Effect of Extracelluar Vesicles Derived from Akkermansia muciniphila on Intestinal Barrier in Colitis Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Akk Culture and Akk EVs Preparation
2.2. Cell Culture
2.3. Measurement of Cellular Uptake of Akk EVs
2.4. Measurement of Cytokine and Nitric Oxide (NO) Production
2.5. Animals and Treatments
2.6. Histomorphological and Immunohistochemical Assessment
2.7. Measurement of Biochemical Parameters
2.8. Quantitative Real-Time PCR
2.9. 16S rRNA Gene Sequencing
2.10. Statistical Analysis
3. Results
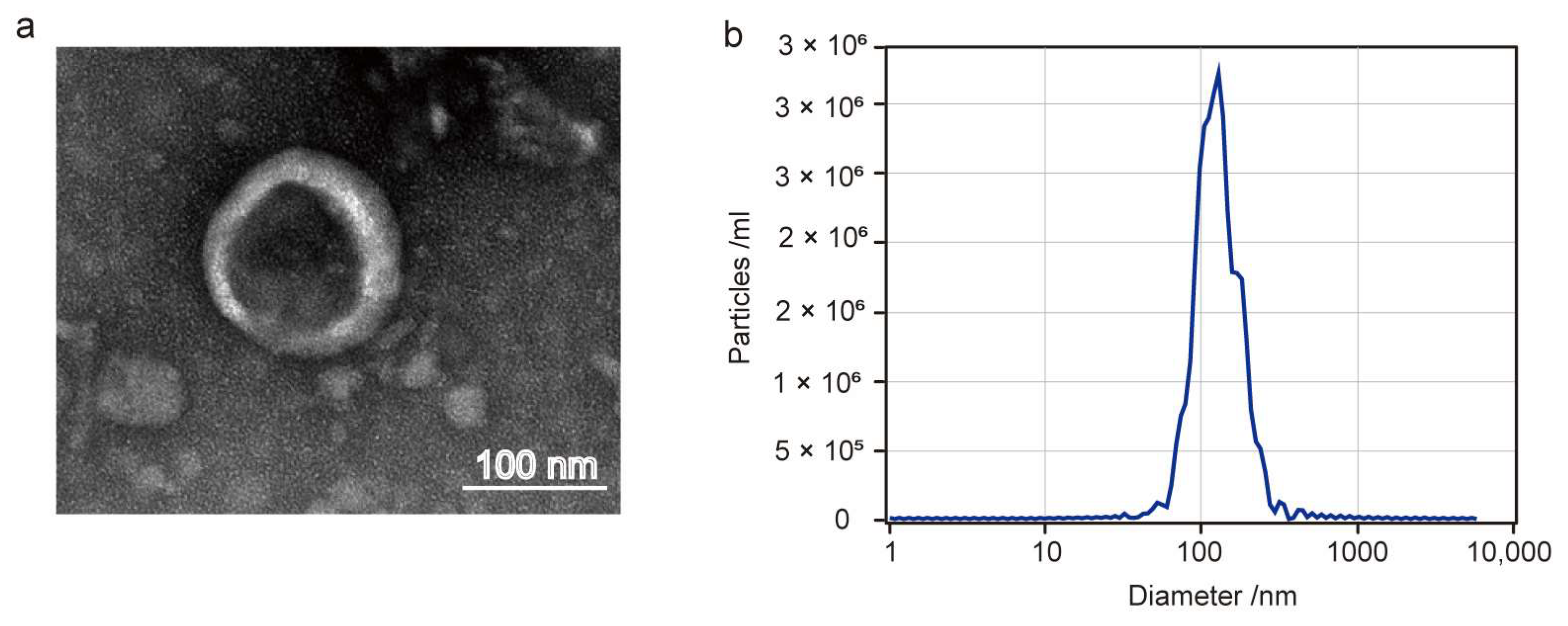
3.1. Characterization of Akk EVs
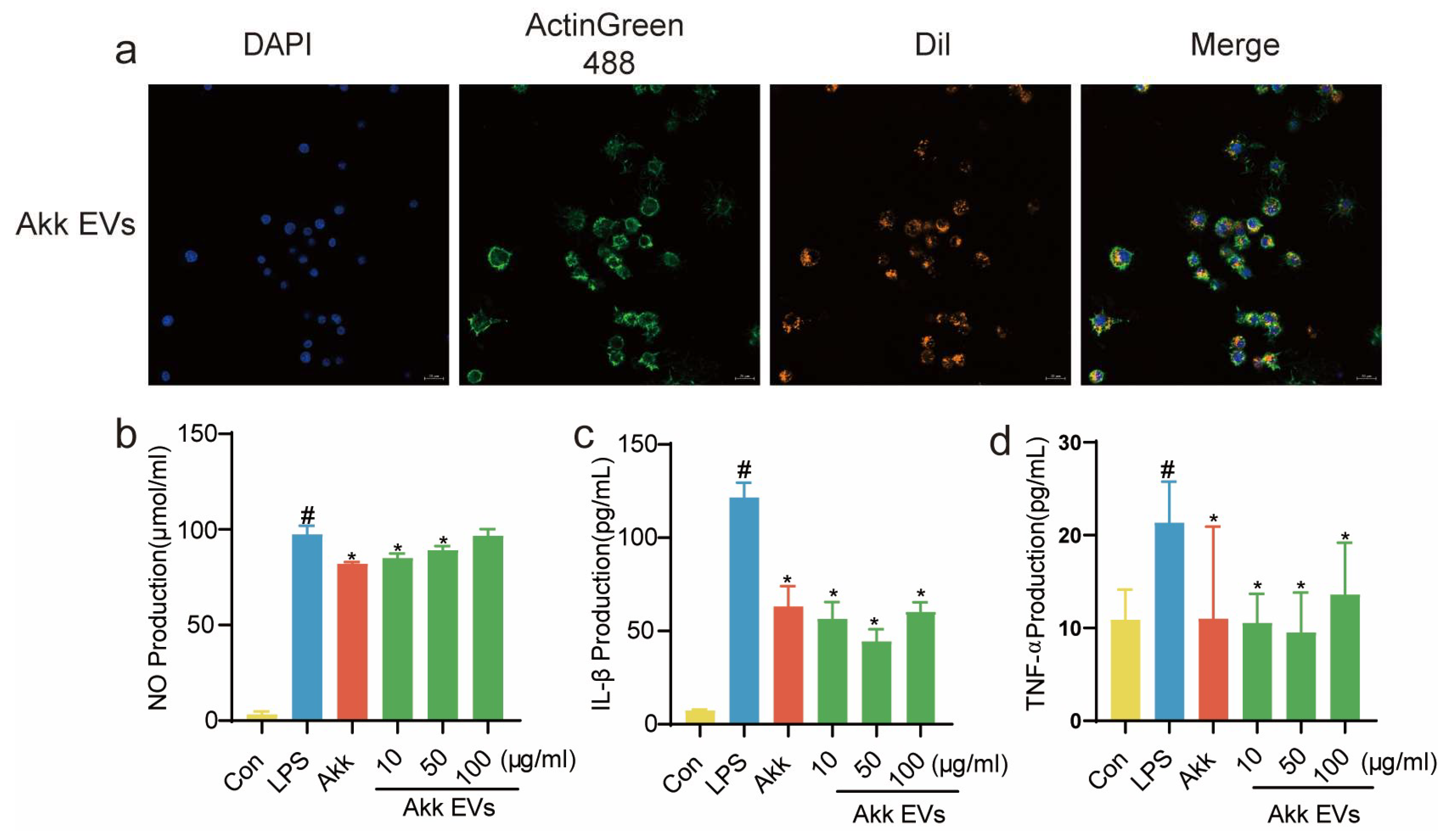
3.2. Cellular Uptake and Anti-Inflammatory Activity of Akk EVs
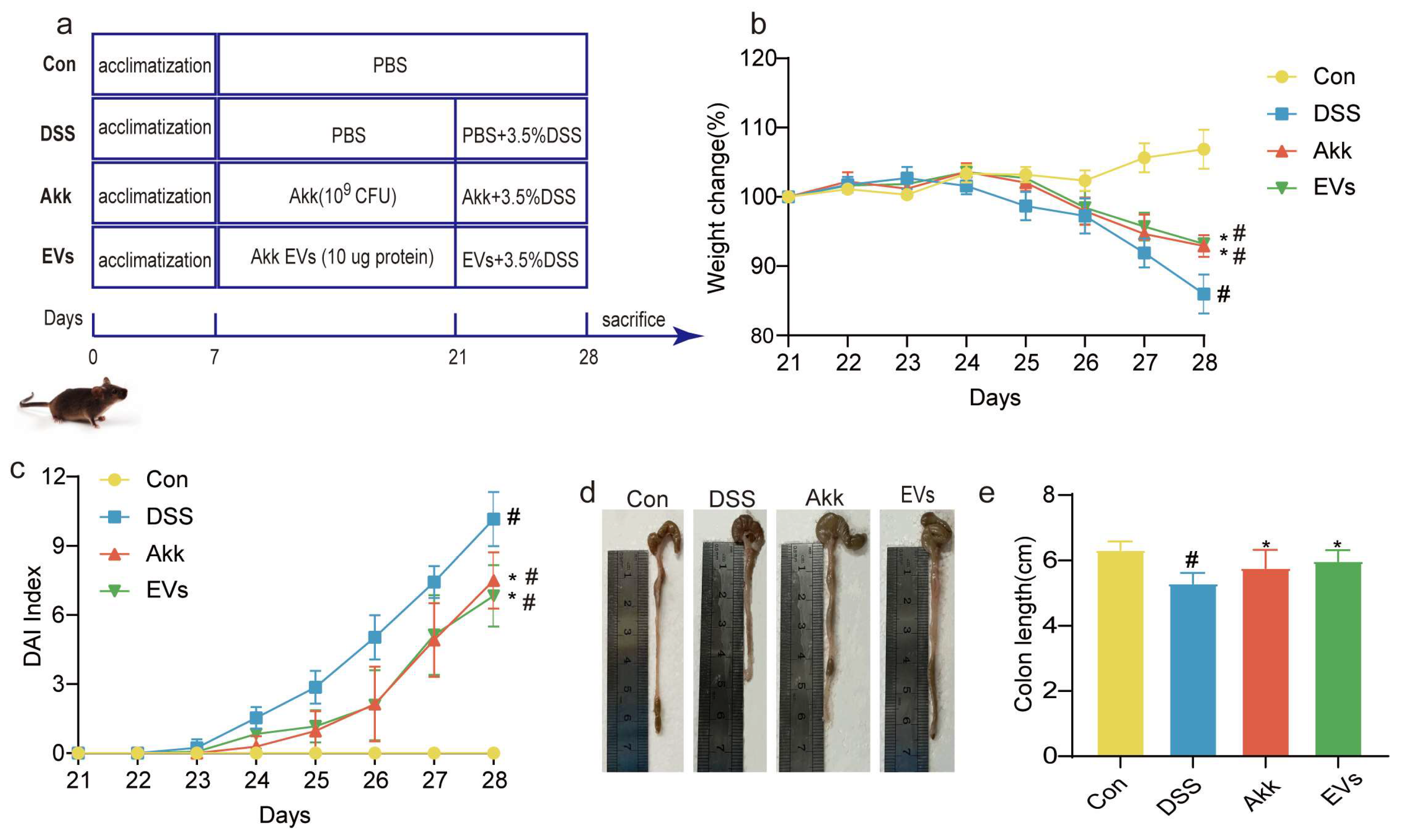
3.3. Effects of Akk EVs on the Symptoms
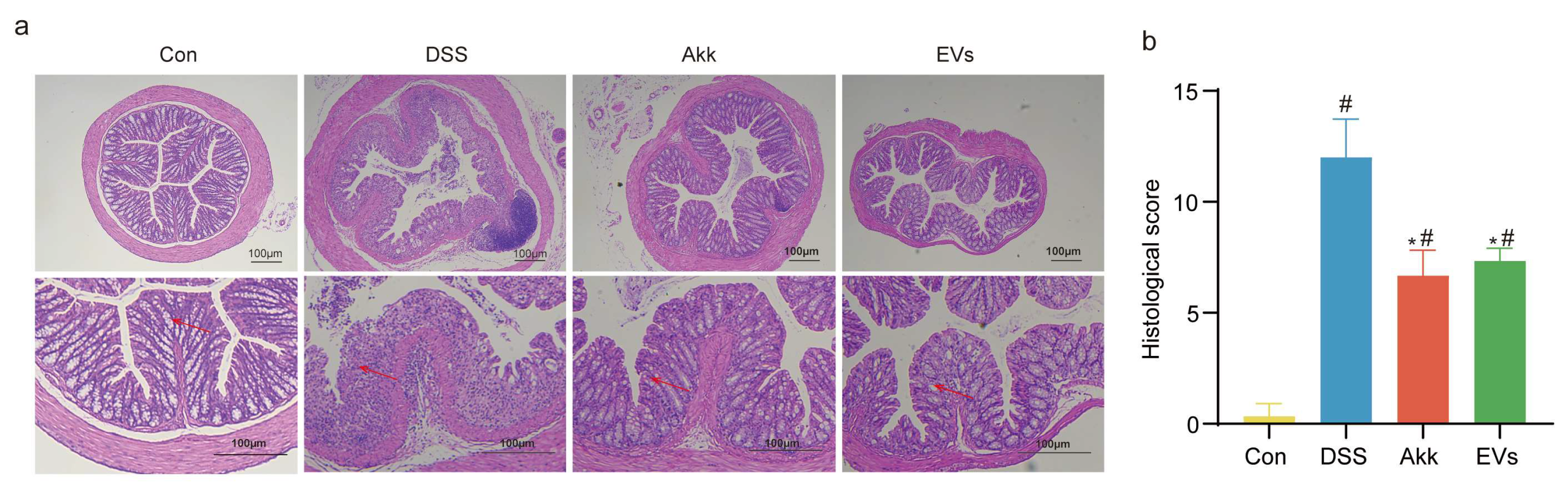
3.4. Effects of Akk EVs on Histopathological Characteristics
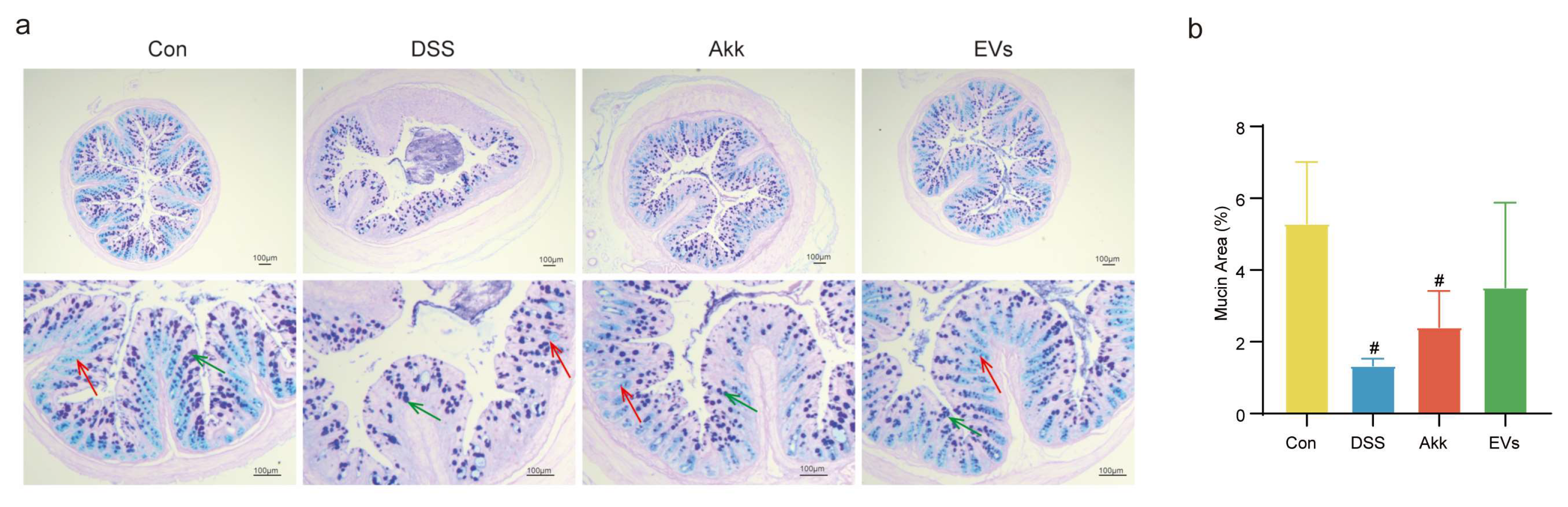
3.5. Effects of Akk EVs on Mucus Destruction and Goblet Cell Exhaustion
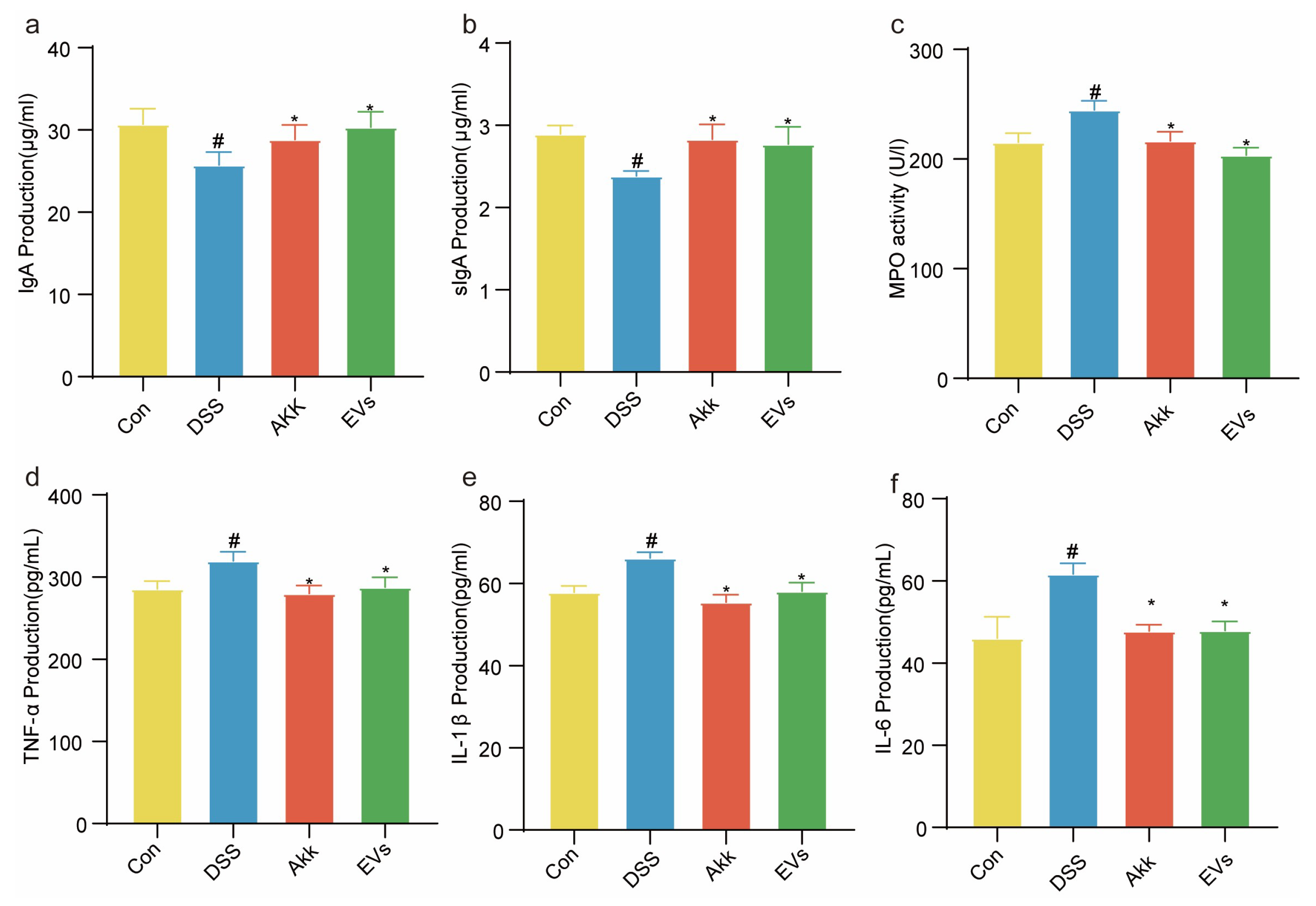
3.6. Effects of Akk EVs on Immunoglobulins and Inflammatory Cytokine
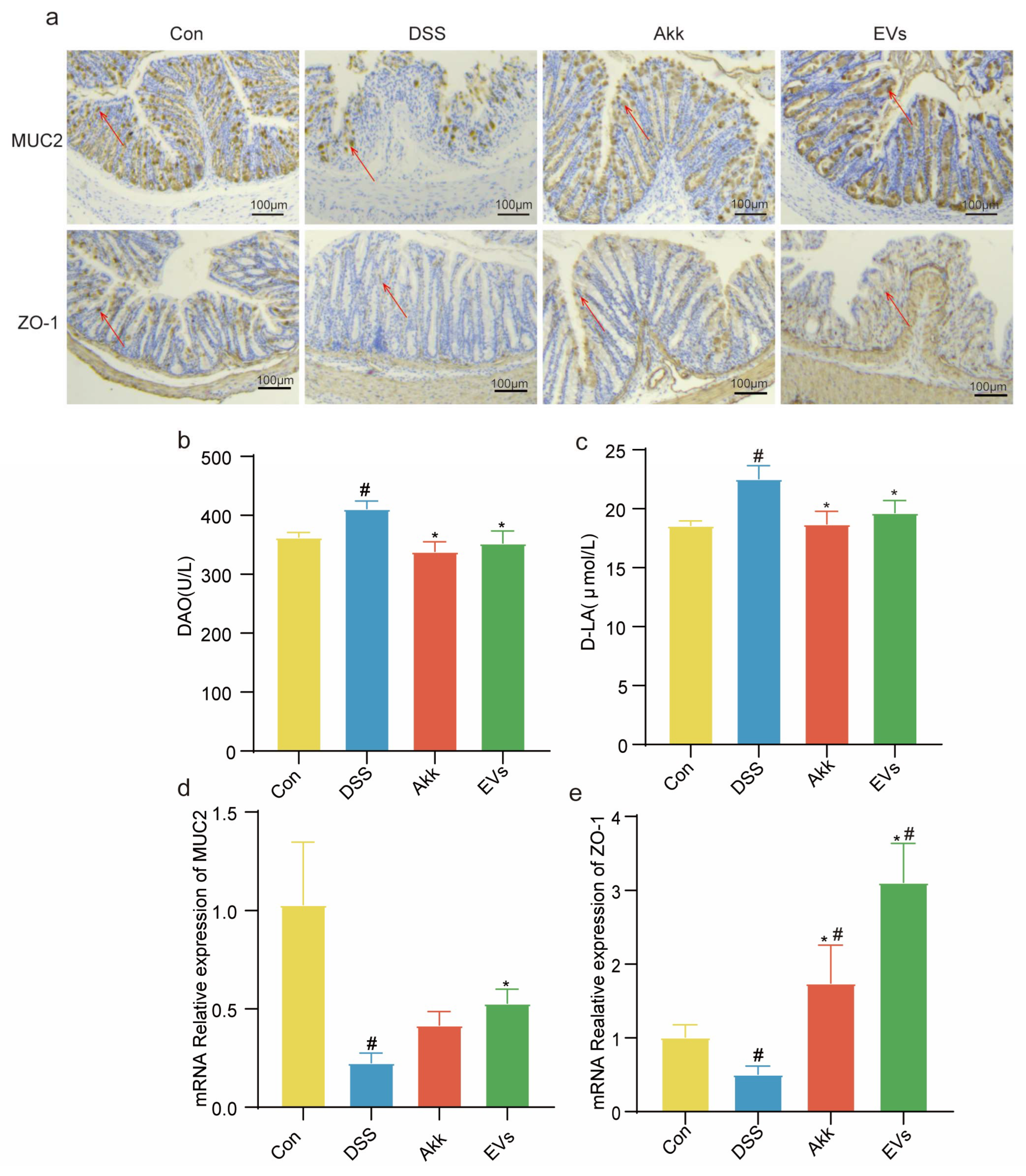
3.7. Effects of Akk EVs on Intestinal Permeability and Barrier Function
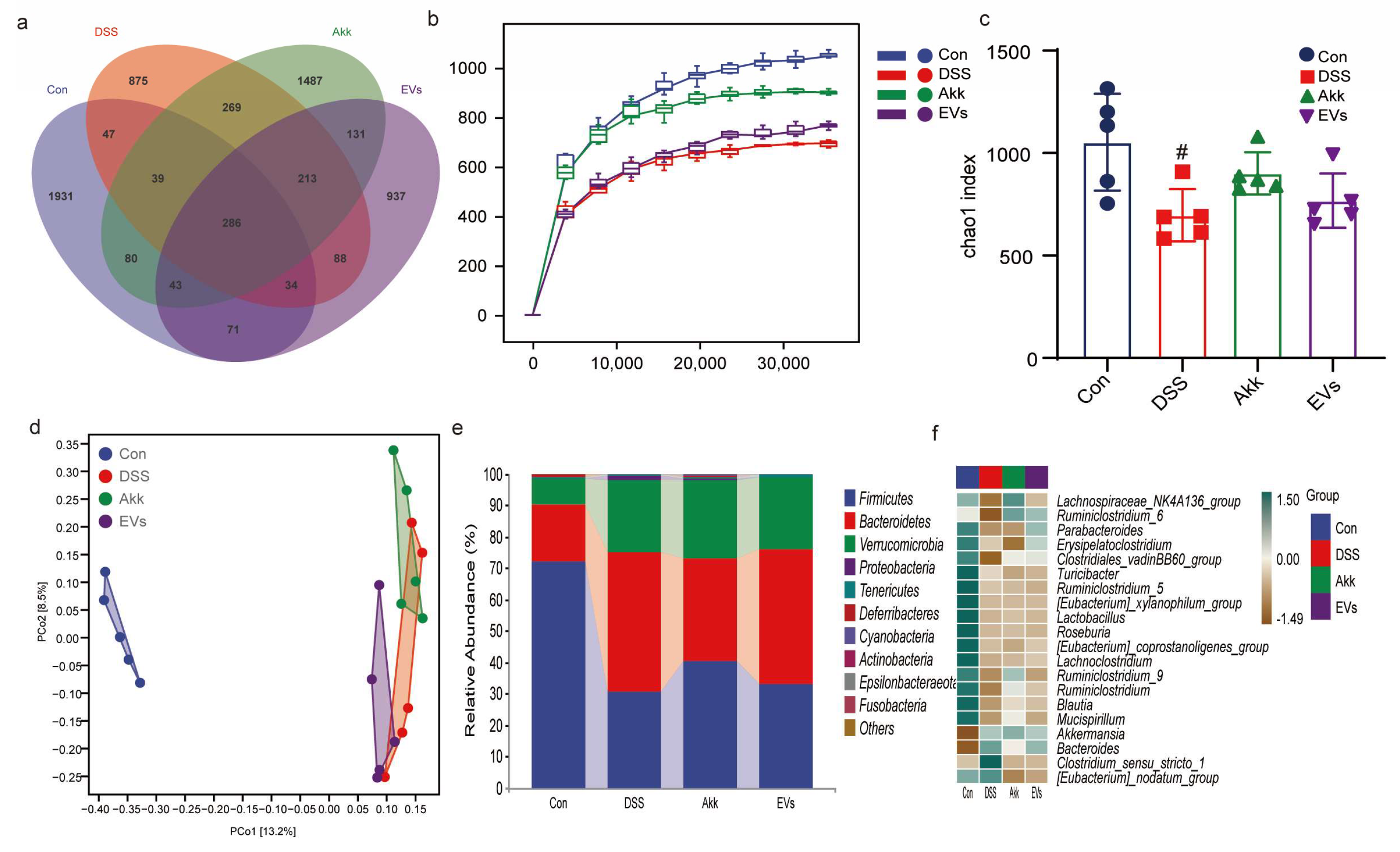
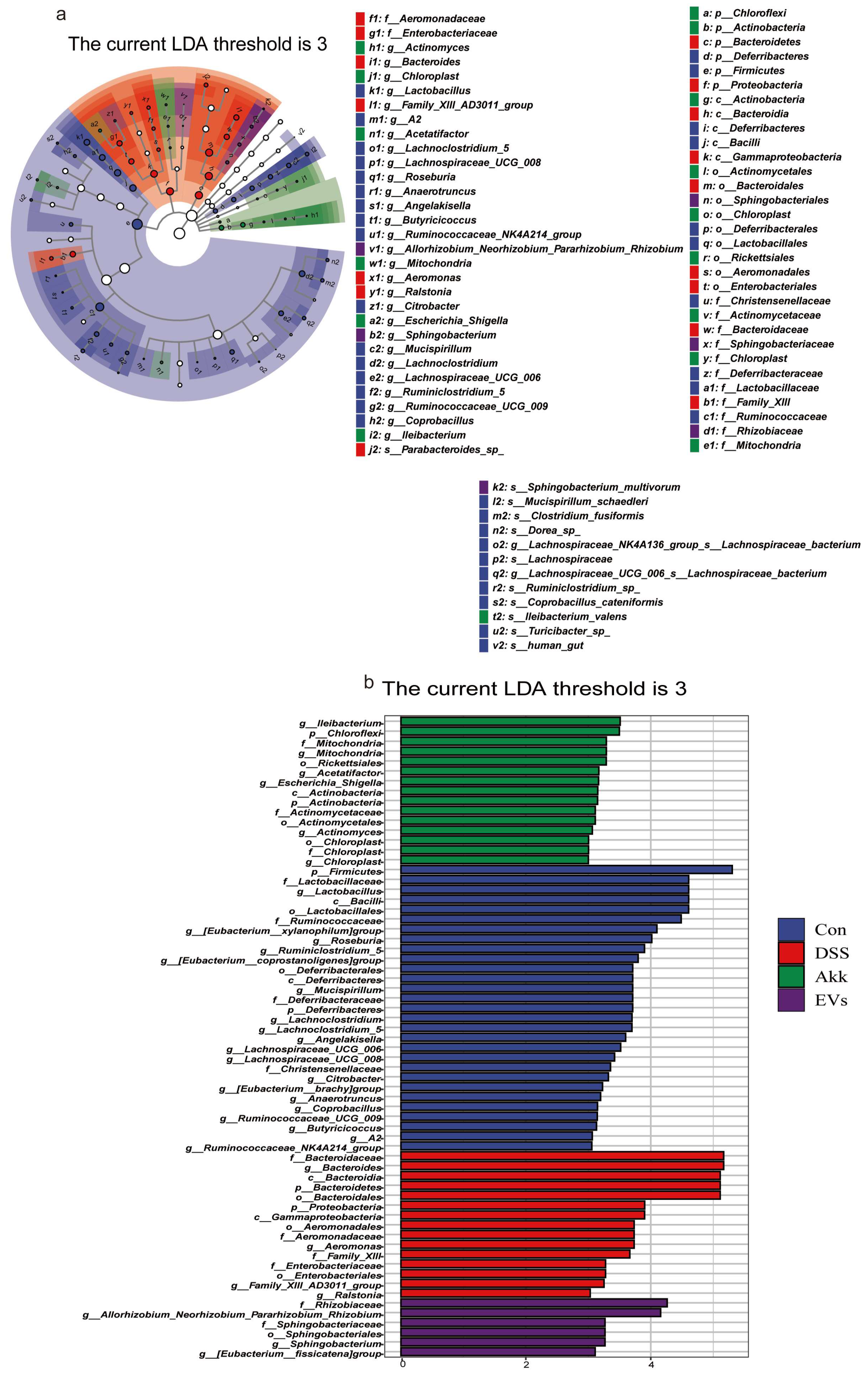
3.8. Effects of Akk EVs on Gut Microbiota
4. Discussion
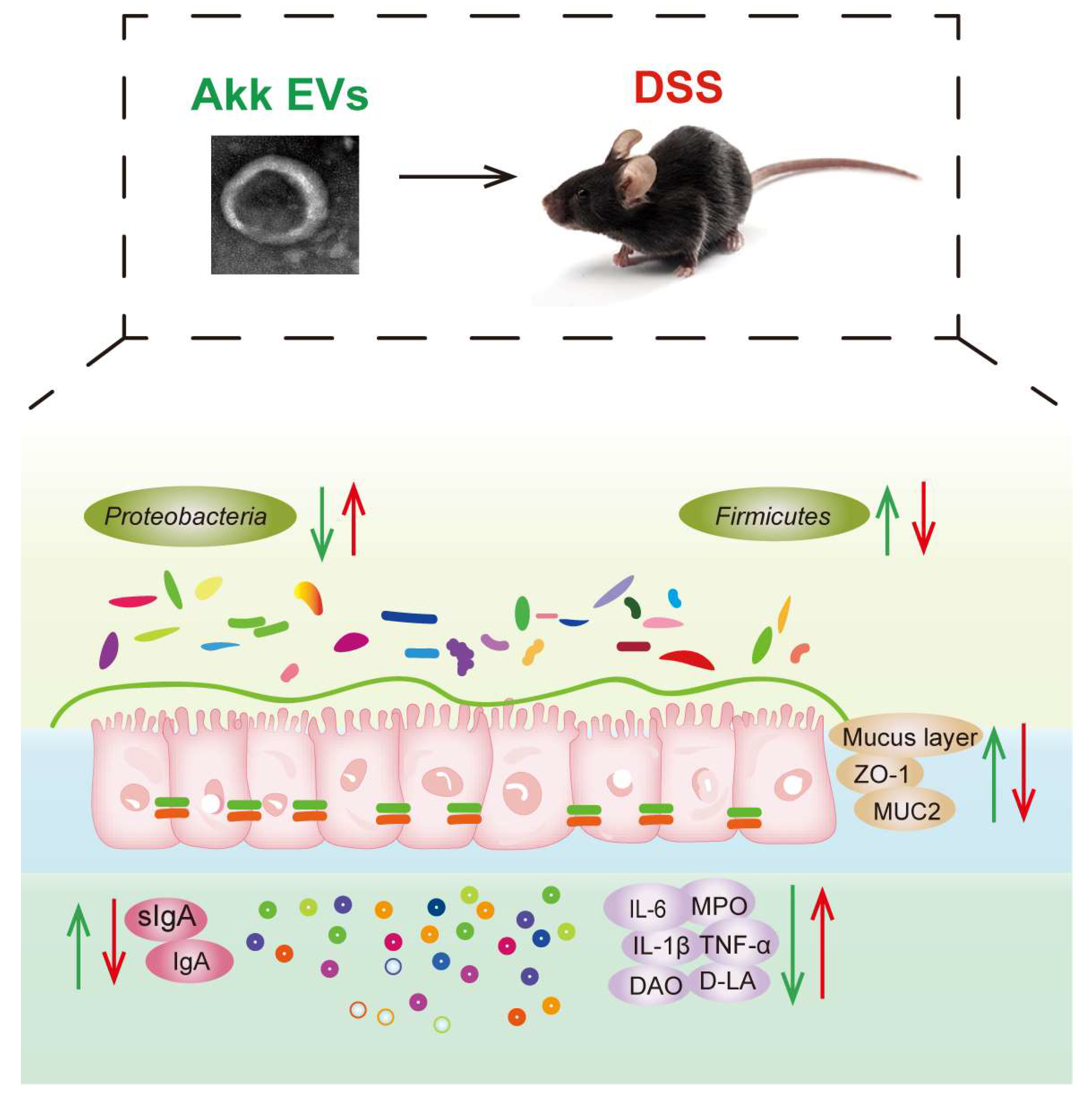
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2018, 4, 293–305. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The Four Epidemiological Stages in the Global Evolution of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xie, J.; Wang, Y.; Jiang, H.; Chen, K.; Li, D.; Wang, J.; Liu, Y.; He, J.; Zhou, J.; et al. Mannose Ameliorates Experimental Colitis by Protecting Intestinal Barrier Integrity. Nat. Commun. 2022, 13, 4804. [Google Scholar] [CrossRef] [PubMed]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the Human Gut Microbiome in Inflammatory Bowel Disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases. Intest. Res. 2015, 13, 11. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current Advances in the Anti-Inflammatory Effects and Mechanisms of Natural Polysaccharides. Crit. Rev. Food Sci. Nutr. 2022, 63, 5890–5910. [Google Scholar] [CrossRef]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.-M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipčík, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia Muciniphila Phospholipid Induces Homeostatic Immune Responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Wade, H.; Pan, K.; Duan, Q.; Kaluzny, S.; Pandey, E.; Fatumoju, L.; Saraswathi, V.; Wu, R.; Harris, E.N.; Su, Q. Akkermansia Muciniphila and Its Membrane Protein Ameliorates Intestinal Inflammatory Stress and Promotes Epithelial Wound Healing via CREBH and miR-143/145. J. Biomed. Sci. 2023, 30, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, R.; Duan, Z.; Yuan, X.; Ding, Y.; Feng, Z.; Bu, F.; Liu, L.; Wang, Q.; Zhou, J.; et al. Akkermansia Muciniphila Protects Against Psychological Disorder-Induced Gut Microbiota-Mediated Colonic Mucosal Barrier Damage and Aggravation of Colitis. Front. Cell. Infect. Microbiol. 2021, 11, 723856. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurised Bacterium Blunts Colitis Associated Tumourigenesis by Modulation of CD8+ T Cells in Mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Liang, X.; Dai, N.; Sheng, K.; Lu, H.; Wang, J.; Chen, L.; Wang, Y. Gut Bacterial Extracellular Vesicles: Important Players in Regulating Intestinal Microenvironment. Gut Microbes 2022, 14, 2134689. [Google Scholar] [CrossRef]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Wang, X.; Lin, S.; Wang, L.; Cao, Z.; Zhang, M.; Zhang, Y.; Liu, R.; Liu, J. Versatility of Bacterial Outer Membrane Vesicles in Regulating Intestinal Homeostasis. Sci. Adv. 2023, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Raftar, S.K.A.; Ashrafian, F.; Abdollahiyan, S.; Yadegar, A.; Moradi, H.R.; Masoumi, M.; Vaziri, F.; Moshiri, A.; Siadat, S.D.; Zali, M.R. The Anti-Inflammatory Effects of Akkermansia Muciniphila and Its Derivates in HFD/CCL4-Induced Murine Model of Liver Injury. Sci. Rep. 2022, 12, 2453. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Ban, M.; Choi, E.-J.; Moon, H.-G.; Jeon, J.-S.; Kim, D.-K.; Park, S.-K.; Jeon, S.G.; Roh, T.-Y.; Myung, S.-J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia Muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef]
- Greer, R.L.; Dong, X.; Moraes, A.C.F.; Zielke, R.A.; Fernandes, G.R.; Peremyslova, E.; Vasquez-Perez, S.; Schoenborn, A.A.; Gomes, E.P.; Pereira, A.C.; et al. Akkermansia Muciniphila Mediates Negative Effects of IFNγ on Glucose Metabolism. Nat. Commun. 2016, 7, 13329. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of Extracellular Vesicles Derived From Lactobacillus Plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dong, L.; Du, H.; Bao, Z.; Lin, S. Potential Mechanisms Underlying the Protective Effects of Tricholoma Matsutake Singer Peptides against LPS-Induced Inflammation in RAW264.7 Macrophages. Food Chem. 2021, 353, 129452. [Google Scholar] [CrossRef]
- Hu, R.; Lin, H.; Wang, M.; Zhao, Y.; Liu, H.; Min, Y.; Yang, X.; Gao, Y.; Yang, M. Lactobacillus Reuteri-Derived Extracellular Vesicles Maintain Intestinal Immune Homeostasis against Lipopolysaccharide-Induced Inflammatory Responses in Broilers. J. Anim. Sci. Biotechnol. 2021, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F.; et al. Milk-Derived Extracellular Vesicles Alleviate Ulcerative Colitis by Regulating the Gut Immunity and Reshaping the Gut Microbiota. Theranostics 2021, 11, 8570–8586. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.-S.; Giménez, R.; Cañas, M.-A.; Vera, R.; Díaz-Garrido, N.; Badia, J.; Baldomà, L. Extracellular Vesicles and Soluble Factors Secreted by Escherichia Coli Nissle 1917 and ECOR63 Protect against Enteropathogenic E. Coli-Induced Intestinal Epithelial Barrier Dysfunction. BMC Microbiol. 2019, 19, 166. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Carreras, J.; Shimizu, T.; Kakizaki, M.; Kikuti, Y.Y.; Roncador, G.; Nakamura, N.; Kotani, A. Anti-HBV Drug Entecavir Ameliorates DSS-Induced Colitis through PD-L1 Induction. Pharmacol. Res. 2022, 179, 105918. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Li, Q.-M.; Zha, X.-Q.; Luo, J.-P. Dendrobium Fimbriatum Hook Polysaccharide Ameliorates Dextran-Sodium-Sulfate-Induced Colitis in Mice via Improving Intestinal Barrier Function, Modulating Intestinal Microbiota, and Reducing Oxidative Stress and Inflammatory Responses. Food Funct. 2022, 13, 143–160. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, S.; Liu, Q.; Huang, C.; Hao, H.; Tan, M.S.; Yu, X.; Lou, C.K.L.; Huang, R.; Zhang, Z.; et al. Milk-Derived Extracellular Vesicles Protect Intestinal Barrier Integrity in the Gut-Liver Axis. Sci. Adv. 2023, 9, eade5041. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, X.; Zhang, Z.; Lv, Y.; Zhang, L.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 2020, 64, 1901251. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Nigam, G.B.; Bate, S.; Hamdy, S.; Limdi, J.K. The Prevalence and Burden of Rome IV Faecal Incontinence in Ulcerative Colitis: A Cross-sectional Study. Aliment. Pharmacol. Ther. 2023, 58, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Faye, A.S.; Allin, K.H.; Iversen, A.T.; Agrawal, M.; Faith, J.; Colombel, J.-F.; Jess, T. Antibiotic Use as a Risk Factor for Inflammatory Bowel Disease across the Ages: A Population-Based Cohort Study. Gut 2023, 72, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.Y.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-First Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef]
- Mehandru, S.; Colombel, J.-F. The Intestinal Barrier, an Arbitrator Turned Provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 83–84. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia Muciniphila: Paradigm for next-Generation Beneficial Microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Fan, L.; Qi, Y.; Xu, C.; Hu, Y.; Chen, S.; Liu, W.; Liu, W.; Si, J. Akkermansia Muciniphila Alleviates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by NLRP3 Activation. Microbiol. Spectr. 2021, 9, e00730-21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Tang, L.; Wang, F.; Huang, S.; Liu, S.; Lei, Y.; Wang, S.; Xie, Z.; Wang, W.; et al. TLR4 Regulates RORγt+ Regulatory T-Cell Responses and Susceptibility to Colon Inflammation through Interaction with Akkermansia Muciniphila. Microbiome 2022, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia Muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-Membrane Vesicles from Gram-Negative Bacteria: Biogenesis and Functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-Derived Extracellular Vesicles in Interkingdom Communication in the Gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef]
- Qing, S.; Lyu, C.; Zhu, L.; Pan, C.; Wang, S.; Li, F.; Wang, J.; Yue, H.; Gao, X.; Jia, R.; et al. Biomineralized Bacterial Outer Membrane Vesicles Potentiate Safe and Efficient Tumor Microenvironment Reprogramming for Anticancer Therapy. Adv. Mater. 2020, 32, 2002085. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and Functions of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lin, H.; Li, J.; Zhao, Y.; Wang, M.; Sun, X.; Min, Y.; Gao, Y.; Yang, M. Probiotic Escherichia Coli Nissle 1917-Derived Outer Membrane Vesicles Enhance Immunomodulation and Antimicrobial Activity in RAW264.7 Macrophages. BMC Microbiol. 2020, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.C.; Clare, S.; Beresford-Jones, B.S.; Harcourt, K.; Notley, G.; Stares, M.D.; Kumar, N.; Soderholm, A.T.; Adoum, A.; Wong, H.; et al. Identification of Gut Microbial Species Linked with Disease Variability in a Widely Used Mouse Model of Colitis. Nat. Microbiol. 2022, 7, 590–599. [Google Scholar] [CrossRef]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of Commensal and Pathogenic Microorganisms with the Intestinal Mucosal Barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, M.; Xu, J.; Xu, H.; Zhu, M.; Liang, Y.; Zhang, Y.; Tian, C.; Nie, Y.; Shi, R.; et al. Extracellular Vesicles: The Next Generation Theranostic Nanomedicine for Inflammatory Bowel Disease. IJN 2022, 17, 3893–3911. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.H.; Nyström, E.E.L.; Johansson, M.E.V.; Hansson, G.C. A Sentinel Goblet Cell Guards the Colonic Crypt by Triggering Nlrp6-Dependent Muc2 Secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Cornick, S.; Kumar, M.; Moreau, F.; Gaisano, H.; Chadee, K. VAMP8-Mediated MUC2 Mucin Exocytosis from Colonic Goblet Cells Maintains Innate Intestinal Homeostasis. Nat. Commun. 2019, 10, 4306. [Google Scholar] [CrossRef]
- Yao, Y.; Kim, G.; Shafer, S.; Chen, Z.; Kubo, S.; Ji, Y.; Luo, J.; Yang, W.; Perner, S.P.; Kanellopoulou, C.; et al. Mucus Sialylation Determines Intestinal Host-Commensal Homeostasis. Cell 2022, 185, 1172–1188.e28. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial Tryptophan Metabolites Regulate Gut Barrier Function via the Aryl Hydrocarbon Receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Zhong, W.; Zheng, Y.; Li, Y.; Guo, L.; Zhang, Y.; Ran, Y.; Zhao, J.; Zhou, L.; et al. Leaky Gut Driven by Dysbiosis Augments Activation and Accumulation of Liver Macrophages via RIP3 Signaling Pathway in Autoimmune Hepatitis. Front. Immunol. 2021, 12, 624360. [Google Scholar] [CrossRef]
- Džidić-Krivić, A.; Kusturica, J.; Sher, E.K.; Selak, N.; Osmančević, N.; Karahmet Farhat, E.; Sher, F. Effects of Intestinal Flora on Pharmacokinetics and Pharmacodynamics of Drugs. Drug Metab. Rev. 2023, 55, 126–139. [Google Scholar] [CrossRef]
- Farhat, E.K.; Sher, E.K.; Džidić-Krivić, A.; Banjari, I.; Sher, F. Functional Biotransformation of Phytoestrogens by Gut Microbiota with Impact on Cancer Treatment. J. Nutr. Biochem. 2023, 118, 109368. [Google Scholar] [CrossRef]
- Hughes, E.R.; Winter, M.G.; Duerkop, B.A.; Spiga, L.; Furtado De Carvalho, T.; Zhu, W.; Gillis, C.C.; Büttner, L.; Smoot, M.P.; Behrendt, C.L.; et al. Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host Microbe 2017, 21, 208–219. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The Gut Microbiota and Inflammatory Bowel Disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What Role Do Proteobacteria Play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wu, J.; Gong, J.; Ke, J.; Ding, T.; Zhao, W.; Cheng, W.M.; Luo, Z.; He, Q.; Zeng, W.; et al. Microbiota in Mesenteric Adipose Tissue from Crohn’s Disease Promote Colitis in Mice. Microbiome 2021, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut Firmicutes: Relationship with Dietary Fiber and Role in Host Homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Hao, W.-J.; Zhou, R.-M.; Huang, C.-L.; Wang, X.-Y.; Liu, Y.-S.; Li, X.-Z. Pretreatment with Bifidobacterium Longum BAA2573 Ameliorates Dextran Sulfate Sodium (DSS)-Induced Colitis by Modulating Gut Microbiota. Front. Microbiol. 2023, 14, 1211259. [Google Scholar] [CrossRef]
- Huang, S.; Hu, S.; Liu, S.; Tang, B.; Liu, Y.; Tang, L.; Lei, Y.; Zhong, L.; Yang, S.; He, S. Lithium Carbonate Alleviates Colon Inflammation through Modulating Gut Microbiota and Treg Cells in a GPR43-Dependent Manner. Pharmacol. Res. 2022, 175, 105992. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, R.; Wang, C.; Lin, S.; Wang, L.; Pang, Y. Fluorescence-Activating and Absorption-Shifting Nanoprobes for Anaerobic Tracking of Gut Microbiota Derived Vesicles. ACS Nano 2023, 17, 2279–2293. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Zhang, Y.; Zhao, H.; Cui, J.; Li, J.; Di, L. Emerging Prospects of Extracellular Vesicles for Brain Disease Theranostics. J. Control. Release 2022, 341, 844–868. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.-Z.; Li, Z.-H.; Bai, X.-F.; Liu, C.-J.; Zhang, X.-Z. Hybrid Vesicles Based on Autologous Tumor Cell Membrane and Bacterial Outer Membrane To Enhance Innate Immune Response and Personalized Tumor Immunotherapy. Nano Letters 2021, 21, 8609–8618. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, T.; Hao, H.; Liu, Q.; Li, J.; Yao, Y.; Liu, Y.; Zhang, T.; Zhang, Z.; Yi, H. Effect of Extracelluar Vesicles Derived from Akkermansia muciniphila on Intestinal Barrier in Colitis Mice. Nutrients 2023, 15, 4722. https://doi.org/10.3390/nu15224722
Zheng T, Hao H, Liu Q, Li J, Yao Y, Liu Y, Zhang T, Zhang Z, Yi H. Effect of Extracelluar Vesicles Derived from Akkermansia muciniphila on Intestinal Barrier in Colitis Mice. Nutrients. 2023; 15(22):4722. https://doi.org/10.3390/nu15224722
Chicago/Turabian StyleZheng, Ting, Haining Hao, Qiqi Liu, Jiankun Li, Yukun Yao, Yisuo Liu, Tai Zhang, Zhe Zhang, and Huaxi Yi. 2023. "Effect of Extracelluar Vesicles Derived from Akkermansia muciniphila on Intestinal Barrier in Colitis Mice" Nutrients 15, no. 22: 4722. https://doi.org/10.3390/nu15224722
APA StyleZheng, T., Hao, H., Liu, Q., Li, J., Yao, Y., Liu, Y., Zhang, T., Zhang, Z., & Yi, H. (2023). Effect of Extracelluar Vesicles Derived from Akkermansia muciniphila on Intestinal Barrier in Colitis Mice. Nutrients, 15(22), 4722. https://doi.org/10.3390/nu15224722






