogenic Substances, Origins, and Effects. In Bioactive Molecules in Food, Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Lee, A.; Beaubernard, L.; Lamothe, V.; Bennetau-Pelissero, C. New Evaluation of Isoflavone Exposure in the French Population. Nutrients 2019, 11, 2308. [Google Scholar] [CrossRef]
- Lee, A.; Bensaada, S.; Lamothe, V.; Lacoste, M.; Bennetau-Pelissero, C. Endocrine disruptors on and in fruits and vegetables: Estimation of the potential exposure of the French population. Food Chem. 2022, 373, 131513. [Google Scholar] [CrossRef] [PubMed]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a Broad Spectrum of Lignans in Cereals, Oilseeds, and Nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Kläring, K.; Heinzmann, S.S.; Platz, S.; Scholz, B.; Engel, K.-H.; Schmitt-Kopplin, P.; Haller, D.; Rohn, S.; Skurk, T.; et al. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol. Nutr. Food Res. 2015, 59, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Franco, B.; García-González, A.; Montero-Bravo, A.M.; Iglesias-Gutiérrez, E.; Úbeda, N.; Maroto-Núñez, L.; Adlercreutz, H.; Peñalvo, J.L. Dietary Alkylresorcinols and Lignans in the Spanish Diet: Development of the Alignia Database. J. Agric. Food Chem. 2011, 59, 9827–9834. [Google Scholar] [CrossRef] [PubMed]
- Kuijsten, A.; Arts, I.C.W.; Vree, T.B.; Hollman, P.C.H. Pharmacokinetics of Enterolignans in Healthy Men and Women Consuming a Single Dose of Secoisolariciresinol Diglucoside. J. Nutr. 2005, 135, 795–801. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C. Risks and benefits of phytoestrogens: Where are we now? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 477–483. [Google Scholar] [CrossRef]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and Their Human Metabolites Show Distinct Agonistic and Antagonistic Properties on Estrogen Receptor α (ERα) and ERβ in Human Cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef]
- Travis, R.C.; A Spencer, E.; E Allen, N.; Appleby, P.N.; Roddam, A.W.; Overvad, K.; Johnsen, N.F.; Olsen, A.; Kaaks, R.; Linseisen, J.; et al. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2009, 100, 1817–1823. [Google Scholar] [CrossRef]
- Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERα transcriptional activation in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2008, 110, 176–185. [Google Scholar] [CrossRef]
- Power, K.A.; Saarinen, N.M.; Chen, J.M.; Thompson, L.U. Mammalian lignans enterolactone and enterodiol, alone and in combination with the isoflavone genistein, do not promote the growth of MCF-7 xenografts in ovariectomized athymic nude mice. Int. J. Cancer 2006, 118, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Touillaud, M.S.; Thiébaut, A.C.M.; Fournier, A.; Niravong, M.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F. Dietary Lignan Intake and Postmenopausal Breast Cancer Risk by Estrogen and Progesterone Receptor Status. J. Natl. Cancer Inst. 2007, 99, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Respective contribution exerted by AF-1 and AF-2 transactivation functions in estrogen receptor α induced transcriptional activity by isoflavones and equol: Consequence on breast cancer cell proliferation. Mol. Nutr. Food Res. 2009, 53, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Uifălean, A.; Schneider, S.; Ionescu, C.; Lalk, M.; Iuga, C.A. Soy Isoflavones and Breast Cancer Cell Lines: Molecular Mechanisms and Future Perspectives. Molecules 2015, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Allred, C.D.; Allred, K.F.; Ju, Y.H.; Virant, S.M.; Helferich, W.G. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001, 61, 5045–5050. [Google Scholar] [PubMed]
- Shike, M.; Doane, A.S.; Russo, L.; Cabal, R.; Reis-Filo, J.; Gerald, W.; Cody, H.; Khanin, R.; Bromberg, J.; Norton, L. The Effects of Soy Supplementation on Gene Expression in Breast Cancer: A Randomized Placebo-Controlled Study. J. Natl. Cancer Inst. 2014, 106, dju189. [Google Scholar] [CrossRef]
- Messina, M. Impact of Soy Foods on the Development of Breast Cancer and the Prognosis of Breast Cancer Patients. Complement. Med. Res. 2016, 23, 75–80. [Google Scholar] [CrossRef]
- Jiang, R.; Botma, A.; Rudolph, A.; Hüsing, A.; Chang-Claude, J. Phyto-oestrogens and colorectal cancer risk: A systematic review and dose–response meta-analysis of observational studies. Br. J. Nutr. 2016, 116, 2115–2128. [Google Scholar] [CrossRef]
- Nusbaum, J.S.; Mirza, I.; Shum, J.; Freilich, R.; Cohen, R.E.; Pillinger, M.; Izmirly, P.; Buyon, J. Sex Differences in Systemic Lupus Erythematosus: Epidemiology, Clinical Considerations, and Disease Pathogenesis. Mayo Clin. Proc. 2020, 95, 384–394. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Costenbader, K.H. Environmental exposures and the development of systemic lupus erythematosus. Curr. Opin. Rheumatol. 2016, 28, 497–505. [Google Scholar] [CrossRef]
- Shinkaruk, S.; Durand, M.; Lamothe, V.; Carpaye, A.; Martinet, A.; Chantre, P.; Vergne, S.; Nogues, X.; Moore, N.; Bennetau-Pelissero, C. Bioavailability of glycitein relatively to other soy isoflavones in healthy young Caucasian men. Food Chem. 2012, 135, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Shinkaruk, S.; Pinot, E.; Lamothe, V.; Schmitter, J.-M.; Baguenard, L.; Bennetau, B.; Pelissero, C.B. Design and validation of a novel immunological test for enterolactone. Talanta 2014, 119, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Le Houérou, C.; Bennetau-Pelissero, C.; Lamothe, V.; Le Menn, F.; Babin, P.; Bennetau, B. Syntheses of Novel Hapten–Protein Conjugates for Production of Highly Specific Antibodies to Formononetin, Daidzein and Genistein. Tetrahedron 2000, 56, 295–301. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C.; Le Houérou, C.; Lamothe, V.; Le Menn, F.; Babin, P.; Bennetau, B. Synthesis of Haptens and Conjugates for ELISAs of Phytoestrogens. Development of the Immunological Tests. J. Agric. Food Chem. 2000, 48, 305–311. [Google Scholar] [CrossRef] [PubMed]
- van der Velpen, V.; Hollman, P.C.; van Nielen, M.; Schouten, E.G.; Mensink, M.; Veer, P.V.; Geelen, A. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur. J. Clin. Nutr. 2014, 68, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Salomé, M.; Huneau, J.F.; Le Baron, C.; Kesse-Guyot, E.; Fouillet, H.; Mariotti, F. Substituting Meat or Dairy Products with Plant-Based Substitutes Has Small and Heterogeneous Effects on Diet Quality and Nutrient Security: A Simulation Study in French Adults (INCA3). J. Nutr. 2021, 151, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Mathey, J.; Lamothe, V.; Coxam, V.; Potier, M.; Sauvant, P.; Pelissero, C.B. Concentrations of isoflavones in plasma and urine of post-menopausal women chronically ingesting high quantities of soy isoflavones. J. Pharm. Biomed. Anal. 2006, 41, 957–965. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; McTiernan, A.; Tworoger, S.S.; Atkinson, C.; Thomas, W.K.; Stanczyk, F.Z.; Marcovina, S.M.; Weigle, D.S.; Weiss, N.S.; Holt, V.L.; et al. Serum steroid hormones, sex hormone-binding globulin concentrations, and urinary hydroxylated estrogen metabolites in post-menopausal women in relation to daidzein-metabolizing phenotypes. J. Steroid Biochem. Mol. Biol. 2004, 88, 399–408. [Google Scholar] [CrossRef]
- Bensaada, S.; Raymond, I.; Breton, M.; Pellegrin, I.; Viallard, J.-F.; Bennetau-Pelissero, C. Development of an Assay for Soy Isoflavones in Women’s Hair. Nutrients 2022, 14, 3619. [Google Scholar] [CrossRef]
- Atkinson, C.; Newton, K.M.; Stanczyk, F.Z.; Westerlind, K.C.; Li, L.; Lampe, J.W. Daidzein-metabolizing phenotypes in relation to serum hormones and sex hormone binding globulin, and urinary estrogen metabolites in premenopausal women in the United States. Cancer Causes Control 2008, 19, 1085–1093. [Google Scholar] [CrossRef]
- Talbot, D.C.; Ogborne, R.M.; Dadd, T.; Adlercreutz, H.; Barnard, G.; Bugel, S.; Kohen, F.; Marlin, S.; Piron, J.; Cassidy, A.; et al. Monoclonal Antibody-Based Time-Resolved Fluorescence Immunoassays for Daidzein, Genistein, and Equol in Blood and Urine: Application to the Isoheart Intervention Study. Clin. Chem. 2007, 53, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Chandrareddy, A.; Muneyyirci-Delale, O.; McFarlane, S.I.; Murad, O.M. Adverse effects of phytoestrogens on reproductive health: A report of three cases. Complement. Ther. Clin. Pract. 2008, 14, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Nishikawa, H.; Suzuki, A.; Kodama, E.; Iida, T.; Mikura, K.; Hashizume, M.; Kigawa, Y.; Tadokoro, R.; Sugisawa, C.; et al. Secondary Hypogonadism due to Excessive Ingestion of Isoflavone in a Man. Intern. Med. 2022, 61, 2899–2903. [Google Scholar] [CrossRef] [PubMed]
- Vergne, S.; Titier, K.; Bernard, V.; Asselineau, J.; Durand, M.; Lamothe, V.; Potier, M.; Perez, P.; Demotes-Mainard, J.; Chantre, P.; et al. Bioavailability and urinary excretion of isoflavones in humans: Effects of soy-based supplements formulation and equol production. J. Pharm. Biomed. Anal. 2007, 43, 1488–1494. [Google Scholar] [CrossRef]
- Vergne, S.; Bennetau-Pelissero, C.; Lamothe, V.; Chantre, P.; Potier, M.; Asselineau, J.; Perez, P.; Durand, M.; Moore, N.; Sauvant, P. Higher bioavailability of isoflavones after a single ingestion of a soya-based supplement than a soya-based food in young healthy males. Br. J. Nutr. 2008, 99, 333–344. [Google Scholar] [CrossRef]
- Mazur, W.M.; Uehara, M.; Wähälä, K.; Adlercreutz, H. Phyto-oestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after a single strawberry-meal in human subjects. Br. J. Nutr. 2000, 83, 381–387. [Google Scholar] [CrossRef]
- Hålldin, E.; Eriksen, A.K.; Brunius, C.; da Silva, A.B.; Bronze, M.; Hanhineva, K.; Aura, A.; Landberg, R. Factors Explaining Interpersonal Variation in Plasma Enterolactone Concentrations in Humans. Mol. Nutr. Food Res. 2019, 63, e1801159. [Google Scholar] [CrossRef]
- Grace, P.B.; Taylor, J.I.; Low, Y.-L.; Luben, R.N.; Mulligan, A.A.; Botting, N.P.; Dowsett, M.; Welch, A.A.; Khaw, K.-T.; Wareham, N.J.; et al. Phytoestrogen Concentrations in Serum and Spot Urine as Biomarkers for Dietary Phytoestrogen Intake and Their Relation to Breast Cancer Risk in European Prospective Investigation of Cancer and Nutrition-Norfolk. Cancer Epidemiol. Biomark. Prev. 2004, 13, 698–708. [Google Scholar] [CrossRef]
- French, M.R.; Thompson, L.U.; Hawker, G.A. Validation of a phytoestrogen food frequency questionnaire with urinary concentrations of isoflavones and lignan metabolites in premenopausal women. J. Am. Coll. Nutr. 2007, 26, 76–82. [Google Scholar] [CrossRef]
- Ritchie, M.R.; Morton, M.S.; Deighton, N.; Blake, A.; Cummings, J.H. Plasma and urinary phyto-oestrogens as biomarkers of intake: Validation by duplicate diet analysis. Br. J. Nutr. 2004, 91, 447–457. [Google Scholar] [CrossRef]
- Verkasalo, P.K.; Appleby, P.N.; Allen, N.E.; Davey, G.; Adlercreutz, H.; Key, T.J. Soya intake and plasma concentrations of daidzein and genistein: Validity of dietary assessment among eighty British women (Oxford arm of the European Prospective Investigation into Cancer and Nutrition). Br. J. Nutr. 2001, 86, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Heald, C.L.; Bolton-Smith, C.; Ritchie, M.R.; Morton, M.S.; E Alexander, F. Phyto-oestrogen intake in Scottish men: Use of serum to validate a self-administered food-frequency questionnaire in older men. Eur. J. Clin. Nutr. 2006, 60, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-H.; Harrison, G.G.; Mohamed, M.M.; Gornbein, J.A.; Henning, S.M.; Go, V.L.W.; Greendale, G.A. Assessing the Accuracy of a Food Frequency Questionnaire for Estimating Usual Intake of Phytoestrogens. Nutr. Cancer 2000, 37, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Skor, H.E.; Fitzgibbons, E.D.; Scholes, D.; Chen, C.; Wähälä, K.; Schwartz, S.M.; Lampe, J.W. Overnight urinary isoflavone excretion in a population of women living in the United States, and its relationship to isoflavone intake. Cancer Epidemiol. Biomark. Prev. 2002, 11, 253–260. [Google Scholar]
- Kim, J.; Kim, H.J.; Joung, H.; Park, M.K.; Li, S.; Song, Y.; Franke, A.A.; Paik, H.-Y. Overnight urinary excretion of isoflavones as an indicator for dietary isoflavone intake in Korean girls of pubertal age. Br. J. Nutr. 2010, 104, 709–715. [Google Scholar] [CrossRef]
- Morimoto, Y.; Beckford, F.; A Franke, A.; Maskarinec, G. Urinary isoflavonoid excretion as a biomarker of dietary soy intake during two randomized soy trials. Asia Pac. J. Clin. Nutr. 2014, 23, 205. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Suárez, K.M.; Ortega-Vélez, M.I.; Valenzuela-Quintanar, A.I.; Galván-Portillo, M.; López-Carrillo, L.; Esparza-Romero, J.; Saucedo-Tamayo, M.S.; Robles-Burgueño, M.R.; Palma-Durán, S.A.; Gutiérrez-Coronado, M.L.; et al. Phytoestrogen Concentrations in Human Urine as Biomarkers for Dietary Phytoestrogen Intake in Mexican Women. Nutrients 2017, 9, 1078. [Google Scholar] [CrossRef]
- Xiang, H.; Schevzov, G.; Gunning, P.; Williams, H.M.; Silink, M. A Comparative Study of Growth-Inhibitory Effects of Isoflavones and Their Metabolites on Human Breast and Prostate Cancer Cell Lines. Nutr. Cancer 2002, 42, 224–232. [Google Scholar] [CrossRef]
- Angeloni, S.; Navarini, L.; Khamitova, G.; Sagratini, G.; Vittori, S.; Caprioli, G. Quantification of lignans in 30 ground coffee samples and evaluation of theirs extraction yield in espresso coffee by HPLC-MS/MS triple quadrupole. Int. J. Food Sci. Nutr. 2019, 71, 193–200. [Google Scholar] [CrossRef]
- Horn-Ross, P.L.; Lee, M.; John, E.M.; Koo, J. Sources of Phytoestrogen Exposure among Non-Asian Women in California, USA. Cancer Causes Control 2000, 11, 299–302. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen Content of Beverages, Nuts, Seeds, and Oils. J. Agric. Food Chem. 2008, 56, 7311–7315. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen Content of Cereals and Cereal-Based Foods Consumed in the UK. Nutr. Cancer 2009, 61, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Joosen, A.M.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of fruits and vegetables commonly consumed in the UK based on LC–MS and 13C-labelled standards. Food Chem. 2009, 116, 542–554. [Google Scholar] [CrossRef]
- Milder, I.E.J.; Feskens, E.J.M.; Arts, I.C.W.; de Mesquita, H.B.B.; Hollman, P.C.H.; Kromhout, D. Intake of the Plant Lignans Secoisolariciresinol, Matairesinol, Lariciresinol, and Pinoresinol in Dutch Men and Women. J. Nutr. 2005, 135, 1202–1207. [Google Scholar] [CrossRef]
- Nurmi, T.; Heinonen, S.; Mazur, W.; Deyama, T.; Nishibe, S.; Adlercreutz, H. Lignans in selected wines. Food Chem. 2003, 83, 303–309. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Haajanen, K.M.; Botting, N.; Adlercreutz, H. Quantification of Lignans in Food Using Isotope Dilution Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 9342–9347. [Google Scholar] [CrossRef]
- Tetens, I.; Turrini, A.; Tapanainen, H.; Christensen, T.; Lampe, J.W.; Fagt, S.; Håkansson, N.; Lundquist, A.; Hallund, J.; Valsta, L.M. Dietary intake and main sources of plant lignans in five European countries. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen Content of Foods Consumed in Canada, Including Isoflavones, Lignans, and Coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef]
- Valsta, L.M.; Kilkkinen, A.; Mazur, W.; Nurmi, T.; Lampi, A.-M.; Ovaskainen, M.-L.; Korhonen, T.; Adlercreutz, H.; Pietinen, P. Phyto-oestrogen database of foods and average intake in Finland. Br. J. Nutr. 2003, 89, S31–S38. [Google Scholar] [CrossRef]
| PlasmaIFs (n = 26) | UrineIfs * (n = 54) | UrineEQ * (n = 46) | PlasmaENL (n= 57) | UrineENL * (n = 57) | |
|---|---|---|---|---|---|
| Mean (µg.L−1) | 74.95 | 8484.94 | 72.99 | 17.89 | 802.53 |
| Standard deviation (µg.L−1) | 147.40 | 61,775.14 | 219.72 | 11.71 | 1438.85 |
| Median (µg.L−1) | 21.21 | 124.32 | 21.63 | 13.68 | 304.73 |
| Min (µg.L−1) | 1.17 | 9.53 | 3.37 | 1.94 | 6.76 |
| Max (µg.L−1) | 729.60 | 454,384.51 | 1469.81 | 57.165 | 8076.13 |
| Producer characteristics (%) | - | - | 56.52 a | 7.01 b | 10.53 b |
| Significant consumers (%) ** | 29.82 | 38.60 | - | 40.35 | 36.84 |
| Dietary Scores | ||||
|---|---|---|---|---|
| PlasmaIFs (n = 26) | UrineIFs (n = 54) | PlasmaENL (n = 57) | UrineENL (n = 57) | |
| Mean | 3.92 | 3.11 | 12.38 | 12.11 |
| Standard deviation | 3.43 | 2.85 | 5.57 | 5.94 |
| Median | 2.62 | 2.40 | 11.73 | 11.40 |
| Min | 0.63 | 0.40 | 3.2 | 3.20 |
| Max | 16.56 | 16.90 | 38.35 | 42.80 |
| Significant consumers (%) * | 42.11 | 38.60 | 40.35 | 59.65 |
| Subjects | Nature of Samples | Biomarkers | Dietary Data (mg.day−1) | Correlation | References |
|---|---|---|---|---|---|
| 80 British volunteers | Plasma | GEN DAI | 7-day food diaries | GEN: r = 0.80; p < 0.001 DAI: r = 0,45; p < 0.001 | [42] |
| 77 volunteers | Plasma | GEN DAI | FFQ | GEN: r = 0,53; p < 0.001 DAI: r = 0,45; p < 0.001 | [29] |
| 14 adults (14% men) | Plasma | IFs | 24 h food record: (11.0) 24 h recall: (12.3) | IFs: r = 0.92; p < 0.001 | [41] |
| 333 volunteers | Serum | IFs | 7-day food diaries | IFs r = 0.31; p < 0.001 | [39] |
| 203 male volunteers | Serum | IFs | FFQ | IFs: r = 0.27; p < 0.001 | [43] |
| 26 French women | Spot plasma | IFs | 24 h and 48 h dietary recall | IFs: r = 0.900; p < 0.001 GEN: r = 0.921; p < 0.001 DAI: r = 0.846; p < 0.001 | Present study |
| 51 Japanese women 18 Caucasian women | 24 h urine | GEN DAI | 48 h dietary recall | GEN: r = 0.54; p < 0.001 DAI: r = 0.58; p < 0.001 | [44] |
| 360 women | 2 overnight urines | IFs | Twice, 24 h recall DAI (µg): (5.0–6.4) GEN (µg): (7.3–9.3) | IFs: r = 0.52; p = 0.001 FFQ: r = 0.29; p < 0.01 | [45] |
| 284 volunteers | Spot urine | IFs | 7-day food diaries | IFs r = 0.27; p < 0.001 | [39] |
| 14 adults (14% men) | 24 h urine | IFs | 24 h food record: (11.0) 24 h recall: (12.3) | IFs: r = 0.97; p < 0.001 | [41] |
| 26 premenopausal Canadian women | 24 h urine | IFs | Habitual record 24 h recall | IFs: r = 0.64, p < 0.001 IFs: r = 0.54, p = 0.004 | [40] |
| 24 pubertal girls | 12 h urine | IFs | 3-day 24 h recall ISO: 3.0–13.3 | lFs: r = 0.72; p < 0.001 | [46] |
| 256 premenopausal women | 12 h urine | IFs | FFQ Low: 0.1–2.3 High: 49.8–74.6 | IFs: r = 0.51; p < 0.001 | [47] |
| 100 healthy women | 12 h urine | IFs | 24 h recall | IFs: r = 0.460; p < 0.001 | [48] |
| 57 French women | Spot urine | IFs | 24 and 48 h dietary recall | IFs: r = 0.921; p < 0.001 GEN: r = 0.885; p < 0.001 DAI: r = 0.919; p < 0.001 | Present study |
| 284 British women of the EPIC-Norfolk study | Spot urine | ENL | Fiber intake over 7-day recall | ENL: r = 0.29; p < 0.001 | [39] |
| 26 premenopausal Canadian women | 24 h urine | ENL | Habitual record 24 h recall | ENL: r = 0.46, p = 0.02 ENL: r = 0.40, p = 0.05 | [40] |
| 100 apparently healthy Mexican women | 12 h urine | ENL | 24 h recall | ENL: r = 0.067; p = 0.580 | [48] |
| 57 healthy premenopausal French women | Spot urine Spot plasma | ENL | 24 and 48 h dietary recalls | ENL: r = 0.764; p < 0.001 ENL: r = 0.723; p < 0.001 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensaada, S.; Raymond, I.; Pellegrin, I.; Viallard, J.-F.; Bennetau-Pelissero, C. Validation of ELISAs for Isoflavones and Enterolactone for Phytoestrogen Intake Assessment in the French Population. Nutrients 2023, 15, 967. https://doi.org/10.3390/nu15040967
Bensaada S, Raymond I, Pellegrin I, Viallard J-F, Bennetau-Pelissero C. Validation of ELISAs for Isoflavones and Enterolactone for Phytoestrogen Intake Assessment in the French Population. Nutrients. 2023; 15(4):967. https://doi.org/10.3390/nu15040967
Chicago/Turabian StyleBensaada, Souad, Isabelle Raymond, Isabelle Pellegrin, Jean-François Viallard, and Catherine Bennetau-Pelissero. 2023. "Validation of ELISAs for Isoflavones and Enterolactone for Phytoestrogen Intake Assessment in the French Population" Nutrients 15, no. 4: 967. https://doi.org/10.3390/nu15040967
APA StyleBensaada, S., Raymond, I., Pellegrin, I., Viallard, J.-F., & Bennetau-Pelissero, C. (2023). Validation of ELISAs for Isoflavones and Enterolactone for Phytoestrogen Intake Assessment in the French Population. Nutrients, 15(4), 967. https://doi.org/10.3390/nu15040967






