New Perspective on Anorexia Nervosa: Tryptophan-Kynurenine Pathway Hypothesis
Abstract
1. Introduction
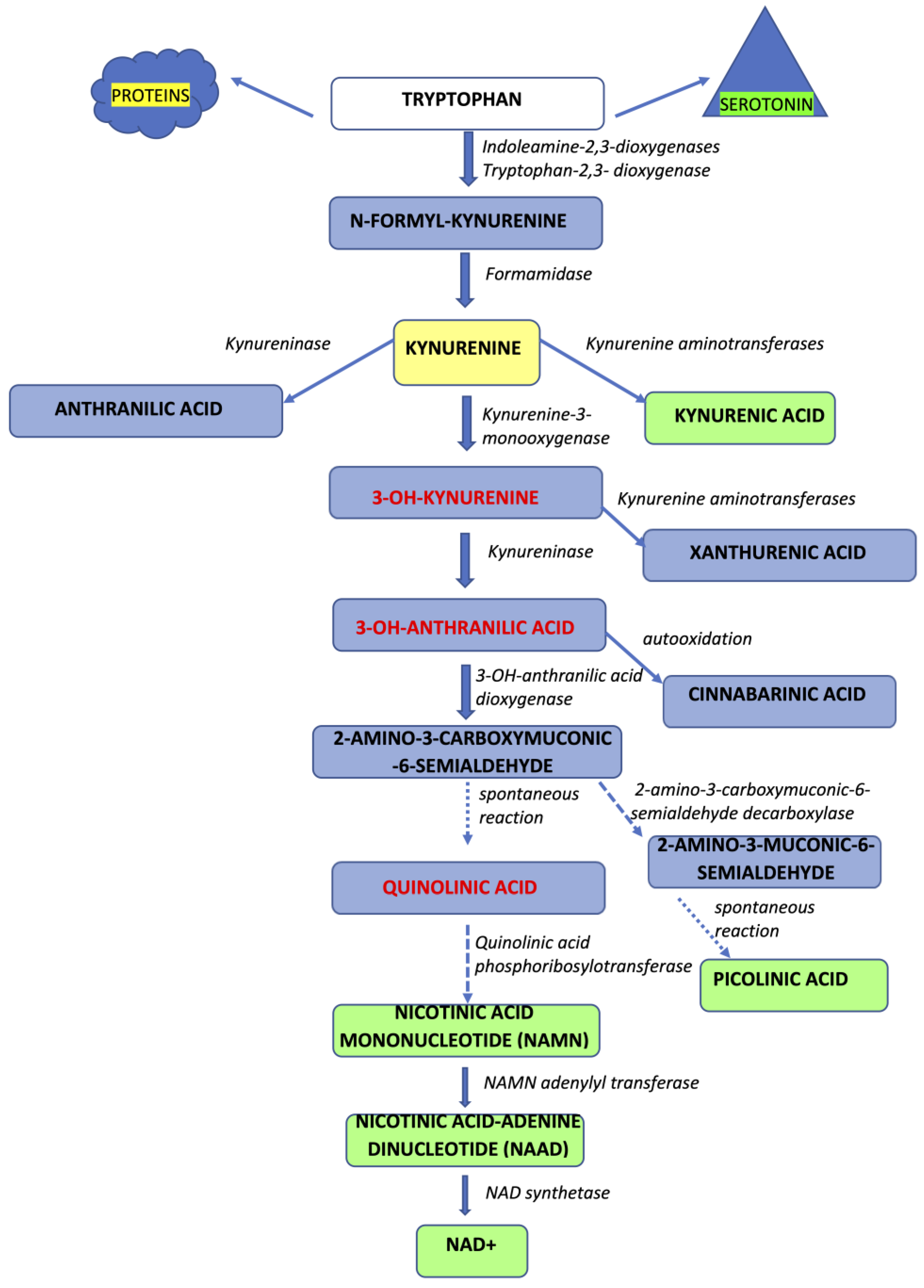
2. The Kynurenine Pathway
3. Tryptophan and Kynurenines in Anorexia Nervosa
3.1. Tryptophan
3.2. L-Kynurenine and Kynurenic Acid
3.3. 3-Hydroxykynurenine and Quinolinic Acid
3.4. Enzymes of the Kynurenine Pathway
4. Dietary Interventions
5. Concluding Remarks and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Eeden, A.E.; van Hoeken, D.; Hoek, H.W. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr. Opin. Psychiatry 2021, 34, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Silén, Y.; Keski-Rahkonen, A. Worldwide prevalence of DSM-5 eating disorders among young people. Curr. Opin. Psychiatry 2022, 35, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F. Mortality in anorexia nervosa. Am. J. Psychiatry 1995, 152, 1073–1074. [Google Scholar] [CrossRef]
- Keel, P.K.; Brown, T.A. Update on course and outcome in eating disorders. Int. J. Eat. Disord. 2010, 43, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ratnasuriya, R.H.; Eisler, I.; Szmukler, G.I.; Russell, G.F. Anorexia nervosa: Outcome and prognostic factors after 20 years. Br. J. Psychiatry 1991, 158, 495–502. [Google Scholar] [CrossRef]
- Zipfel, S.; Löwe, B.; Reas, D.L.; Deter, H.C.; Herzog, W. Long-term prognosis in anorexia nervosa: Lessons from a 21-year follow-up study. Lancet 2000, 355, 721–722. [Google Scholar] [CrossRef]
- Moore, C.A.; Bokor, B.R. Anorexia Nervosa. In StatPearls; StatPearls Publishing. Copyright © 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Walsh, B.T. The enigmatic persistence of anorexia nervosa. Am. J. Psychiatry 2013, 170, 477–484. [Google Scholar] [CrossRef]
- Di Lodovico, L.; Hanachi, M.; Duriez, P.; Gorwood, P. The Fitter I Am, the Larger I Feel-The Vicious Circle of Physical Exercise in Anorexia Nervosa. Nutrients 2022, 14, 4507. [Google Scholar] [CrossRef]
- Titova, O.E.; Hjorth, O.C.; Schiöth, H.B.; Brooks, S.J. Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: A meta-analysis of VBM studies. BMC Psychiatry 2013, 13, 110. [Google Scholar] [CrossRef]
- Seitz, J.; Bühren, K.; von Polier, G.G.; Heussen, N.; Herpertz-Dahlmann, B.; Konrad, K. Morphological changes in the brain of acutely ill and weight-recovered patients with anorexia nervosa. A meta-analysis and qualitative review. Z Kinder Jugendpsychiatr. Psychother. 2014, 42, 7–17; quiz 17–18. [Google Scholar] [CrossRef]
- King, J.A.; Geisler, D.; Ritschel, F.; Boehm, I.; Seidel, M.; Roschinski, B.; Soltwedel, L.; Zwipp, J.; Pfuhl, G.; Marxen, M.; et al. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biol. Psychiatry 2015, 77, 624–632. [Google Scholar] [CrossRef]
- Nickel, K.; Joos, A.; Tebartz van Elst, L.; Matthis, J.; Holovics, L.; Endres, D.; Zeeck, A.; Hartmann, A.; Tüscher, O.; Maier, S. Recovery of cortical volume and thickness after remission from acute anorexia nervosa. Int. J. Eat. Disord. 2018, 51, 1056–1069. [Google Scholar] [CrossRef]
- Riva, G. Neurobiology of Anorexia Nervosa: Serotonin Dysfunctions Link Self-Starvation with Body Image Disturbances through an Impaired Body Memory. Front. Hum. Neurosci. 2016, 10, 600. [Google Scholar] [CrossRef]
- Russo, S.; Kema, I.P.; Bosker, F.; Haavik, J.; Korf, J. Tryptophan as an evolutionarily conserved signal to brain serotonin: Molecular evidence and psychiatric implications. World J. Biol. Psychiatry 2009, 10, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Fudge, J.L.; Paulus, M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 2009, 10, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Seitz, J.; Kipp, M. The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa. J. Clin. Med. 2021, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Ogbechi, J.; Clanchy, F.I.; Williams, R.O.; Stone, T.W. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front. Immunol. 2020, 11, 388. [Google Scholar] [CrossRef]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci. 2022, 28, 19–35. [Google Scholar] [CrossRef]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: Disease and healthy States. Int. J. Tryptophan Res. 2009, 2, IJTR-S2097. [Google Scholar] [CrossRef]
- Fujigaki, H.; Saito, K.; Fujigaki, S.; Takemura, M.; Sudo, K.; Ishiguro, H.; Seishima, M. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: Involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J. Biochem. 2006, 139, 655–662. [Google Scholar] [CrossRef]
- Asp, L.; Johansson, A.S.; Mann, A.; Owe-Larsson, B.; Urbanska, E.M.; Kocki, T.; Kegel, M.; Engberg, G.; Lundkvist, G.B.; Karlsson, H. Effects of pro-inflammatory cytokines on expression of kynurenine pathway enzymes in human dermal fibroblasts. J. Inflamm. (Lond) 2011, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef]
- Acharyya, S.; Ladner, K.J.; Nelsen, L.L.; Damrauer, J.; Reiser, P.J.; Swoap, S.; Guttridge, D.C. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Invest. 2004, 114, 370–378. [Google Scholar] [CrossRef]
- Dafny, N.; Yang, P.B. Interferon and the central nervous system. Eur. J. Pharm. 2005, 523, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef]
- Sathyasaikumar, K.V.; Tararina, M.; Wu, H.Q.; Neale, S.A.; Weisz, F.; Salt, T.E.; Schwarcz, R. Xanthurenic Acid Formation from 3-Hydroxykynurenine in the Mammalian Brain: Neurochemical Characterization and Physiological Effects. Neuroscience 2017, 367, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hirota, K.; Christensen, J.; O’Garra, A.; Stockinger, B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 2009, 206, 43–49. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef]
- Rossi, F.; Miggiano, R.; Ferraris, D.M.; Rizzi, M. The Synthesis of Kynurenic Acid in Mammals: An Updated Kynurenine Aminotransferase Structural KATalogue. Front. Mol. Biosci. 2019, 6, 7. [Google Scholar] [CrossRef]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef]
- Fellendorf, F.T.; Bonkat, N.; Dalkner, N.; Schönthaler, E.M.D.; Manchia, M.; Fuchs, D.; Reininghaus, E.Z. Indoleamine 2,3-dioxygenase (IDO)-activity in Severe Psychiatric Disorders: A Systemic Review. Curr. Top Med. Chem. 2022, 22, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Corcos, M.; Guilbaud, O.; Paterniti, S.; Moussa, M.; Chambry, J.; Chaouat, G.; Consoli, S.M.; Jeammet, P. Involvement of cytokines in eating disorders: A critical review of the human literature. Psychoneuroendocrinology 2003, 28, 229–249. [Google Scholar] [CrossRef]
- Haleem, D.J. Decreases of plasma tryptophan concentrations following restricted feeding do not decrease serotonin and its metabolite in rat brain. Nahrung 1994, 38, 606–611. [Google Scholar] [CrossRef]
- Haleem, D.J.; Jabeen, B.; Parveen, T. Inhibition of restraint-induced anorexia by injected tryptophan. Life Sci. 1998, 63, PL205–PL212. [Google Scholar] [CrossRef]
- Haider, S.; Haleem, D.J. Decreases of brain serotonin following a food restriction schedule of 4 weeks in male and female rats. Med. Sci. Monit. 2000, 6, 1061–1067. [Google Scholar]
- Lemieux, G.A.; Cunningham, K.A.; Lin, L.; Mayer, F.; Werb, Z.; Ashrafi, K. Kynurenic acid is a nutritional cue that enables behavioral plasticity. Cell 2015, 160, 119–131. [Google Scholar] [CrossRef]
- Hassanyeh, F.; Marshall, E.F. Measures of serotonin metabolism in anorexia nervosa. Acta Psychiatr. Scand. 1991, 84, 561–563. [Google Scholar] [CrossRef]
- Favaro, A.; Caregaro, L.; Burlina, A.B.; Santonastaso, P. Tryptophan levels, excessive exercise, and nutritional status in anorexia nervosa. Psychosom. Med. 2000, 62, 535–538. [Google Scholar] [CrossRef]
- Bossola, M.; Scribano, D.; Colacicco, L.; Tavazzi, B.; Giungi, S.; Zuppi, C.; Luciani, G.; Tazza, L. Anorexia and plasma levels of free tryptophan, branched chain amino acids, and ghrelin in hemodialysis patients. J. Ren. Nutr. 2009, 19, 248–255. [Google Scholar] [CrossRef]
- Attia, E.; Wolk, S.; Cooper, T.; Glasofer, D.; Walsh, B.T. Plasma tryptophan during weight restoration in patients with anorexia nervosa. Biol. Psychiatry 2005, 57, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Franke, L.; Schneider, N.; Salbach-Andrae, H.; Schott, R.; Craciun, E.M.; Pfeiffer, E.; Uebelhack, R.; Lehmkuhl, U. Aromatic amino acids in weight-recovered females with anorexia nervosa. Int. J. Eat. Disord. 2009, 42, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Hassler, C.; Mattar, L.; Launay, J.M.; Callebert, J.; Steiger, H.; Melchior, J.C.; Falissard, B.; Berthoz, S.; Mourier-Soleillant, V.; et al. Symptoms of depression and anxiety in anorexia nervosa: Links with plasma tryptophan and serotonin metabolism. Psychoneuroendocrinology 2014, 39, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Askenazy, F.; Candito, M.; Caci, H.; Myquel, M.; Chambon, P.; Darcourt, G.; Puech, A.J. Whole blood serotonin content, tryptophan concentrations, and impulsivity in anorexia nervosa. Biol. Psychiatry 1998, 43, 188–195. [Google Scholar] [CrossRef]
- Comai, S.; Bertazzo, A.; Carretti, N.; Podfigurna-Stopa, A.; Luisi, S.; Costa, C.V. Serum levels of tryptophan, 5-hydroxytryptophan and serotonin in patients affected with different forms of amenorrhea. Int. J. Tryptophan Res. 2010, 3, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Barbarich, N.C.; Putnam, K.; Gendall, K.A.; Fernstrom, J.; Fernstrom, M.; McConaha, C.W.; Kishore, A. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int. J. Eat. Disord. 2003, 33, 257–267. [Google Scholar] [CrossRef]
- Demitrack, M.A.; Heyes, M.P.; Altemus, M.; Pigott, T.A.; Gold, P.W. Cerebrospinal fluid levels of kynurenine pathway metabolites in patients with eating disorders: Relation to clinical and biochemical variable. Biol. Psychiatry 1995, 37, 512–520. [Google Scholar] [CrossRef]
- Dudzińska, E.; Szymona, K.; Kloc, R.; Kocki, T.; Gil-Kulik, P.; Bogucki, J.; Kocki, J.; Paduch, R.; Urbańska, E.M. Fractalkine, sICAM-1 and Kynurenine Pathway in Restrictive Anorexia Nervosa-Exploratory Study. Nutrients 2021, 13, 339. [Google Scholar] [CrossRef]
- Franzago, M.; Orecchini, E.; Porreca, A.; Mondanelli, G.; Orabona, C.; Dalla Ragione, L.; Di Nicola, M.; Stuppia, L.; Vitacolonna, E.; Beccari, T.; et al. SLC6A4 DNA Methylation Levels and Serum Kynurenine/Tryptophan Ratio in Eating Disorders: A Possible Link with Psychopathological Traits? Nutrients 2023, 15, 406. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Nobis, S.; Breton, J.; Jésus, P.; Belmonte, L.; Maurer, B.; Legrand, R.; Bôle-Feysot, C.; do Rego, J.L.; Goichon, A.; et al. Maintaining physical activity during refeeding improves body composition, intestinal hyperpermeability and behavior in anorectic mice. Sci. Rep. 2016, 6, 21887. [Google Scholar] [CrossRef] [PubMed]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. (Lausanne) 2019, 10, 158. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Wurtman, R.J. Brain serotonin content: Increase following ingestion of carbohydrate diet. Science 1971, 174, 1023–1025. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Wurtman, R.J. Brain serotonin content: Physiological regulation by plasma neutral amino acids. Science 1972, 178, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J.; Wurtman, J.J. Brain serotonin, carbohydrate-craving, obesity and depression. Obes. Res. 1995, 3 (Suppl. 4), 477s–480s. [Google Scholar] [CrossRef]
- Molfino, A.; Muscaritoli, M.; Cascino, A.; Fanfarillo, F.; Fava, A.; Bertini, G.; Citro, G.; Rossi Fanelli, F.; Laviano, A. Free tryptophan/large neutral amino acids ratios in blood plasma do not predict cerebral spinal fluid tryptophan concentrations in interleukin-1-induced anorexia. Pharm. Biochem. Behav. 2008, 89, 31–35. [Google Scholar] [CrossRef]
- Pajak, B.; Orzechowska, S.; Pijet, B.; Pijet, M.; Pogorzelska, A.; Gajkowska, B.; Orzechowski, A. Crossroads of cytokine signaling--the chase to stop muscle cachexia. J. Physiol. Pharm. 2008, 59 (Suppl. 9), 251–264. [Google Scholar]
- Asarian, L.; Langhans, W. A new look on brain mechanisms of acute illness anorexia. Physiol. Behav. 2010, 100, 464–471. [Google Scholar] [CrossRef]
- van Donkelaar, E.L.; Blokland, A.; Ferrington, L.; Kelly, P.A.; Steinbusch, H.W.; Prickaerts, J. Mechanism of acute tryptophan depletion: Is it only serotonin? Mol. Psychiatry 2011, 16, 695–713. [Google Scholar] [CrossRef]
- Ehrlich, S.; Salbach-Andrae, H.; Weiss, D.; Burghardt, R.; Goldhahn, K.; Craciun, E.M.; Franke, L.; Uebelhack, R.; Klapp, B.F.; Lehmkuhl, U. S100B in underweight and weight-recovered patients with anorexia nervosa. Psychoneuroendocrinology 2008, 33, 782–788. [Google Scholar] [CrossRef]
- Gerner, R.H.; Cohen, D.J.; Fairbanks, L.; Anderson, G.M.; Young, J.G.; Scheinin, M.; Linnoila, M.; Shaywitz, B.A.; Hare, T.A. CSF neurochemistry of women with anorexia nervosa and normal women. Am. J. Psychiatry 1984, 141, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.; Griffin, É.W.; O’Loughlin, E.; Lyons, A.; Sherwin, E.; Ahmed, S.; Stevenson, N.J.; Harkin, A.; Cunningham, C. Interdependent and independent roles of type I interferons and IL-6 in innate immune, neuroinflammatory and sickness behaviour responses to systemic poly I:C. Brain Behav. Immun. 2015, 48, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Campion, S.; Teeling, J.; Felton, L.; Perry, V.H. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C). Brain Behav. Immun. 2007, 21, 490–502. [Google Scholar] [CrossRef]
- Field, R.; Campion, S.; Warren, C.; Murray, C.; Cunningham, C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav. Immun. 2010, 24, 996–1007. [Google Scholar] [CrossRef]
- Wilinski, D.; Winzeler, J.; Duren, W.; Persons, J.L.; Holme, K.J.; Mosquera, J.; Khabiri, M.; Kinchen, J.M.; Freddolino, P.L.; Karnovsky, A.; et al. Rapid metabolic shifts occur during the transition between hunger and satiety in Drosophila melanogaster. Nat. Commun. 2019, 10, 4052. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Summers, K.L.; Meinitzer, A.; Zelzer, S.; Almer, G.; Prassl, R.; Schnedl, W.J.; Reininghaus, E.; Paulmichl, K.; Weghuber, D.; et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: Role of age and parameters of the metabolic syndrome. Obesity (Silver Spring) 2014, 22, 195–201. [Google Scholar] [CrossRef]
- Connick, J.; Lombardi, G.; Beni, M.; Moroni, F. Decrease in rat cerebral quinolinic acid concentration following chronic hydrocortisone treatment. Neurosci. Lett. 1988, 88, 216–220. [Google Scholar] [CrossRef]
- Yates, J.R.; Gay, E.A.; Heyes, M.P.; Blight, A.R. Effects of methylprednisolone and 4-chloro-3-hydroxyanthranilic acid in experimental spinal cord injury in the guinea pig appear to be mediated by different and potentially complementary mechanisms. Spinal. Cord. 2014, 52, 662–666. [Google Scholar] [CrossRef]
- Saito, K.; Markey, S.P.; Heyes, M.P. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience 1992, 51, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Laugeray, A.; Launay, J.M.; Callebert, J.; Surget, A.; Belzung, C.; Barone, P.R. Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behav. Brain Res. 2010, 210, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Okuno, E.; Schwarcz, R. Characterization of rat brain kynurenine aminotransferases I and II. J. Neurosci. Res. 1997, 50, 457–465. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Liesbrock, J.; Leunissen, C.; Kempermann, J.; Etdöger, S.; Kas, M.J.; Tolba, R.; Heussen, N.; Neulen, J.; et al. The reduction of astrocytes and brain volume loss in anorexia nervosa-the impact of starvation and refeeding in a rodent model. Transl. Psychiatry 2019, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Haro, D. Glial cells in anorexia. Front. Cell Neurosci. 2022, 16, 983577. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef]
- Gul, S.; Saleem, D.; Haleem, M.A.; Haleem, D.J. Inhibition of hormonal and behavioral effects of stress by tryptophan in rats. Nutr. Neurosci. 2019, 22, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Laviano, A.; Meguid, M.M.; Chen, C.; Rossi-Fanelli, F.; Hatakeyama, K. Involvement of plasma leptin, insulin and free tryptophan in cytokine-induced anorexia. Clin. Nutr. 2003, 22, 139–146. [Google Scholar] [CrossRef]
- Hernandez, L.; Parada, M.; Baptista, T.; Schwartz, D.; West, H.L.; Mark, G.P.; Hoebel, B.G. Hypothalamic serotonin in treatments for feeding disorders and depression as studied by brain microdialysis. J. Clin. Psychiatry 1991, 52, 32–40. [Google Scholar]
- Saeed, R.; Mahmood, K.; Ali, S.B.; Haleem, D.J. Prevention of diet restriction induced hyperactivity but not body-weight reduction in rats co-treated with tryptophan: Relationship with striatal serotonin and dopamine metabolism and serotonin-1A auto-receptor expression. Nutr. Neurosci. 2022, 25, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Silverstone, T.; Goodall, E. Serotoninergic mechanisms in human feeding: The pharmacological evidence. Appetite 1986, 7, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Hill, A.J. Influence of tryptophan on appetite and food selection in man. In Amino Acids in Health and Disease: New Perspectives; Kaufman, S., Alan, R., Eds.; Liss: New York, NY, USA, 1987; Volume 55, pp. 403–419. [Google Scholar]
- Poesen, R.; Mutsaers, H.A.; Windey, K.; van den Broek, P.H.; Verweij, V.; Augustijns, P.; Kuypers, D.; Jansen, J.; Evenepoel, P.; Verbeke, K.; et al. The Influence of Dietary Protein Intake on Mammalian Tryptophan and Phenolic Metabolites. PLoS ONE 2015, 10, e0140820. [Google Scholar] [CrossRef] [PubMed]
- Dencker, M.; Björgell, O.; Hlebowicz, J. Effect of food intake on 92 neurological biomarkers in plasma. Brain Behav. 2017, 7, e00747. [Google Scholar] [CrossRef]
- Barbarich, N.C.; McConaha, C.W.; Halmi, K.A.; Gendall, K.; Sunday, S.R.; Gaskill, J.; La Via, M.; Frank, G.K.; Brooks, S.; Plotnicov, K.H.; et al. Use of nutritional supplements to increase the efficacy of fluoxetine in the treatment of anorexia nervosa. Int. J. Eat. Disord. 2004, 35, 10–15. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Shapiro, C.M.; Bennie, J.; Dick, H.; Carroll, S.; Fink, G. The neuroendocrine responses and psychological effects of infusion of L-tryptophan in anorexia nervosa. Psychol. Med. 1989, 19, 857–864. [Google Scholar] [CrossRef]
- Van der Does, A.J. The effects of tryptophan depletion on mood and psychiatric symptoms. J. Affect Disord 2001, 64, 107–119. [Google Scholar] [CrossRef]
- Boehm, I.; Hennig, J.; Ritschel, F.; Geisler, D.; King, J.A.; Lesch, I.; Roessner, V.; Zepf, F.D.; Ehrlich, S. Acute tryptophan depletion balances altered resting-state functional connectivity of the salience network in female patients recovered from anorexia nervosa. J. Psychiatry Neurosci. 2022, 47, E351–E358. [Google Scholar] [CrossRef]
- Weinert, T.; Bernardoni, F.; King, J.; Steding, J.; Boehm, I.; Mannigel, M.; Ritschel, F.; Zepf, F.; Roessner, V.; Ehrlich, S. No effects of acute tryptophan depletion on anxiety or mood in weight-recovered female patients with anorexia nervosa. Eur. Arch. Psychiatry Clin. Neurosci. 2022. [Google Scholar] [CrossRef]
- Steding, J.; Ritschel, F.; Boehm, I.; Geisler, D.; King, J.A.; Roessner, V.; Smolka, M.N.; Zepf, F.D.; Ehrlich, S. The effects of acute tryptophan depletion on instrumental reward learning in anorexia nervosa—An fMRI study. Psychol. Med. 2022, Mar 28, 1–11. [Google Scholar] [CrossRef]
- Kerley-Hamilton, J.S.; Trask, H.W.; Ridley, C.J.; Dufour, E.; Ringelberg, C.S.; Nurinova, N.; Wong, D.; Moodie, K.L.; Shipman, S.L.; Moore, J.H.; et al. Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a Western diet. Env. Health Perspect. 2012, 120, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Moyer, B.J.; Rojas, I.Y.; Kerley-Hamilton, J.S.; Hazlett, H.F.; Nemani, K.V.; Trask, H.W.; West, R.J.; Lupien, L.E.; Collins, A.J.; Ringelberg, C.S.; et al. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGFβ, and IDO1. Toxicol. Appl. Pharm. 2016, 300, 13–24. [Google Scholar] [CrossRef] [PubMed]
| Metabolites/Enzymes | Conditions | Type of Samples | Results | Reference Number |
|---|---|---|---|---|
| Tryptophan | Four-day starvation in rats | Brain | ↓ | [39,40] |
| Four-week starvation in rats | Plasma | ↓ | [41] | |
| Brain (hypothalamus) | ↓ | [41] | ||
| Caenorhabditis elegans, in culture, 2 h fasting | Extracts from C. elegans | ←→ | [42] | |
| AN (n = 35) | Plasma | ←→ | [43] | |
| AN (n = 16) | Blood | ←→ | [44] | |
| Anorectic, dialyzed (n = 13) | Plasma | ←→ | [45] | |
| AN (n = 26) | Plasma | ↓ | [46] | |
| Acute AN (n = 32), Recovered AN (n = 32) | Plasma | ↓ | [47] | |
| AN (n = 42) | Plasma | ↓ | [48] | |
| AN (n = 19) | Whole blood | ↓ | [49] | |
| AN (n = 16) | Serum | ↓ | [50] | |
| Acute AN (n = 14), Recovered AN (n = 14) | Plasma | ↓ acute AN vs. recovered ←→ vs. CTR | [51] | |
| Medication-free AN (n = 10) | CSF | ←→ | [52] | |
| Restrictive AN (n = 20) | Serum | ←→ | [53] | |
| Purging AN (n = 21) | Serum | ↑ | [54] | |
| Tryptophan/LNAA ratio | AN (n = 16) | Blood | ↑ in more severe catabolic status | [44] |
| Anorectic, dialyzed (n = 13) | Plasma | ←→ | [45] | |
| AN (n = 19) | Whole blood | ↓ | [49] | |
| Acute AN (n = 14), Recovered AN (n = 14) | Plasma | ↑ | [51] | |
| AN (n = 42) | Plasma | ↓ | [48] | |
| L-kynurenine | Caenorhabditis elegans, in culture, 2 h fasting | Extracts from C. elegans | ↓ | [42] |
| Medication-free AN (n = 10) | CSF | ←→ | [52] | |
| Restrictive AN (n = 20) | Serum | ←→ | [53] | |
| Restrictive AN (n = 45), purging AN (n = 21) | Serum | ←→ | [54] | |
| 3-OH-kynurenine | Caenorhabditis elegans, in culture, 2 h fasting | Extracts from C. elegans | ↓ | [42] |
| Restrictive AN (n = 20) | Serum | ←→ | [53] | |
| Kynurenic acid | Medication-free AN (n = 10) | CSF | ↓ | [52] |
| Restrictive AN (n = 20) | Serum | ←→ | [53] | |
| Quinolinic acid | Medication-free AN (n = 10) | CSF | ←→ | [52] |
| KAT1 gene expression | Restrictive AN (n = 20) | Serum | ←→ | [53] |
| KAT2 gene expression | Restrictive AN (n = 20) | Serum | ←→ | [53] |
| KAT3 gene expression | C57BL/6 mice, activity-based anorexia | Muscles | ↑ | [55] |
| Restrictive AN (n = 20) | Serum | ↑ | [53] | |
| KAT4 gene expression | C57BL/6 mice, activity-based anorexia | Muscles | ↑ | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberts, C.; Owe-Larsson, M.; Urbanska, E.M. New Perspective on Anorexia Nervosa: Tryptophan-Kynurenine Pathway Hypothesis. Nutrients 2023, 15, 1030. https://doi.org/10.3390/nu15041030
Alberts C, Owe-Larsson M, Urbanska EM. New Perspective on Anorexia Nervosa: Tryptophan-Kynurenine Pathway Hypothesis. Nutrients. 2023; 15(4):1030. https://doi.org/10.3390/nu15041030
Chicago/Turabian StyleAlberts, Charl, Maja Owe-Larsson, and Ewa M. Urbanska. 2023. "New Perspective on Anorexia Nervosa: Tryptophan-Kynurenine Pathway Hypothesis" Nutrients 15, no. 4: 1030. https://doi.org/10.3390/nu15041030
APA StyleAlberts, C., Owe-Larsson, M., & Urbanska, E. M. (2023). New Perspective on Anorexia Nervosa: Tryptophan-Kynurenine Pathway Hypothesis. Nutrients, 15(4), 1030. https://doi.org/10.3390/nu15041030







