Rifaximin Ameliorates Loperamide-Induced Constipation in Rats through the Regulation of Gut Microbiota and Serum Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. Physiological Indices for the Rats
2.2.1. Body Weight Changes and Food Intake
2.2.2. Fecal Parameters
2.2.3. Feces, Blood, and Colon Tissue Collection
2.2.4. Intestinal Propulsive Rate
2.3. Hematoxylin and Eosin Stain (H&E)
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT–qPCR)
2.6. 16S rRNA High-Throughput Sequencing of Fecal Microbiota
2.7. Metabolomics Analysis
2.8. Statistical Analysis
3. Results
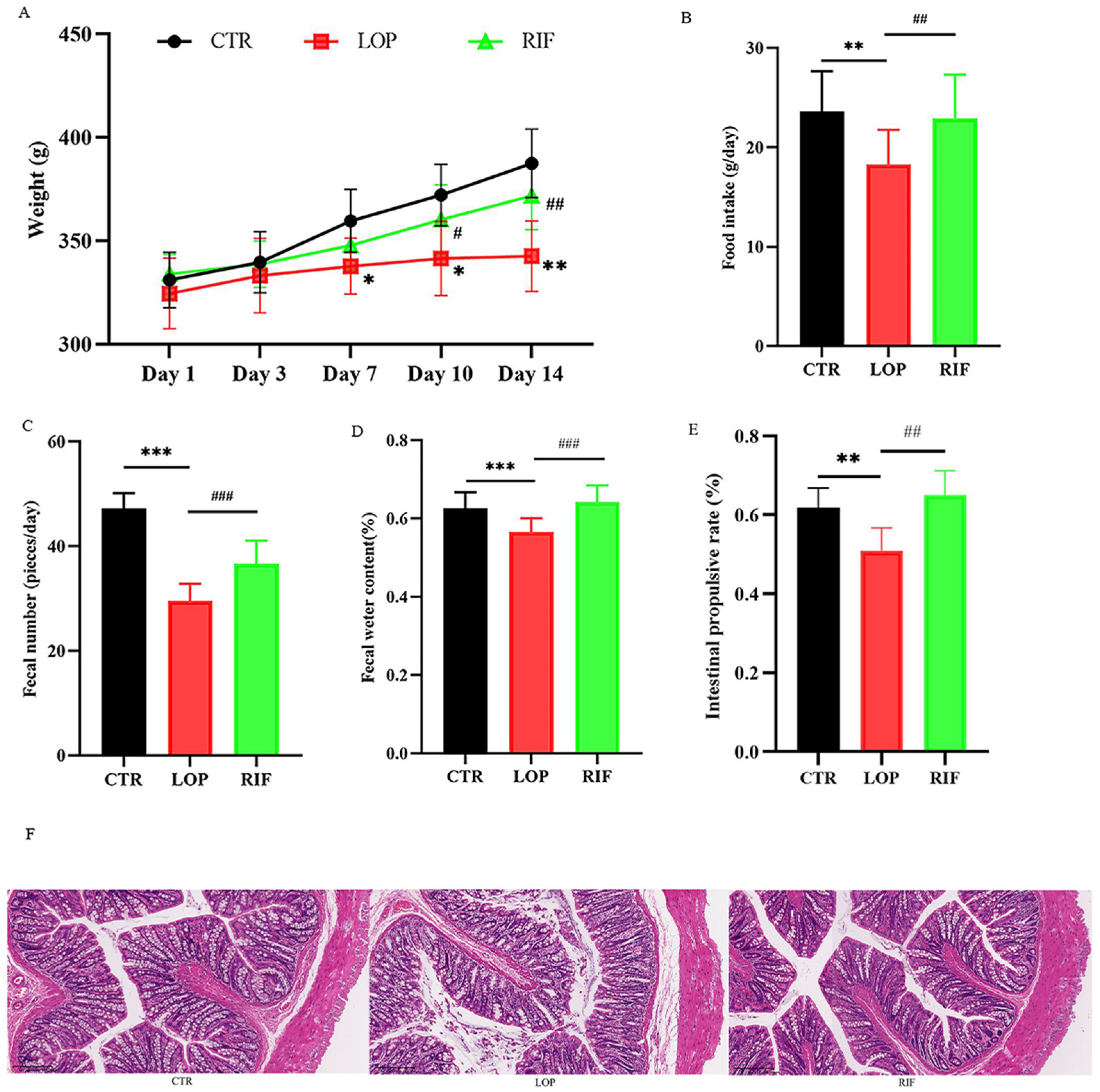
3.1. Rifaximin Ameliorates Physiological Indices in Constipated Rats
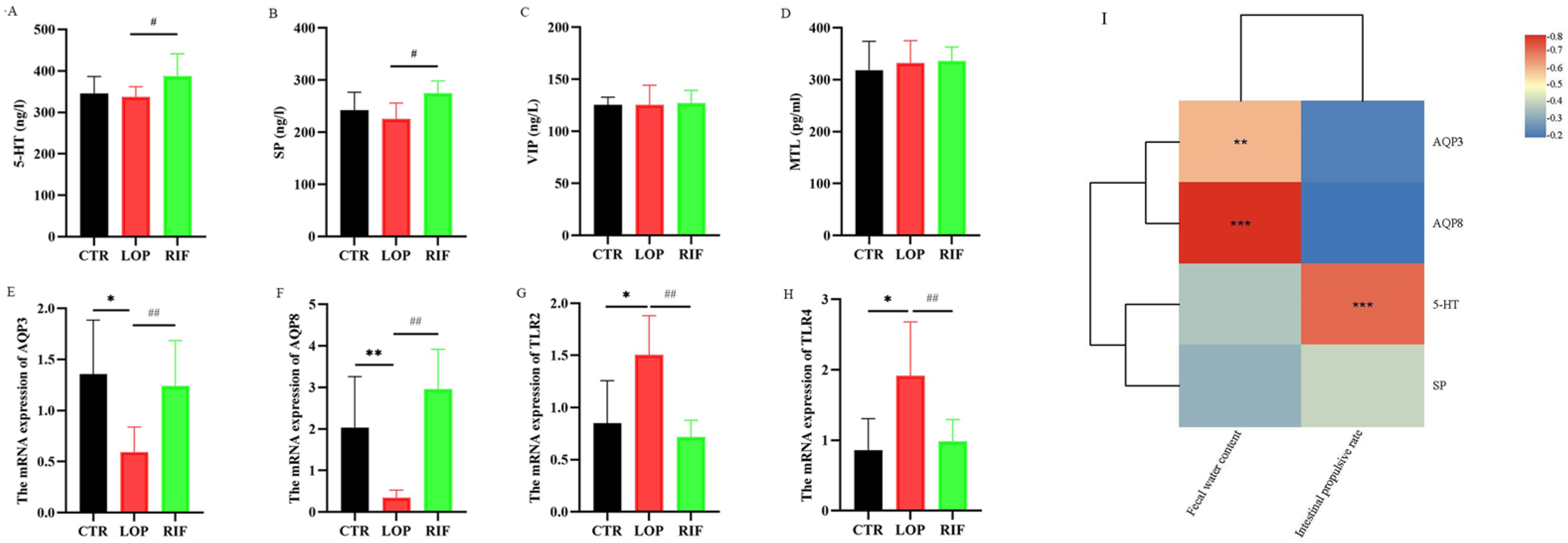
3.2. Rifaximin Affects Serum Neurotransmitters, Neuropeptides, and mRNA Expression of Inflammatory Cytokines and Aquaporins in Colon Tissues
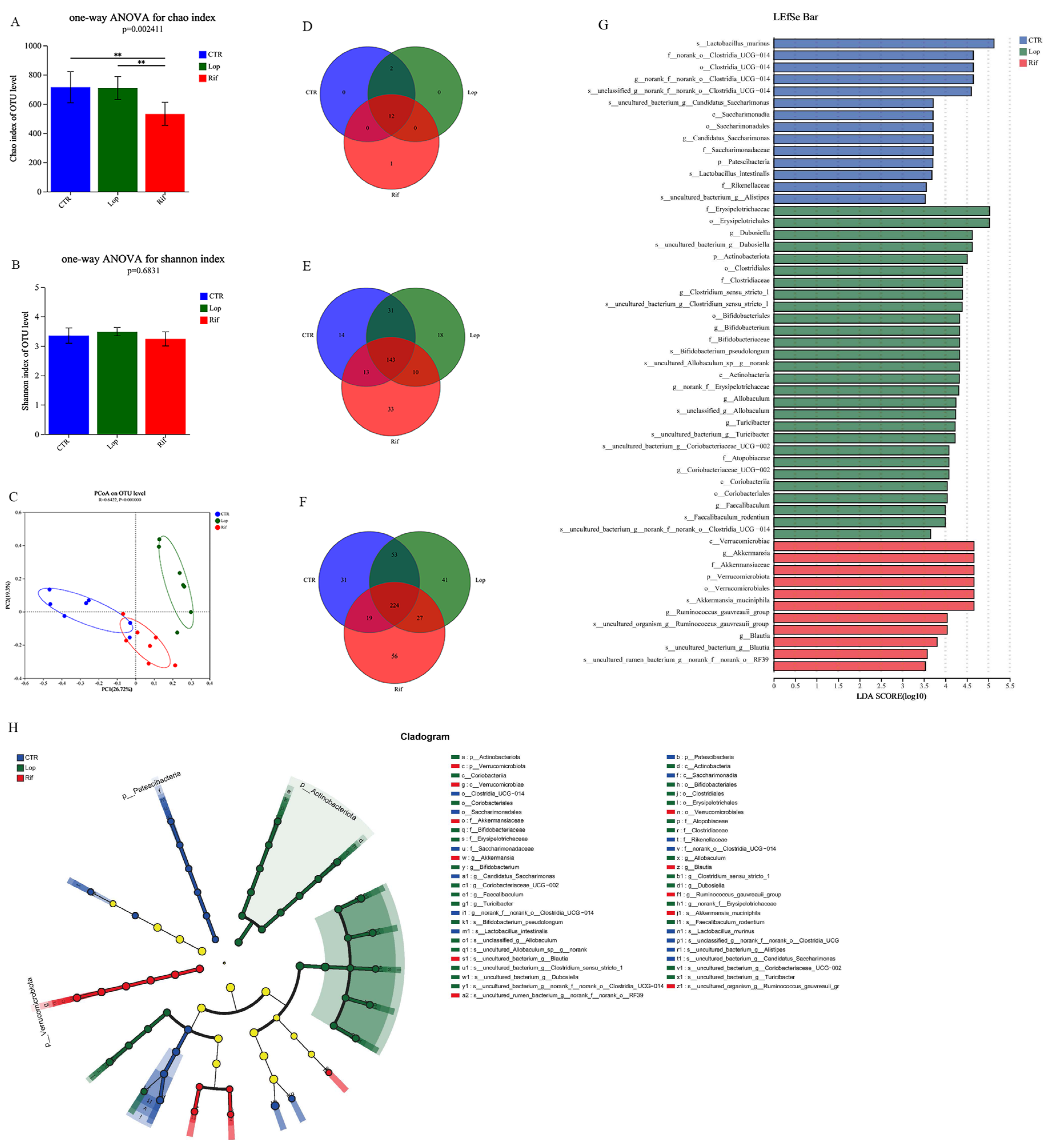
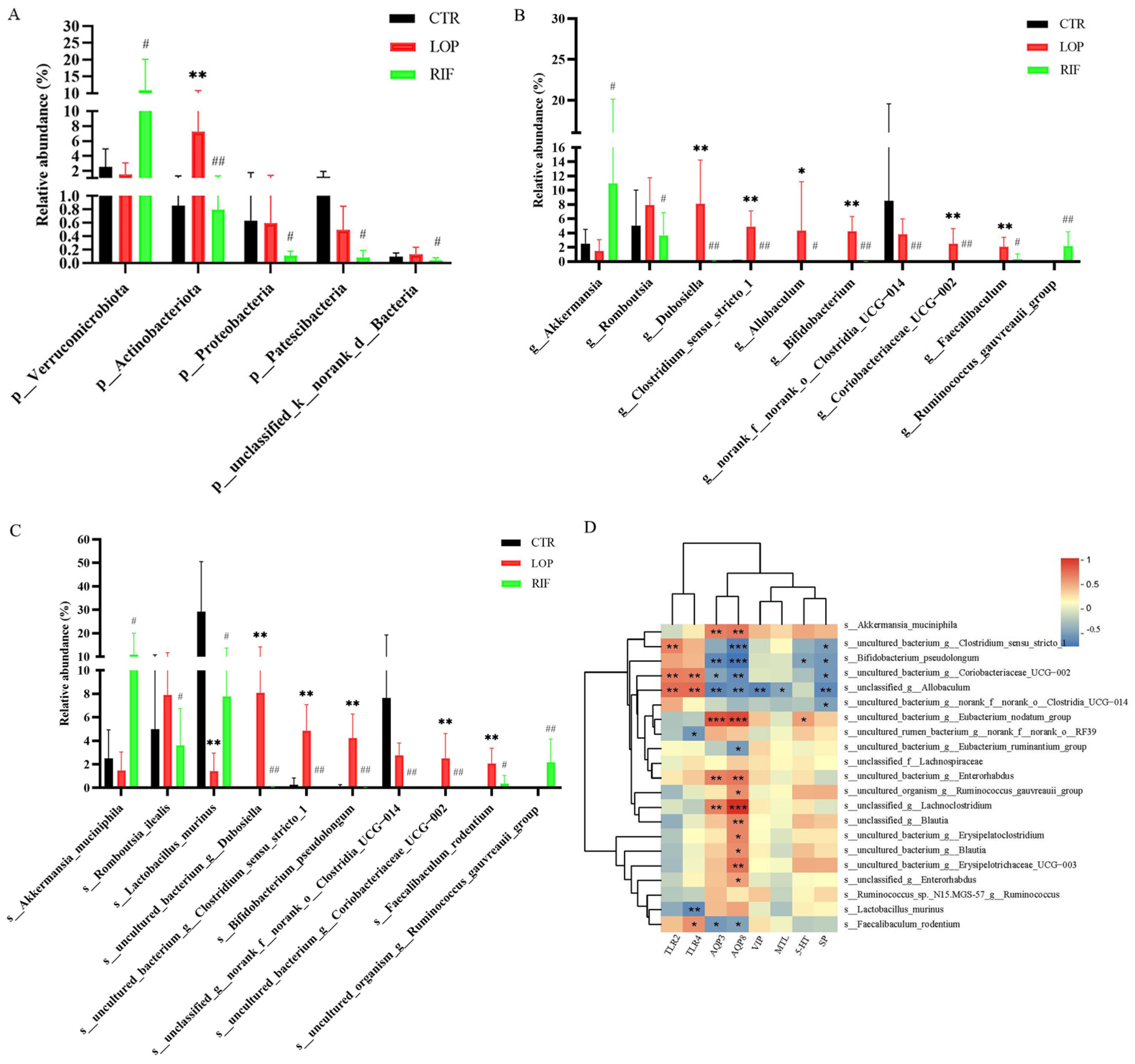
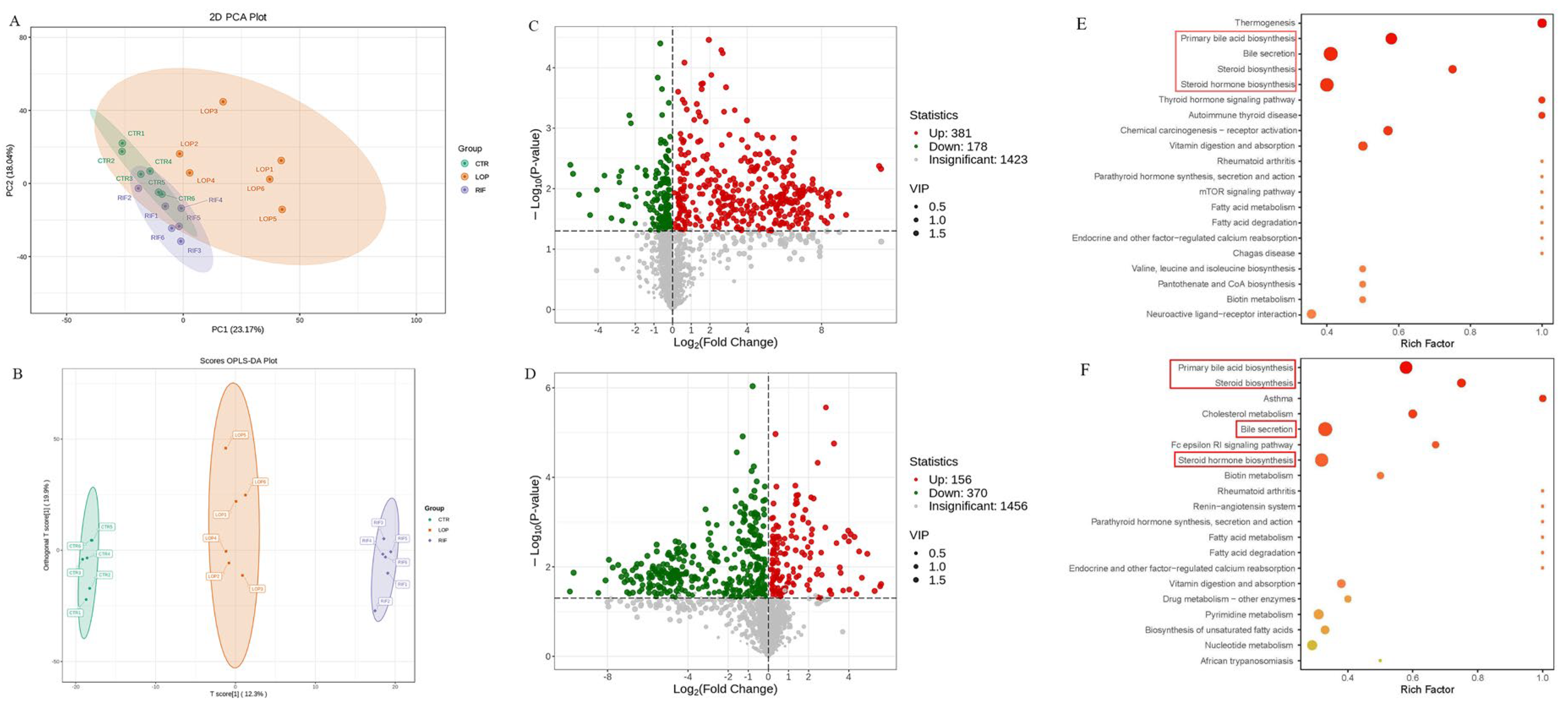
3.3. Rifaximin Regulates the Structures of Gut Microbiota in Constipated Rats
3.4. Functional Prediction Analysis of Gut Microbiota
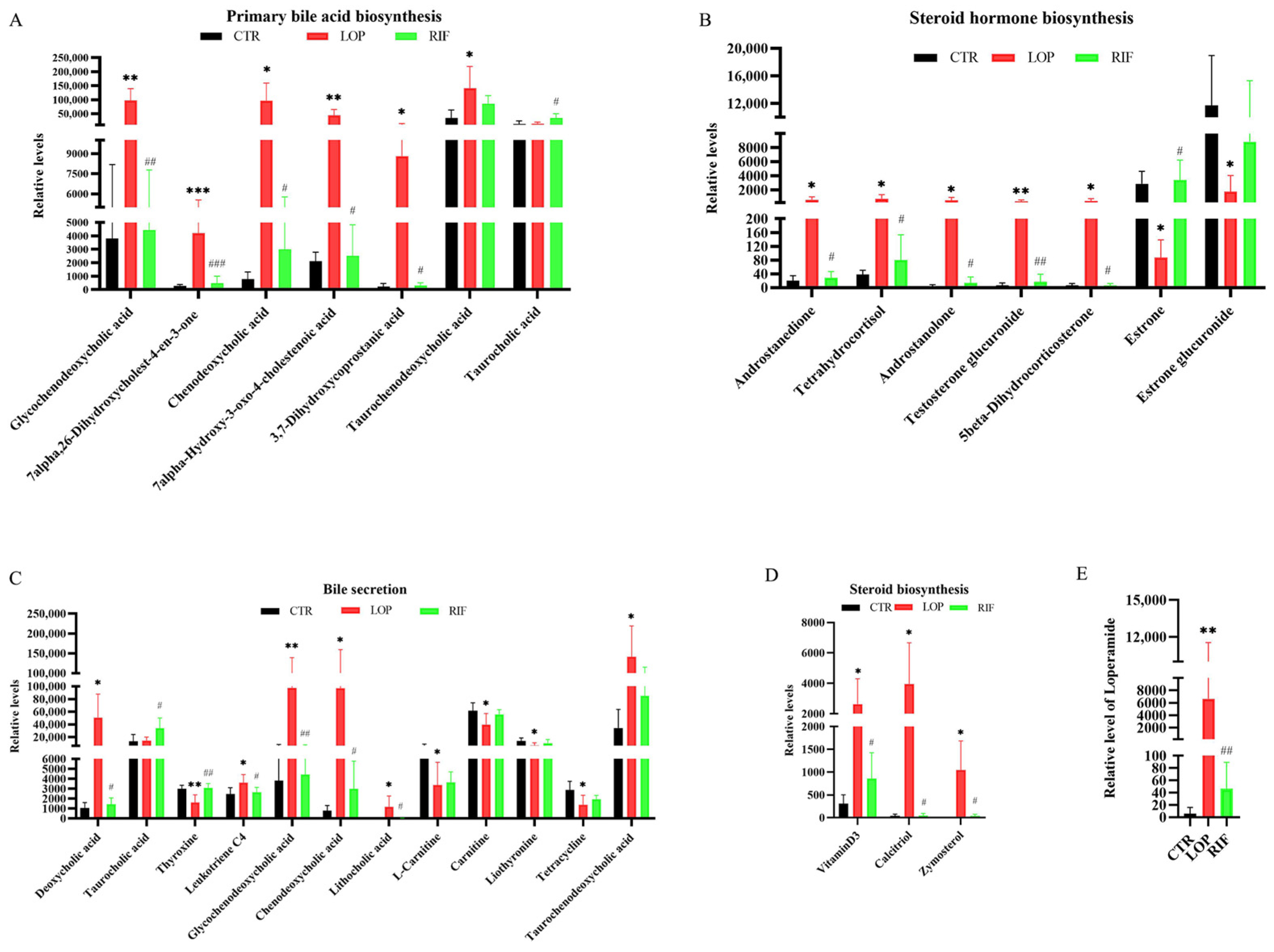
3.5. Rifaximin Affects Serum Metabolites in Constipated Rats
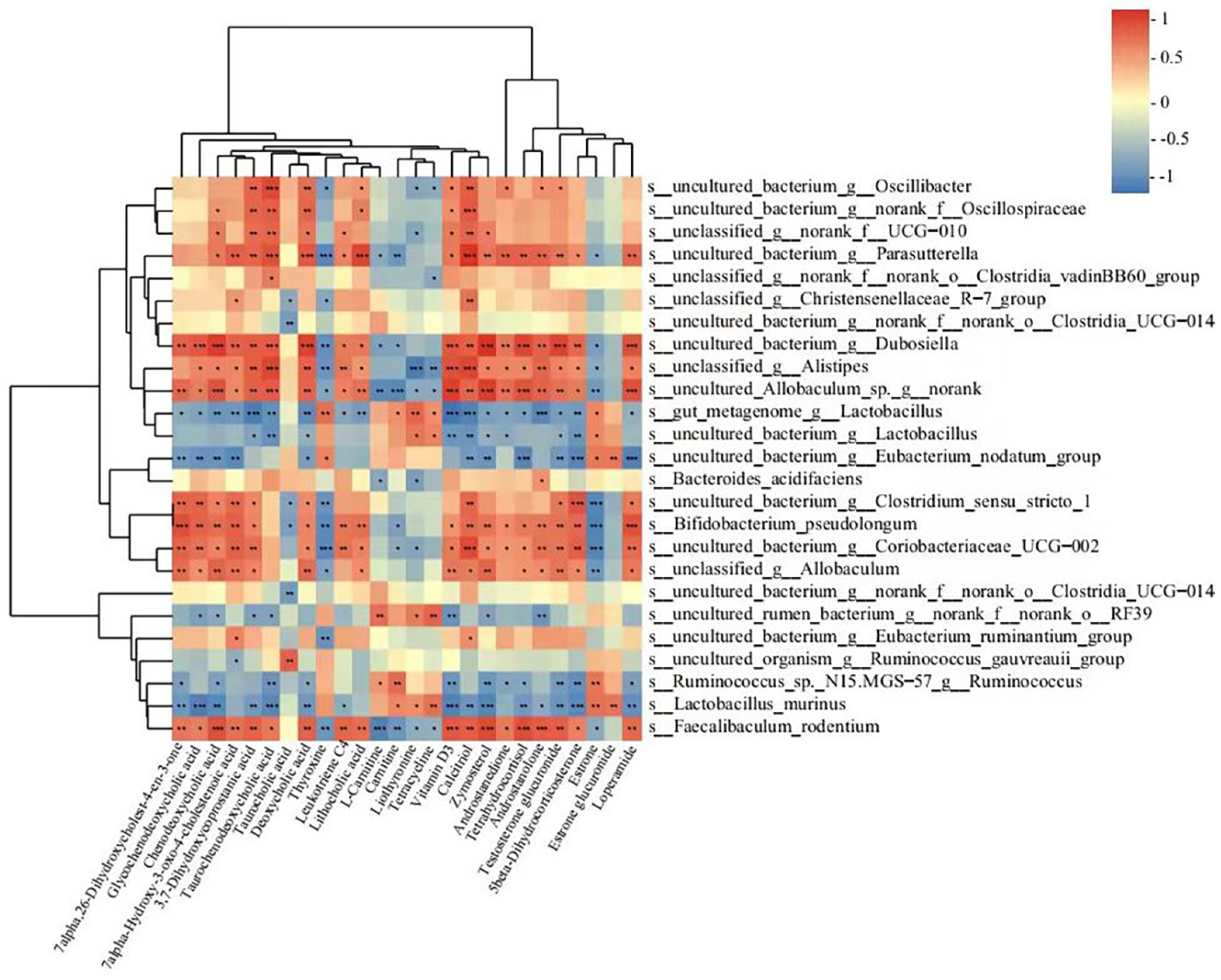
3.6. Correlations between Gut Microbiota and the Metabolites Involved in the Primary Bile Acid Biosynthesis, Bile Secretion, Steroid Hormone Biosynthesis, and Steroid Biosynthesis Metabolic Pathways
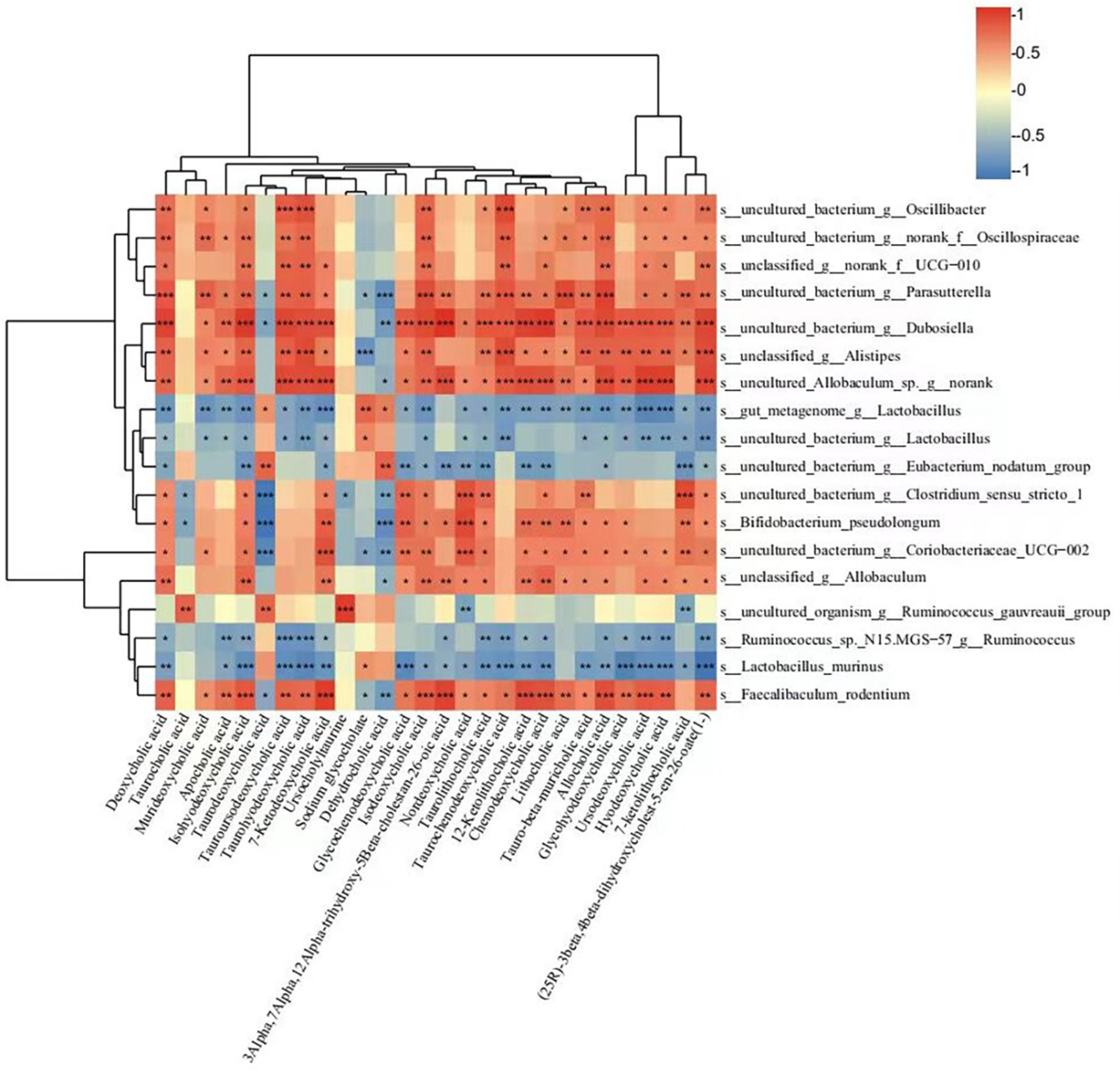
3.7. Rifaximin Affects Serum Bas in Constipated Rats
3.8. Correlations between Gut Microbiota and BAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Camilleri, M.; Ford, A.C.; Mawe, G.M.; Dinning, P.G.; Rao, S.S.; Chey, W.D.; Simrén, M.; Lembo, A.; Young-Fadok, T.M.; Chang, L. Chronic constipation. Nat. Rev. Dis. Primers 2017, 3, 17095. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Barbaro, M.R.; Marasco, G.; Cremon, C. Chronic constipation: From pathophysiology to management. Minerva Gastroenterol. 2023, 69, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Wald, A. Chronic Constipation. Mayo Clin. Proc. 2019, 94, 2340–2357. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Gong, X.; Siah, K.T.; Pratap, N.; Ghoshal, U.C.; Abdullah, M.; Syam, A.F.; Bak, Y.T.; Choi, M.G.; Lu, C.L.; et al. Rome foundation Asian working team report: Real world treatment experience of Asian patients with functional bowel disorders. J. Gastroenterol. Hepatol. 2017, 32, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Khalif, I.L.; Quigley, E.M.; Konovitch, E.A.; Maximova, I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef]
- Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Zhang, Y.; Li, W.; Jiang, S.; Qian, D.; Duan, J. Gut microbiota: A new avenue to reveal pathological mechanisms of constipation. Appl. Microbiol. Biotechnol. 2022, 106, 6899–6913. [Google Scholar] [CrossRef]
- Zoppi, G.; Cinquetti, M.; Luciano, A.; Benini, A.; Muner, A.; Bertazzoni Minelli, E. The intestinal ecosystem in chronic functional constipation. Acta Paediatr. 1998, 87, 836–841. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural changes in the gut microbiome of constipated patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, A.; Jackson, M.; Valestin, J.; Rao, S.S. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am. J. Gastroenterol. 2010, 105, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Camilleri, M.; Vijayvargiya, P.; Busciglio, I.; Burton, D.; Ryks, M.; Rhoten, D.; Lueke, A.; Saenger, A.; Girtman, A.; et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 1270–1275.e1271. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Lauritano, E.C.; Barbaro, F.; Migneco, A.; Ainora, M.E.; Fontana, L.; Gabrielli, M.; Gasbarrini, A. Rifaximin pharmacology and clinical implications. Expert. Opin. Drug Metab. Toxicol. 2009, 5, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.M.; et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflamm. 2021, 18, 254. [Google Scholar] [CrossRef]
- Scarpignato, C.; Pelosini, I. Rifaximin, a poorly absorbed antibiotic: Pharmacology and clinical potential. Chemotherapy 2005, 51 (Suppl. 1), 36–66. [Google Scholar] [CrossRef]
- Gatta, L.; Scarpignato, C. Systematic review with meta-analysis: Rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment. Pharmacol. Ther. 2017, 45, 604–616. [Google Scholar] [CrossRef]
- Boltin, D.; Perets, T.T.; Shporn, E.; Aizic, S.; Levy, S.; Niv, Y.; Dickman, R. Rifaximin for small intestinal bacterial overgrowth in patients without irritable bowel syndrome. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 49. [Google Scholar] [CrossRef]
- Chojnacki, C.; Popławski, T.; Konrad, P.; Fila, M.; Chojnacki, J.; Błasiak, J. Serotonin Pathway of Tryptophan Metabolism in Small Intestinal Bacterial Overgrowth-A Pilot Study with Patients Diagnosed with Lactulose Hydrogen Breath Test and Treated with Rifaximin. J. Clin. Med. 2021, 10, 2065. [Google Scholar] [CrossRef]
- Jian, J.; Nie, M.T.; Xiang, B.; Qian, H.; Yin, C.; Zhang, X.; Zhang, M.; Zhu, X.; Xie, W.F. Rifaximin Ameliorates Non-alcoholic Steatohepatitis in Mice Through Regulating gut Microbiome-Related Bile Acids. Front. Pharmacol. 2022, 13, 841132. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.T.; Chan, L.; Chen, K.Y.; Lee, H.H.; Huang, L.K.; Yang, Y.S.H.; Liu, Y.R.; Hu, C.J. Rifaximin Modifies Gut Microbiota and Attenuates Inflammation in Parkinson’s Disease: Preclinical and Clinical Studies. Cells 2022, 11, 3468. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Scaldaferri, F.; Petito, V.; Paroni Sterbini, F.; Pecere, S.; Lopetuso, L.R.; Palladini, A.; Gerardi, V.; Masucci, L.; Pompili, M.; et al. The Role of Antibiotics in Gut Microbiota Modulation: The Eubiotic Effects of Rifaximin. Dig. Dis. 2016, 34, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Gerardi, V.; Pecere, S.; D’Aversa, F.; Lopetuso, L.; Zocco, M.A.; Pompili, M.; Gasbarrini, A. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J. Gastroenterol. 2015, 21, 12322–12333. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Srivastava, D.; Misra, A. A randomized double-blind placebo-controlled trial showing rifaximin to improve constipation by reducing methane production and accelerating colon transit: A pilot study. Indian. J. Gastroenterol. 2018, 37, 416–423. [Google Scholar] [CrossRef]
- Hajji, N.; Wannes, D.; Jabri, M.A.; Rtibi, K.; Tounsi, H.; Abdellaoui, A.; Sebai, H. Purgative/laxative actions of Globularia alypum aqueous extract on gastrointestinal-physiological function and against loperamide-induced constipation coupled to oxidative stress and inflammation in rats. Neurogastroenterol. Motil. 2020, 32, e13858. [Google Scholar] [CrossRef]
- Jo, H.G.; Kim, M.J.; Moon, B.Y.; Cheong, S.H. Antioxidant and laxative effects of taurine-xylose, a synthetic taurine-carbohydrate derivative, in loperamide-induced constipation in Sprague-Dawley rats. J. Exerc. Nutr. Biochem. 2019, 23, 6–13. [Google Scholar] [CrossRef]
- Wang, J.K.; Yao, S.K. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid.-Based Complement. Altern. Med. 2021, 2021, 5560310. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Li, D.; Zhao, L.; Zhu, L. Role of gut microbiota in functional constipation. Gastroenterol. Rep. 2021, 9, 392–401. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Li, G.; Zhang, M.; Niu, T.; Kang, X.; Zhao, H.; Chen, J.; Sun, E.; Li, Y. Effect of Bifidobacterium animalis subsp. lactis MN-Gup on constipation and the composition of gut microbiota. Benef. Microbes 2021, 12, 31–42. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, X.; Zhao, Y.; Liu, S.; Luo, K.; Fu, X.; Li, J.; Sheng, J.; Tian, Y.; Fan, Y. Flavonoids in Amomum tsaoko Crevost et Lemarie Ameliorate Loperamide-Induced Constipation in Mice by Regulating Gut Microbiota and Related Metabolites. Int. J. Mol. Sci. 2023, 24, 7191. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Li, Y.; He, Y.; Chen, F.; Mi, B.; Li, J.; Xie, J.; Ma, G.; Yang, J.; Xu, K.; et al. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: A double-blinded randomized placebo trial. Gut Microbes 2023, 15, 2197837. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Wang, J.; Jiang, Y.; Zhu, X.; Zhang, Z.; Wang, Y.; Shu, X.; Deng, Y.; Zhang, F. Bifidobacterium lactis TY-S01 Prevents Loperamide-Induced Constipation by Modulating Gut Microbiota and Its Metabolites in Mice. Front. Nutr. 2022, 9, 890314. [Google Scholar] [CrossRef]
- Zhou, Q.; Costinean, S.; Croce, C.M.; Brasier, A.R.; Merwat, S.; Larson, S.A.; Basra, S.; Verne, G.N. MicroRNA 29 targets nuclear factor-κB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology 2015, 148, 158–169.e158. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Yamamoto, T.; Tani, T.; Nihei, K.; Kondo, D.; Funaki, H.; Yaoita, E.; Kawasaki, K.; Sato, N.; Hatakeyama, K.; et al. Expression and localization of aquaporins in rat gastrointestinal tract. Am. J. Physiol. 1999, 276, C621–C627. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Yuan, W.T. Expression of aquaporin 3, 4, and 8 in colonic mucosa of rat models with slow transit constipation. Zhonghua Wei Chang. Wai Ke Za Zhi 2011, 14, 459–461. [Google Scholar]
- Shi, Y.; Chen, F.; Wang, Z.; Cao, J.; Li, C. Effect and mechanism of functional compound fruit drink on gut microbiota in constipation mice. Food Chem. 2023, 401, 134210. [Google Scholar] [CrossRef]
- Kim, J.E.; Yun, W.B.; Lee, M.L.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Hong, J.T.; Song, H.K.; Hwang, D.Y. Synergic Laxative Effects of an Herbal Mixture of Liriope platyphylla, Glycyrrhiza uralensis, and Cinnamomum cassia in Loperamide-Induced Constipation of Sprague Dawley Rats. J. Med. Food 2019, 22, 294–304. [Google Scholar] [CrossRef]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef]
- Suo, H.; Zhao, X.; Qian, Y.; Li, G.; Liu, Z.; Xie, J.; Li, J. Therapeutic effect of activated carbon-induced constipation mice with Lactobacillus fermentum Suo on treatment. Int. J. Mol. Sci. 2014, 15, 21875–21895. [Google Scholar] [CrossRef]
- Ivashkin, V.; Shifrin, O.; Maslennikov, R.; Poluektova, E.; Korolev, A.; Kudryavtseva, A.; Krasnov, G.; Benuni, N.; Barbara, G. Eubiotic effect of rifaximin is associated with decreasing abdominal pain in symptomatic uncomplicated diverticular disease: Results from an observational cohort study. BMC Gastroenterol. 2023, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021, 12, 3597–3610. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Watanabe, K.; Tanaka, R.; Fukuda, M.; Oyaizu, H. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 1999, 65, 4506–4512. [Google Scholar] [CrossRef]
- Tatsuoka, M.; Osaki, Y.; Ohsaka, F.; Tsuruta, T.; Kadota, Y.; Tochio, T.; Hino, S.; Morita, T.; Sonoyama, K. Consumption of indigestible saccharides and administration of Bifidobacterium pseudolongum reduce mucosal serotonin in murine colonic mucosa. Br. J. Nutr. 2022, 127, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Tatsuoka, M.; Shimada, R.; Ohsaka, F.; Sonoyama, K. Administration of Bifidobacterium pseudolongum suppresses the increase of colonic serotonin and alleviates symptoms in dextran sodium sulfate-induced colitis in mice. Biosci. Microbiota Food Health 2023, 42, 186–194. [Google Scholar] [CrossRef]
- Yang, C.; Bai, X.; Hu, T.; Xue, X.; Su, X.; Zhang, X.; Wu, T.; Zhang, M.; Shen, X.; Dong, X. Integrated metagenomics and targeted-metabolomics analysis of the effects of phenylalanine on loperamide-induced constipation in rats. Front. Microbiol. 2022, 13, 1018008. [Google Scholar] [CrossRef]
- Kubota, M.; Ito, K.; Tomimoto, K.; Kanazaki, M.; Tsukiyama, K.; Kubota, A.; Kuroki, H.; Fujita, M.; Vandenplas, Y. Lactobacillus reuteri DSM 17938 and Magnesium Oxide in Children with Functional Chronic Constipation: A Double-Blind and Randomized Clinical Trial. Nutrients 2020, 12, 225. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, J.; Diao, W.; Zhou, X.; Mu, J.; Pang, L.; Tan, F.; Zhao, X. Lactobacillus plantarum KSFY06 and geniposide counteract montmorillonite-induced constipation in Kunming mice. Food Sci. Nutr. 2020, 8, 5128–5137. [Google Scholar] [CrossRef]
- Jiang, C.; Xu, Q.; Wen, X.; Sun, H. Current developments in pharmacological therapeutics for chronic constipation. Acta Pharm. Sin. B 2015, 5, 300–309. [Google Scholar] [CrossRef]
- James, S.C.; Fraser, K.; Young, W.; Heenan, P.E.; Gearry, R.B.; Keenan, J.I.; Talley, N.J.; Joyce, S.A.; McNabb, W.C.; Roy, N.C. Concentrations of Fecal Bile Acids in Participants with Functional Gut Disorders and Healthy Controls. Metabolites 2021, 11, 612. [Google Scholar] [CrossRef]
- Dior, M.; Delagrèverie, H.; Duboc, H.; Jouet, P.; Coffin, B.; Brot, L.; Humbert, L.; Trugnan, G.; Seksik, P.; Sokol, H.; et al. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol. Motil. 2016, 28, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Bajor, A.; Törnblom, H.; Rudling, M.; Ung, K.A.; Simrén, M. Increased colonic bile acid exposure: A relevant factor for symptoms and treatment in IBS. Gut 2015, 64, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Chedid, V.; Vijayvargiya, P.; Camilleri, M. Elobixibat for the treatment of constipation. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.; Wong, B.S.; Camilleri, M.; Odunsi-Shiyanbade, S.T.; McKinzie, S.; Ryks, M.; Burton, D.; Carlson, P.; Lamsam, J.; Singh, R.; et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: A pharmacodynamic and pharmacogenetic analysis. Gastroenterology 2010, 139, 1549–1558.e1541. [Google Scholar] [CrossRef]
- Maneerattanaporn, M.; Chey, W.D. Targeting bile acids in the treatment of constipation. Expert. Rev. Gastroenterol. Hepatol. 2011, 5, 657–659. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]

| Compounds name | Mean CTR | Mean LOP | VIP | p-Value | Fold Change | Log2FC | Change in LOP |
|---|---|---|---|---|---|---|---|
| Deoxycholic acid | 1066.260 | 50,606.155 | 1.633 | 0.022 | 47.461 | 5.569 | up |
| Apocholic acid | 11,102.901 | 106,959.609 | 1.330 | 0.031 | 9.633 | 3.268 | up |
| Isohyodeoxycholic acid | 178.559 | 16,957.735 | 1.651 | 0.012 | 94.970 | 6.569 | up |
| Taurodeoxycholic acid | 6364.034 | 970.079 | 1.634 | 0.034 | 0.152 | −2.714 | down |
| Tauroursodeoxycholic acid | 677.763 | 19,139.782 | 1.604 | 0.045 | 28.240 | 4.820 | up |
| Taurohyodeoxycholic acid | 3784.726 | 25,107.742 | 1.550 | 0.014 | 6.634 | 2.730 | up |
| 7-Ketodeoxycholic acid | 259.556 | 165,052.412 | 1.748 | 0.027 | 635.902 | 9.313 | up |
| Dehydrocholic acid | 21,229.988 | 12,196.397 | 1.256 | 0.015 | 0.574 | −0.800 | down |
| Glycochenodeoxycholic acid | 3807.062 | 97,532.070 | 1.784 | 0.003 | 25.619 | 4.679 | up |
| Isodeoxycholic acid | 3700.985 | 202,609.462 | 1.712 | 0.017 | 54.745 | 5.775 | up |
| 3Alpha,7Alpha,12Alpha-trihydroxy-5Beta-cholestan-26-oic acid | 2776.560 | 20,266.751 | 1.777 | 0.000 | 7.299 | 2.868 | up |
| Nordeoxycholic acid | 456.004 | 7336.268 | 1.574 | 0.020 | 16.088 | 4.008 | up |
| Taurochenodeoxycholic acid | 34,228.022 | 141,577.639 | 1.440 | 0.017 | 4.136 | 2.048 | up |
| 12-Ketolithocholic acid | 1168.491 | 43,504.507 | 1.654 | 0.024 | 37.231 | 5.218 | up |
| Chenodeoxycholic acid | 783.305 | 96,992.442 | 1.766 | 0.013 | 123.825 | 6.952 | up |
| Lithocholic acid | 30.397 | 1182.747 | 1.304 | 0.047 | 38.910 | 5.282 | up |
| Tauro-beta-muricholic acid | 22.559 | 1594.160 | 1.710 | 0.009 | 70.667 | 6.143 | up |
| Allocholic acid | 18.281 | 1759.077 | 1.555 | 0.016 | 96.226 | 6.588 | up |
| Glycohyodeoxycholic acid | 7268.141 | 44,534.381 | 1.651 | 0.000 | 6.127 | 2.615 | up |
| Ursodeoxycholic acid | 35,044.860 | 466,779.821 | 1.573 | 0.035 | 13.319 | 3.735 | up |
| Hyodeoxycholic acid | 25,480.018 | 360,940.644 | 1.522 | 0.034 | 14.166 | 3.824 | up |
| 7-ketolithocholic acid | 61.692 | 4127.493 | 1.655 | 0.016 | 66.905 | 6.064 | up |
| (25R)-3beta,4beta-dihydroxycholest-5-en-26-oate(1-) | 10.766 | 1369.281 | 1.723 | 0.012 | 127.187 | 6.991 | up |
| Compounds Name | Mean LOP | Mean RIF | VIP | p-Value | Fold Change | Log2FC | Change in RIF |
|---|---|---|---|---|---|---|---|
| Deoxycholic acid | 50,606.155 | 1425.796 | 1.650 | 0.022 | 0.028 | −5.149 | down |
| Taurocholic acid | 14,283.205 | 34,218.129 | 1.465 | 0.025 | 2.396 | 1.260 | up |
| Isohyodeoxycholic acid | 16,957.735 | 699.985 | 1.592 | 0.014 | 0.041 | −4.598 | down |
| Taurodeoxycholic acid | 970.079 | 18,803.560 | 1.862 | 0.002 | 19.384 | 4.277 | up |
| 7-Ketodeoxycholic acid | 165,052.412 | 3830.114 | 1.597 | 0.029 | 0.023 | −5.429 | down |
| Ursocholyltaurine | 20,896.284 | 66,936.404 | 1.734 | 0.005 | 3.203 | 1.680 | up |
| Sodium glycocholate | 5364.930 | 6095.290 | 1.097 | 0.050 | 1.136 | 0.184 | up |
| Dehydrocholic acid | 12,196.397 | 26,944.344 | 1.494 | 0.002 | 2.209 | 1.144 | up |
| Glycochenodeoxycholic acid | 97,532.070 | 4435.374 | 1.839 | 0.003 | 0.045 | −4.459 | down |
| Isodeoxycholic acid | 202,609.462 | 4877.642 | 1.736 | 0.018 | 0.024 | −5.376 | down |
| Nordeoxycholic acid | 7336.268 | 79.043 | 1.891 | 0.016 | 0.011 | −6.536 | down |
| 12-Ketolithocholic acid | 43,504.507 | 3121.753 | 1.528 | 0.029 | 0.072 | −3.801 | down |
| Chenodeoxycholic acid | 96,992.442 | 2994.280 | 1.676 | 0.014 | 0.031 | −5.018 | down |
| Lithocholic acid | 1182.747 | 24.644 | 1.442 | 0.046 | 0.021 | −5.585 | down |
| Tauro-beta-muricholic acid | 1594.160 | 29.811 | 1.771 | 0.010 | 0.019 | −5.741 | down |
| Allocholic acid | 1759.077 | 72.740 | 1.385 | 0.018 | 0.041 | −4.596 | down |
| Glycohyodeoxycholic acid | 44,534.381 | 21,644.455 | 1.270 | 0.012 | 0.486 | −1.041 | down |
| 7-ketolithocholic acid | 4127.493 | 30.843 | 1.767 | 0.015 | 0.007 | −7.064 | down |
| (25R)-3beta,4beta-dihydroxycholest-5-en-26-oate(1-) | 1369.281 | 33.189 | 1.669 | 0.013 | 0.024 | −5.367 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Xie, P.; Deng, X.; Fan, J.; Xiong, L. Rifaximin Ameliorates Loperamide-Induced Constipation in Rats through the Regulation of Gut Microbiota and Serum Metabolites. Nutrients 2023, 15, 4502. https://doi.org/10.3390/nu15214502
Luo M, Xie P, Deng X, Fan J, Xiong L. Rifaximin Ameliorates Loperamide-Induced Constipation in Rats through the Regulation of Gut Microbiota and Serum Metabolites. Nutrients. 2023; 15(21):4502. https://doi.org/10.3390/nu15214502
Chicago/Turabian StyleLuo, Mei, Peiwei Xie, Xuehong Deng, Jiahui Fan, and Lishou Xiong. 2023. "Rifaximin Ameliorates Loperamide-Induced Constipation in Rats through the Regulation of Gut Microbiota and Serum Metabolites" Nutrients 15, no. 21: 4502. https://doi.org/10.3390/nu15214502
APA StyleLuo, M., Xie, P., Deng, X., Fan, J., & Xiong, L. (2023). Rifaximin Ameliorates Loperamide-Induced Constipation in Rats through the Regulation of Gut Microbiota and Serum Metabolites. Nutrients, 15(21), 4502. https://doi.org/10.3390/nu15214502





