Abstract
The effects of regular physical activity on two important anti-atherosclerosis functions of high-density lipoprotein (HDL), namely its capacity to receive both forms of cholesterol and its anti-oxidant function, were investigated in this study comparing older adults with young individuals. One-hundred and eight healthy adult individuals were enrolled and separated into the following groups: active older (60–80 yrs, n = 24); inactive older (60–79 yrs, n = 21); active young (20–34 yrs, n = 39); and inactive young (20–35 yrs, n = 24). All performed cardiopulmonary tests. Blood samples were collected in order to assess the following measures: lipid profile, HDL anti-oxidant capacity, paraoxonase-1 activity, HDL subfractions, and lipid transfer to HDL. Comparing active older and active young groups with inactive older and inactive young groups, respectively, the active groups presented higher HDL-C levels (p < 0.01 for both comparisons), unesterified cholesterol transfer (p < 0.01, p < 0.05), and intermediate and larger HDL subfractions (p < 0.001, p < 0.01) than the respective inactive groups. In addition, the active young group showed higher esterified cholesterol transfer than the inactive young group (p < 0.05). As expected, the two active groups had higher VO2peak than the inactive groups; VO2peak was higher in the two younger than in the two older groups (p < 0.05). No differences in unesterified and esterified cholesterol transfers and HDL subfractions were found between active young and active older groups. HDL anti-oxidant capacity and paraoxonase-1 activity were equal in all four study groups. Our data highlight and strengthen the benefits of regular practice of physical activity on an important HDL function, the capacity of HDL to receive cholesterol, despite the age-dependent decrease in VO2peak.
1. Introduction
The transfer of cholesterol to high-density lipoprotein (HDL) in either unesterified or esterified forms from the other lipoprotein classes, such as very-low density lipoprotein (VLDL) or low-density lipoprotein (LDL), continuously occurs in the plasma compartment [1]. Unesterified cholesterol transferred to HDL is esterified by the action of lecithin–cholesterol acyltransferase (LCAT) using apolipoprotein A-I, the main HDL protein, as a co-factor. Unesterified cholesterol is thereby sequestered from HDL particles’ surfaces to HDL cores [2]. Esterified cholesterol in the apolipoprotein B-containing lipoproteins can be transferred to HDL by the action of cholesteryl ester transfer protein (CETP) and stored in the lipoprotein core. The esterified cholesterol pool in HDL is eventually taken up by the liver and excreted in the bile. Alternatively, HDL esterified cholesterol can be transferred to the apolipoprotein B-containing lipoproteins by the action of CETP. Those fluxes are essential for the homeostasis of cholesterol in the plasma and are also important for reverse cholesterol transport. In reverse cholesterol transport, unesterified cholesterol pumped out from the cells of peripheral tissues is transferred to HDL, where it is esterified and undergoes the above-described cholesterol fluxes among lipoproteins [1].
Previously, we showed through an in vitro assay that reduced cholesterol transfer from a model lipoprotein to HDL is related with the presence of atherosclerotic diseases or conditions that facilitate atherosclerosis development [3,4]. In this respect, lower cholesterol transfer to HDL occurred in physically inactive individuals, whereas physical activity increased those transfer rates. The effects of physical activity on cholesterol transfer to HDL were elicited regardless of the modality of training [5,6].
Physical activity is a major non-pharmacologic resource to achieve improvement in lipid profile, especially through increased HDL cholesterol (HDL-C) levels, including in the older adult population [6,7]. In this regard, we previously showed that older women who regularly engaged in exercise training not only presented higher HDL-C levels but also increased cholesterol transfer to HDL as compared to a group of sedentary older women [6]. Nonetheless, higher HDL-C is not necessarily accompanied by higher cholesterol transfer rates to HDL. For example, in patients with metabolic syndrome, exercise training elicited an increase in cholesterol transfer without increasing HDL-C [5].
In a previous study, we observed that older women engaging in regular exercise had a higher capacity to transfer cholesterol to HDL than sedentary women [6]. That finding encouraged us to test the process of cholesterol transfer in both genders and in different age ranges in an attempt to acquire a novel global parameter to assess the metabolic effects of regular exercise. A second test of HDL’s functional properties, specifically involving its antioxidant action on LDL lipids, was also performed to investigate whether more than one HDL property could be simultaneously changed by the stimulus of exercise practice. Other parameters related to HDL metabolism, such as CETP and LCAT concentrations, the activity of paraoxonase 1 (PON-1), HDL size and subfractions, and lipid and apolipoprotein plasma profiles, were determined, aiming to establish correlations with the functional properties of HDL.
2. Materials and Methods
2.1. Participants
This study evaluated older (aged > 60 yrs) and young adults (20–35 yrs) of both sexes selected from the Mané Garrincha Sports Education Center of the Department of Sports of São Paulo City, Brazil, and from the employees and students of the Heart Institute of the University of São Paulo Medical School Hospital (InCor-HCFMUSP). All the enrolled participants were thoroughly inquired about their physical activity routine, specifically whether they were engaged or not in an exercise training program for at least the last twelve months [8]. Based on their answers, they were allocated to four study groups: active older (n = 24), inactive older (n = 21), active young (n = 39), and inactive young (n = 24) individuals. The selected volunteers were then submitted to a treadmill exercise test. The predicted VO2peak for age was used to confirm the inclusion by the inquiry of each participant in the active or in the inactive group.
Dietary habits were not scrutinized in the inclusion inquiry, and no recommendations on food consumption before blood sampling were made in addition to the required 12 h fasting. None of the participants were smokers, addicted to alcohol consumption, or were using lipid-lowering drugs, beta-blockers, or anabolic steroids. The exclusion criteria were as follows: individuals with diabetes, previous cardiac or cerebrovascular events, pulmonary, cardiovascular, renal, metabolic, inflammatory, or neoplastic diseases.
The study was conducted in accordance with the Declaration of Helsinki and Ethical Standards in Sport and Exercise Science Research [9] and approved by the Institutional Review Board and Ethics Committee of the Hospital das Clínicas da Universidade de São Paulo (protocol code no. 2.903.252; date of approval 19 September 2018). A written informed consent was obtained from all individuals after a complete description of the protocol.
2.2. Cardiopulmonary Exercise Test
The test was performed on a programmable treadmill (T2100 Model, GE Healthcare, Chicago, IL, USA) using a ramp protocol with increments in workload each minute until volitional exhaustion [9]. Oxygen uptake was measured in a cardiopulmonary exercise test breath-to-breath to determine cardiorespiratory fitness by the VO2peak (SensorMedics—Vmax Analyzer Assembly, Palm Springs, CA, USA). VO2 ventilatory threshold, absolute VO2, and VO2peak were determined [10]. Age-predicted VO2 was calculated according to gender [11], metabolic equivalent (MET) of peak [12], and oxygen uptake efficiency slope (OUES) [13]. The effectiveness of the cardiopulmonary test was assessed and included those reaching values of respiratory exchange ratio (VO2/VCO2) equal to or higher than 1 [9] and by the request of the participant to stop the test by gestures, which indicate maximal effort. All subjects were motivated for maximal performance in the test, and none showed signals of myocardial ischemia during the exercise test.
2.3. Blood Sampling, Plasma Lipids, and Apolipoproteins
Blood samples were collected after fasting 12 h overnight in tubes containing EDTA anticoagulant (ethylenediamine tetra-acetic acid) or not, and plasma/serum samples were obtained after centrifugation (300× g, 10 min at 4 °C).
Plasma lipids were determined by the colorimetric–enzymatic method (Merck KGaA, Darmstadt, Germany). LDL-C was estimated by the Friedewald formula [14]. Apolipoproteins were measured by the immunonephelometric method (ProSpec-Tany TechnoGene Ltd., Rehovot, Israel).
2.4. HDL Size and Subfractions
The particle size of HDL was measured in fresh plasma after chemical precipitation of apolipoprotein B-containing lipoproteins by the laser light scattering method (Malvern Instr., Worcestershire, UK) [4]. The cholesterol content of the HDL subfractions was analyzed by electrophoresis (Lipoprint System Quantimetrix Corporation, Redondo Beach, CA, USA).
2.5. CETP and LCAT Concentrations
The plasma concentrations of CETP and LCAT were determined by ELISA immunoassays (ALPCO Diagnostics, Salem, MA, USA).
2.6. Paraoxonase 1 (PON1) Activity
PON1 activity was measured by adding 1M Tris-HCl buffer (100 mmol/L, pH 8.0) containing 2 mmol/L CaCl2 and 5.5 mmol/L paraoxon (Sigma Chemical Company, St. Louis, MO, USA) to the serum samples. The generation of p-nitrophenol was measured at 405 nm at 37 °C in a plate reader (Victor X3, PerkinElmer, Shelton, CT, USA) [15].
2.7. HDL’s Antioxidant Capacity
HDL’s antioxidant capacity was determined by a modified lag time method using CuSO4 as the oxidizing agent [16]. This method uses standard LDL purified by ultracentrifugation. HDL was precipitated using phosphotungstic acid and magnesium chloride (HDL Cholesterol kit, Labtest, Minas Gerais, Brazil). LDL (83 ug protein/mL), HDL (200 ug protein/mL), and CuSO4 (30 μM) were incubated. The antioxidant capacity of HDL was monitored at 234 nm for 5 h at 37 °C. The following parameters were calculated: lag time; DOmax, defined as the maximum production of conjugated dienes; Tmax, the time needed to achieve DOmax; Vmax, defined as the maximum rate of conjugated dienes; and time to Vmax, the time needed to achieve Vmax.
2.8. Cholesterol Transfer Assay
A nanoemulsion was prepared from a lipid mixture composed of 40 mg cholesteryl oleate, 20 mg egg phosphatidylcholine, 1 mg triolein, and 0.5 mg cholesterol (Sigma Chemical Co.), as described previously [3,4]. Trace amounts of 4-14C-cholesterol or [1α,2α(n)-3H]-cholesteryl oleate (Amersham, Little Chalfont, Buckinghamshire, UK) were added to the initial solution. The emulsification of lipids by prolonged ultrasonic irradiation in aqueous media and the two-step procedure of ultracentrifugating the crude emulsion and adjusting its density with KBr to obtain a nanoemulsion were carried out.
The in vitro assay to measure cholesterol transfer from the nanoemulsion to HDL has been previously described [3,4]. Briefly, the nanoemulsion is incubated with plasma. This incubation is followed by chemical precipitation of the nanoemulsion and apolipoprotein B-containing lipoproteins. The radioactivity of the supernatant containing HDL is measured by a liquid scintillation counter (Liquid Scintillation Analyzer Tri-Carb2100TR, PerkinElmer). The transfer of cholesterol to HDL is expressed as a percentage of the total radioactivity of the nanoemulsion incubated with the plasma.
2.9. Statistical Analysis
The normality and the homogeneity of variance of the data obtained in this study were determined by the Kolmogorov–Smirnov and Levene’s tests, respectively. Parametric data are expressed as mean ± standard deviation, and non-parametric data are expressed as median (minimum:maximum).
Categorical variables were analyzed by the chi-squared test, whereas numerical data were analyzed by using one-way ANOVA followed by multiple comparisons of Bonferroni or by using a Kruskal–Wallis test followed by multiple comparisons of Dunn.
Pearson or Spearman’s rank correlation coefficient tests were used to determine the occurrence of correlation among the variables assessed in this study. In addition, a multivariate regression analysis adjusted for BMI was performed.
Significance was set as p ˂ 0.05 for all analyses, and GraphPad Prism version 5.00 was used for statistical analysis.
3. Results
As shown in Table 1, body mass index (BMI) and waist circumference were higher in the inactive older group compared to the inactive young group but was equal to the active older group. BMI was also higher in the inactive older group than the active older group. Waist–hip ratio (WHR) was higher in the active older group than in the active young group and higher in the inactive older group than in the inactive young group.

Table 1.
Sex distribution, age, anthropometric, and ventilatory data of the study groups.
VO2 ventilatory threshold, peak, age-predicted VO2, and MET were higher in the active older group than in the inactive older group. VO2 absolute values, ventilatory threshold, peak, age-predicted VO2, MET, and OUES were higher in the active young group than the inactive young group. VO2 absolute values, ventilatory threshold, peak, MET, and OUES were higher in the active young group in comparison to the active older group. VO2 of ventilatory threshold, peak, age-predicted VO2, and MET were higher in the inactive young group than the inactive older group.
As shown in Table 2, HDL-C was higher in the active older group than in the inactive older group and in the active young group than the inactive young group. LDL-C and non-HDL-C were higher in the active older group than in the active young group. Non-HDL-C, LDL-C, and triglycerides were higher in the inactive older group than in the inactive young group. Apolipoprotein A-I was similar between the active older group and the inactive older group but higher in the active young group than in the inactive young group. Apolipoprotein A-I was higher in the inactive older group than in the inactive young group. Apolipoprotein B concentration did not differ between the active older and inactive older groups or between the active young and inactive young groups. However, apolipoprotein B was higher in the active older group than the active young group and in the inactive older group than the inactive young group.

Table 2.
Plasma lipids, apolipoproteins, and structural and functional HDL parameters of the study groups.
In Table 2, it is also shown that the transfer of unesterified cholesterol to HDL was higher in the active older group than in the inactive older group, but that of esterified cholesterol was equal. Transfers of unesterified and esterified cholesterol were higher in the active young group than in the inactive young group. CETP concentrations were higher in the active older group than the active young group. LCAT concentration and HDL particle size were not different between the groups (Table 2).
HDL anti-oxidant capacity and PON1 activity were equal among the study groups (Table 3).

Table 3.
PON1 activity and HDL anti-oxidant capacity of study groups.
Figure 1 shows the data of HDL subfractions. The larger HDL subfraction was equal in the active older group and the inactive older group. However, intermediate HDL was higher in the active older group than in the inactive older group. Moreover, larger HDL was higher in the active young group than in the inactive young group, without differences among groups regarding intermediate HDL. Additionally, there were no differences in HDL subfractions between the active older group and the active young group. Larger HDL was higher in the inactive older group than in the inactive young group. Groups had no differences in small HDL.
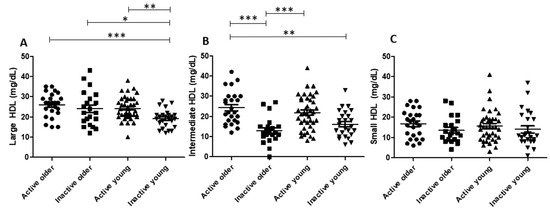
Figure 1.
HDL subfractions in study groups. Large (A), intermediate (B), and small (C) HDL subfractions in study groups. Abbreviations: HDL: high-density lipoprotein. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
Supplementary Table S1 shows the correlation analysis performed with different parameters measured in the study. VO2peak was negatively correlated with concentrations of LDL-C (r = −0.3447; p < 0.001), non-HDL-C (r = −0.4109; p < 0.001), triglycerides (r = −0.3558; p < 0.01), apolipoprotein B (r = −0.3630; p = 0.001), and CETP (r = −0.2417; p < 0.05). Esterified cholesterol transfer was positively correlated with HDL-C (r = 0.4597; p < 0.001), LDL-C (r = 0.3024; p < 0.01), non-HDL-C (r = 0.3363; p < 0.001), triglycerides (r = 0.2597; p < 0.01), apolipoprotein A-I (r = 0.6168; p < 0.001), apolipoprotein B (r = 0.3159; p < 0.001), and unesterified cholesterol transfer (r = 0.8685; p < 0.001). Esterified cholesterol transfer was also positively correlated with the concentrations of CETP (r = 0.3137; p < 0.001) and LCAT (r = 0.3641; p < 0.001), HDL particle size (r = 0.3471; p < 0.001), and the intermediate (r = 0.3910; p < 0.001), and large HDL subfractions (r = 0.4447; p < 0.001). Unesterified cholesterol transfer to HDL was positively correlated with HDL-C (r = 0.5415; p < 0.001), apolipoprotein A-I (r = 0.6652; p < 0.001), HDL particle size (r = 0.3315; p < 0.001), and the concentrations of CETP (r = 0.2467; p < 0.01) and LCAT (r = 0.4060; p < 0.001). Unesterified cholesterol transfer also positively correlated with the small (r = 0.2365; p < 0.05), intermediate (r = 0.4843; p < 0.001), and large HDL subfractions (r = 0.4098; p < 0.001).
Since BMI could impact either metabolic or physical capacity parameters, we additionally performed a multivariate regression analysis adjusted for BMI. In this respect, the BMI observed in the active older group showed a significant effect on MET (β = −1.223; 95% CI −0.2278 to −0.1676; p = 0.0237; R2 = 0.9967); on larger HDL (mg/dL; β = 4.318; 95% CI 0.7100 to 7.926; p = 0.0263; R2 = 0.9978); and on intermediate HDL (mg/d; β = 4.207; 95% CI 0.9046 to 7.510; p = 0.0176; R2 = 0.9986). Moreover, the BMI observed in the young active group and in the young inactive group showed a significant effect on age-predicted VO2 (%; β = −0.2739; 95% CI −0.3856 to −0.1622; p < 0.0001; R2 = 0.8589; and β = −0.3581; 95% CI −0.6077 to −0.1084; p = 0.0096; R2 = 0.9459, respectively).
4. Discussion
Our data showed that several parameters of HDL metabolism assessed in the present study were better in active compared to inactive individuals, regardless of whether they were older (>60 yrs) or younger (20–35 yrs).
In this regard, an increase in HDL-C by regular participation in exercise, a classical all-age observation [17,18,19], was confirmed here, along with higher levels of intermediate or larger HDL subfractions in the two active groups compared to the two inactive groups. According to the literature, larger HDL subfractions are more protective against atherosclerosis than smaller subfractions [20,21], and exercise training has been associated with increments in larger HDL particles [22]. This finding was also reported in subjects with different morbidities that performed exercise training, such as men and women with overweight and obesity [22,23], as well as in young obese women: exercise training was able to reduce the small HDL subfraction [23]. Taken together, our finding of a higher intermediate HDL subfraction in the active older group than in the inactive older group and of increased larger HDL subfractions in the active young group compared to the inactive young group suggests that physical activity improves the quality of the HDL subfraction profile, either in young or older individuals.
Of note was the fact that in our study, the inactive older group had a higher large HDL subfraction than the inactive young group. This is somehow in disagreement with the description by Otrane et al. [24] that found higher large HDL and lower small HDL subfractions in younger compared to older subjects. These discrepant findings can putatively be related to dietary habits, since Otrane et al. [24] mentioned that adherence to a Mediterranean-type diet, and specifically the intake of extra virgin olive oil, was able to increase the large and intermediate HDL subfractions in older individuals. Here, we did not explore the dietary habits of the participants, which can be considered a limitation of this study.
The multivariate analysis yielded an unexpected correlation, i.e., the higher BMI and the higher presence of the large and intermediate HDL particle subfractions in the active older group. The large subfraction is considered the most protective subfraction, and higher BMI is consistently harmful to health.
Here, PON1 activity and HDL antioxidant capacity were not different among all groups. Previously, we had shown that patients with metabolic syndrome have increased PON1 activity after an exercise training program performed over 12 weeks [5]. Our finding that HDL antioxidant capacity was not higher in exercise practitioners is supported by a previous study [25].
The active older group demonstrated higher transfer of unesterified cholesterol to HDL than the inactive older group, although the transfer of esterified cholesterol was similar in both groups. In previous studies, it was shown that lower unesterified cholesterol transfer was associated with manifested atherosclerotic diseases, such as in precocious CAD and in ischemic cerebrovascular events (for review, see [3]). Furthermore, in aged individuals and in patients with type 2 diabetes, the unesterified cholesterol transfer to HDL was lower in those with CAD compared with those without CAD [4]. Therefore, the high transfer of unesterified cholesterol to HDL found in the active older group is suggestive that regular physical activity can favor the mechanisms of HDL protection against atherogenesis. However, it is important to mention that, in accordance with the literature, HDL-C is a complex lipoprotein that can be useful as a biomarker in CAD risk, but its direct causal role in CAD remains controversial [26].
The HDL fraction is the main uptake site for cholesterol esterification in the plasma, a fundamental process for cholesterol homeostasis: unesterified cholesterol from the apolipoprotein B-containing lipoproteins and from the peripheral tissues is transferred to HDL. In HDL, cholesterol is esterified by the action of LCAT associated with the HDL fraction, using apolipoprotein A-I as a co-factor. In previous studies, CAD patients showed increased removal of unesterified cholesterol from the plasma, suggesting that this lipid would dissociate from the lipoprotein particle and independently precipitate in the arterial wall [3]. This finding highlights the importance of unesterified cholesterol transfer to HDL fractions as a mechanism that stabilizes the plasma cholesterol pool. Therefore, the finding of a higher transfer of unesterified cholesterol to HDL in the active older group than in the inactive older group and in the active young group than in the inactive young group is suggestive that physical activity has a protective action against atherogenesis. By transferring unesterified cholesterol from the other lipoprotein classes to HDL, wherein unesterified cholesterol is esterified and stored in the HDL core, unesterified cholesterol dissociation from the non-HDL lipoproteins and direct deposit in the arterial wall is avoided.
In previous studies, higher unesterified cholesterol transfer to HDL was observed in physically active subjects, such as active aged women [6]. Unesterified cholesterol transfer was increased in patients with metabolic syndrome after they underwent an exercise training program, although HDL-C did not increase [5]. Here, esterified cholesterol transfer to HDL was higher in the active young group than the inactive young group, but in the active older group and the inactive older group, esterified cholesterol transfer was not different. Whether esterified cholesterol transfer could be consistently considered beneficial is difficult to establish in view of the results of previous studies [3].
In a previous study, it was shown that omnivorous individuals had higher cholesterol transfer rates than vegans, whereas in lacto-ovo-vegetarians. transfer values were intermediate between the two [27]. Thus, it is tempting to investigate in future studies whether nutritional habits could influence cholesterol transfer in subjects that engage in regular physical activity in view of the major influence of both diet and physical exercise on human health.
In our current study, the concentration of LCAT was not affected by physical activity, in agreement with previous reports [28,29]. Regarding CETP concentration, the active older group and the inactive older group, as well as the active young group and the inactive young group, did not differ. This is a debatable issue in the literature, since some studies found lower CETP concentrations were associated with exercise training [30], while others did not [29].
As expected, physical activity did not change LDL-C or apolipoprotein B values. However, this does not imply that being physically active does not impact the metabolism of apolipoprotein B-containing lipoproteins. Athletes showed pronouncedly increased removal of an LDL nanoemulsion model compared to sedentary controls, despite LDL-C being equal in both groups [31]. This finding suggests that athletes had accelerated turnover of LDL and exercise training decreased small, dense LDL subfractions [22,32]. Consequently, the shortened residence time of LDL in the plasma exposes the lipoprotein to lower levels of peroxidation or other modifying processes that lead LDL to pro-atherogenic catabolic pathways [33].
It is widely accepted that cardiorespiratory fitness, which is assessed by VO2, is not only a pivotal feature of cardiovascular health but is an independent predictor of all-cause mortality [34]. VO2 declines over the years [35], but regular engagement in exercise training can attenuate VO2 decrease [36], as was also observed in the present study. In fact, active individuals—older or young—showed higher VO2peak values than inactive individuals. Additionally, the maintenance of VO2 at satisfactory levels for age, according to the equations used here, has an important role in delaying the development of metabolic disorders, such as dyslipidemia [37,38].
5. Conclusions
Our results show that, regardless of whether the individuals are younger or older, regular physical activity increases the transfer of cholesterol from the other lipoprotein classes to HDL. Cholesterol transfer to HDL in the plasma is one of the key features of HDL’s metabolism and anti-atherosclerosis function and was tested here by a novel and straightforward in vitro method. Remarkably, regular physical activity, while increasing cholesterol transfer, did not increase the antioxidant function of HDL. This is an important finding because it suggests that the several functions of HDL can be independent of each other. Consequently, to seize a global understanding of HDL’s impact on reducing atherosclerosis risk, it would be necessary to go far beyond the determination of HDL-cholesterol and to systematically evaluate each one of the putative HDL functions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15234871/s1, Table S1. Correlation analysis of data with all participants.
Author Contributions
P.G.S.B.: conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing; F.R.F.: methodology, software, validation, formal analysis, data curation and writing—review and editing; A.L.L.B.: conceptualization, investigation and writing—review and editing; G.R.A.: investigation; R.V.B.: investigation and data curation; M.J.N.N.A.: methodology and investigation; R.P.V.: resources; M.W.V.: conceptualization; M.N.A.: methodology; R.K.F.: resources; A.M.F.N.: resources; N.R.T.D.: methodology, software, resources; T.M.T.: conceptualization, methodology, formal analysis, investigation, writing—original draft preparation and supervision; R.C.M.: conceptualization, methodology, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Sao Paulo Research Foundation (FAPESP, Brazil; grant numbers: 2014/03742-0, 2014/50983-3, 2020/16215-0) and the National Institute of Science and Technology Complex Fluids (INCT-FCx, Brazil). Dr Maranhão has an A-1 Research Carrier Award from the National Council for Scientific and Technological Development (CNPq). PGSB was supported by a doctoral scholarship from CNPq.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board and Ethics Committee of the Hospital das Clínicas da Universidade de São Paulo (protocol code no. 2.903.252; date of approval, 19 September 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adorni, M.P.; Ronda, N.; Bernini, F.; Zimetti, F. High Density Lipoprotein Cholesterol Efflux Capacity and Atherosclerosis in Cardiovascular Disease: Pathophysiological Aspects and Pharmacological Perspectives. Cells 2021, 10, 574. [Google Scholar] [CrossRef]
- Glomset, J.A. The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 1968, 9, 155–167. [Google Scholar] [CrossRef]
- Maranhão, R.C.; Freitas, F.R. HDL Metabolism and Atheroprotection: Predictive Value of Lipid Transfer. Adv. Clin. Chem. 2014, 65, 1–41. [Google Scholar]
- Sprandel, M.C.O.; Hueb, W.A.; Segre, A.; Ramires, J.A.F.; Kalil-Filho, R.; Maranhão, R.C. Alterations in lipid transfers to HDL associated with the presence of coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Casella-Filho, A.; Chagas, A.C.P.; Maranhão, R.C.; Trombetta, I.C.; Cesena, F.H.Y.; Silva, V.M.; Tanus-Santos, J.E.; Negrão, C.E.; da Luz, P.L. Effect of exercise training on plasma levels and functional properties of high-density lipoprotein cholesterol in the metabolic syndrome. Am. J. Cardiol. 2011, 107, 1168–1172. [Google Scholar] [CrossRef]
- Bachi, A.L.; Rocha, G.A.; Sprandel, M.C.; Ramos, L.R.; Gravina, C.F.; Pithon-Curi, T.C.; Vaisberg, M.; Maranhão, R.C. Exercise Training Improves Plasma Lipid Inflammatory Profiles and Increases Cholesterol Transfer to High-Density Lipoprotein in Elderly Women. J. Am. Geriatric. Soc. 2015, 63, 1247–1249. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behavior. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Hallal, P.C.; Gomez, L.F.; Parra, D.C.; Lobelo, F.; Mosquera, J.; Florindo, A.A.; Reis, R.S.; Pratt, M.; Sarmiento, O.L. Lessons learned after 10 years of IPAQ use in Brazil and Colombia. J. Phys. Act Health 2010, 7 (Suppl. S2), S259–S264. [Google Scholar] [CrossRef]
- Harriss, D.J.; Atkinson, G. International Journal of Sports Medicine—Ethical standards. Int. J. Sports Med. 2009, 30, 701–702. [Google Scholar] [CrossRef]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef]
- Myers, J.; Kaminsky, L.A.; Lima, R.; Christle, J.W.; Ashely, E.; Arena, R. A Reference Equation for Normal Standards for VO2 Max: Analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry). Prog. Cardiovasc. Dis. 2017, 60, 21–29. [Google Scholar] [CrossRef]
- Skinner, S.; McLellan, T. The transition from aerobic to anaerobic metabolism. Res Exerc Sport. 1980, 51, 234–248. [Google Scholar] [CrossRef]
- Hollenberg, M.; Tager, I.B. Oxygen uptake efficiency slope: An index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J. Am. Coll. Cardiol. 2000, 36, 194–201. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Sentí, M.; Tomás, M.; Fitó, M.; Weinbrenner, T.; Covas, M.I.; Sala, J.; Masiá, R.; Marrugat, J. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 5422–5426. [Google Scholar] [CrossRef]
- Esterbauer, H.; Striegl, G.; Puhl, H.; Rotheneder, M. Continuous monitoring of in vitro oxidation of human low-density lipoprotein. Free Radic. Res. Commun. 1989, 6, 67–75. [Google Scholar] [CrossRef]
- Wood, P.D.; Stefanick, M.L.; Dreon, D.M.; Frey-Hewitt, B.; Garay, S.C.; Williams, P.T.; Superko, H.R.; Fortmann, S.P.; Albers, J.J.; Vranizan, K.M. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N. Engl. J. Med. 1988, 319, 915–924. [Google Scholar] [CrossRef]
- Sunami, Y.; Motoyama, M.; Kinoshita, F.; Mizooka, Y.; Sueta, K.; Matsunaga, A.; Sasaki, J.; Tanaka, H.; Shindo, M. Effects of low-intensity aerobic training on the high-density lipoprotein cholesterol concentration in healthy elderly subjects. Metabolism 1999, 48, 984–988. [Google Scholar] [CrossRef]
- Couillard, C.; Després, J.P.; Lamarche, B.; Bergeron, J.; Gagnon, J.; Leon, A.S.; Rai, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: Evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler Thromb. Vasc. Biol. 2001, 21, 1226–1232. [Google Scholar] [CrossRef]
- Xu, R.X.; Li, S.; Li, X.L.; Zhang, Y.; Guo, Y.L.; Zhu, C.G.; Wu, N.Q.; Qing, P.; Sun, J.; Dong, Q.; et al. High-density lipoprotein subfractions in relation with the severity of coronary artery disease: A Gensini score assessment. J. Clin. Lipidol. 2015, 9, 26–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.G.; Xu, R.X.; Li, S.; Li, X.L.; Guo, Y.L.; Wu, N.Q.; Gao, Y.; Qing, P.; Cui, C.J.; et al. HDL subfractions and very early CAD: Novel findings from untreated patients in a Chinese cohort. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Sarzynski, M.A.; Burton, J.; Rankinen, T.; Blair, S.N.; Church, T.S.; Després, J.P.; Hagberg, J.M.; Landers-Ramos, R.; Leon, A.S.; Mikus, C.R.; et al. The effects of exercise on the lipoprotein subclass profile: A meta-analysis of 10 intervention. Atherosclerosis 2015, 243, 364–372. [Google Scholar] [CrossRef]
- Woudberg, N.J.; Mendham, A.E.; Katz, A.A.; Goedecke, J.H.; Lecour, S. Exercise alters HDL subclasses distribution and function in obese women. Lipids Health Dis. 2018, 17, 232–244. [Google Scholar] [CrossRef]
- Otrane, A.; Trigui, A.; Walha, R.; Berrougui, H.; Fulop, T.; Khalil, A. Extra Virgin Olive Oil Prevents the Age-Related Shifts of the Distribution of HDL Subclasses and Improves Their Functionality. Nutrients 2021, 13, 2235. [Google Scholar] [CrossRef]
- Brites, F.; Zago, V.; Verona, J.; Muzzio, M.L.; Wikinski, R.; Schreier, L. HDL capacity to inhibit LDL oxidation in well-trained triathletes. Life Sci. 2006, 78, 3074–3081. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Barter, P.J.; Björkegren, J.L.M.; Chapman, M.J.; Gaudet, D.; Kim, D.S.; Niesor, E.; Rye, K.A.; Sacks, F.M.; et al. HDL and atherosclerotic cardiovascular disease: Genetic insights into complex biology. Nat. Rev. 2018, 15, 9–19. [Google Scholar] [CrossRef]
- Vinagre, J.C.; Vinagre, C.G.; Pozzi, F.S.; Zácari, C.Z.; Maranhão, R.C. Plasma kinetics of chylomicron-like emulsion and lipid transfers to high-density lipoprotein (HDL) in lacto-ovo vegetarian and in omnivorous subjects. Eur. J. Nutr. 2014, 53, 981–987. [Google Scholar] [CrossRef]
- Marniemi, J.; Dahlström, S.; Kvist, M.; Seppänen, A.; Hietanen, E. Dependence of serum lipid and lecithin: Cholesterol acyltransferase levels on physical training in young men. Eur. J. Appl. Physiol. 1982, 49, 25–35. [Google Scholar] [CrossRef]
- Olchawa, B.; Kingwell, B.A.; Hoang, A.; Schneider, L.; Miyazaki, O.; Nestel, P.; Sviridov, D. Physical fitness and reverse cholesterol transport. Arterioscler Thromb. Vasc. Biol. 2004, 24, 1087–1091. [Google Scholar] [CrossRef]
- Seip, R.L.; Moulin, P.; Cocke, T.; Tall, A.; Kohrt, W.M.; Mankowitz, K.; Semekovich, C.F.; Ostlund, R.; Schonfeld, G. Exercise training decreases plasma cholesteryl ester transfer protein. Aterioscler Thromb. 1993, 13, 1359–1367. [Google Scholar] [CrossRef]
- Vinagre, C.G.C.; Ficker, E.S.; Finazzo, C.; Alves, M.J.N.N.; Angelis, K.; Irigoyen, M.C.; Negrão, C.E.; Maranhão, R.C. Enhanced removal from the plasma of LDL-like nanoemulsion cholesteryl ester in trained men compared with sedentary healthy men. J. Appl. Physiol. 2007, 103, 1166–1171. [Google Scholar] [CrossRef]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Orvos, J.D.; et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Betik, A.C.; Hepple, R.T. Determinants of VO2 max decline with aging: An integrated perspective. Appl. Physiol. Nutr. Metab. 2008, 33, 130–140. [Google Scholar] [CrossRef]
- Breneman, C.B.; Polinski, K.; Sarzynski, M.A.; Lavie, C.J.; Kokkinos, P.F.; Ahmed, A.; Sui, X. The Impact of Cardiorespiratory Fitness Levels on the Risk of Developing Atherogenic Dyslipidemia. Am. J. Med. 2016, 129, 1060–1066. [Google Scholar] [CrossRef]
- Park, Y.M.M.; Sui, X.; Liu, J.; Zhou, H.; Kokkinos, P.F.; Lavie, C.J.; Hardin, J.W.; Blair, S.N. The impact of Cardiorespiratory Fitness on Age-Related Lipids and Lipoproteins. J. Am. Coll. Cardiol. 2015, 19, 2091–2100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).