Role of Baseline Gut Microbiota on Response to Fiber Intervention in Individuals with Irritable Bowel Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment
2.2. Study Design and Intervention
2.3. Questionnaires
2.4. Stool Microbiome Profiling
2.5. Statistical Analysis
3. Results
3.1. Recruitment
3.2. IBS Symptoms Severity Scores
3.3. Differential Microbiome Composition
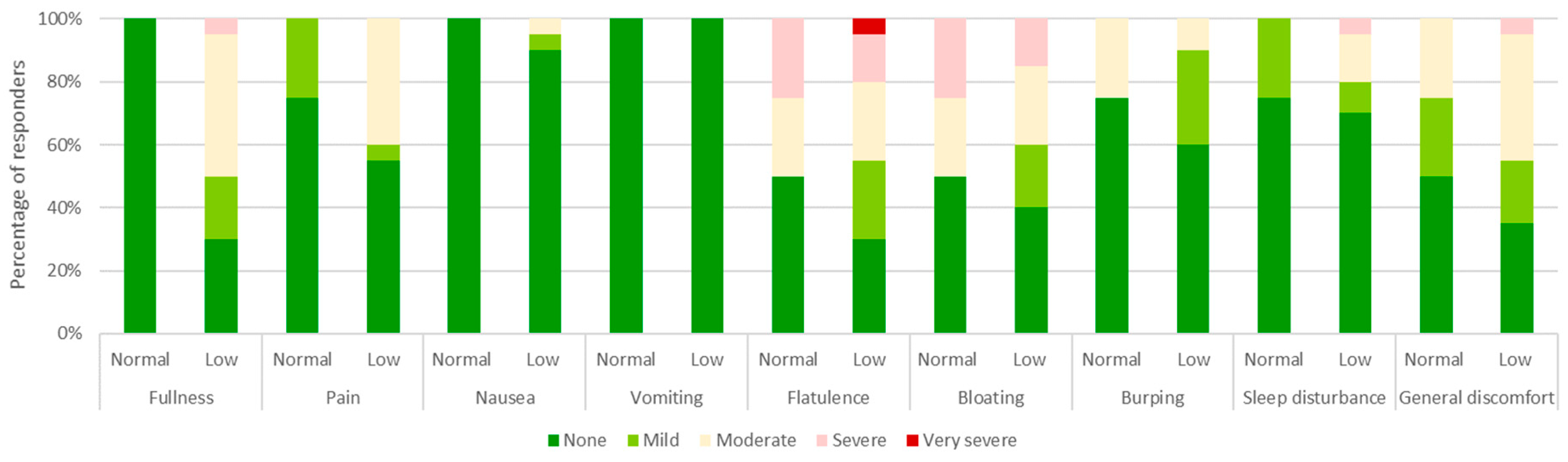
3.4. Tolerability
3.5. Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Whitehead, W.E.; Van Tilburg, M.A.; Chang, L.; Chey, W.; Crowell, M.D.; Keefer, L.; Lembo, A.J.; Parkman, H.P.; Rao, S.S.; et al. Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. Gastroenterology 2016, S0016-5085. [Google Scholar]
- Suriano, F.; Nyström, E.E.L.; Sergi, D.; Gustafsson, J.K. Diet, microbiota, and the mucus layer: The guardians of our health. Front. Immunol. 2022, 13, 953196. [Google Scholar] [CrossRef]
- Luis, A.S.; Hansson, G.C. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe 2023, 31, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, Q.; Song, L.; Duan, L. Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e14513. [Google Scholar] [CrossRef]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Meier, R.; Gassull, M.A. Consensus recommendations on the effects and benefits of fibre in clinical practice. Clin. Nutr. Suppl. 2004, 1, 73–80. [Google Scholar] [CrossRef]
- Suresh, H.; Ho, V.; Zhou, J. Rheological Characteristics of Soluble Fibres during Chemically Simulated Digestion and their Suitability for Gastroparesis Patients. Nutrients 2020, 12, 2479. [Google Scholar] [CrossRef]
- Suresh, H.; Zhou, J.; Ho, V. The Short-Term Effects and Tolerability of Low-Viscosity Soluble Fibre on Gastroparesis Patients: A Pilot Clinical Intervention Study. Nutrients 2021, 13, 4298. [Google Scholar] [CrossRef]
- Vulevic, J.; Rastall, R.A.; Gibon, G.R. Developing a quantitative approach for determining the in vitro prebiotic potential of dietary oligosaccharides. FEMS Micobiol Lett. 2004, 236, 153–159. [Google Scholar] [CrossRef]
- Noack, J.; Timm, D.; Hospattankar, A.; Slavin, J.L. Fermentation profiles of wheat dextrin, inulin and partially hydorlyzed guar gum using an in vitro digestion pretreatment and in vitro batch fermentation system model. Nutrients 2013, 5, 1500–1510. [Google Scholar] [CrossRef]
- Ohashi, Y.; Harada, K.; Tokunaga, M.; Ishihara, N.; Okubo, T.; Ogasawara, Y.; Juneja, L.R.; Fujisawa, T. Faecal fermentation of partially hydrolyzed guar gum. J. Funct. Foods 2012, 4, 398–402. [Google Scholar] [CrossRef]
- Okubo, T.; Ishihara, N.; Takahashi, H.; Fujisawa, T.; Kim, M.; Yamamoto, T.; Mitsuoka, T. Effects of partially hydrolysed guar gum intake on human intestine microflora and its metabolism. Biosci. Bitech. Biochem. 1994, 58, 1364–1369. [Google Scholar] [CrossRef]
- Carlson, J.; Esparza, J.; Swan, J.; Taussig, D.; Combs, J.; Slavin, J. In vitro analysis of partially hydrolyzed guar gum fermentation differences between six individuals. Food Funct. 2016, 7, 1833–1838. [Google Scholar] [CrossRef]
- López-Almela, I.; Romaní-Pérez, M.; Bullich-Vilarrubias, C.; Benítez-Páez, A.; Del Pulgar, E.M.G.; Francés, R.; Liebisch, G.; Sanz, Y. Bacteroides uniformis combined with fiber amplifies metabolic and immune benefits in obese mice. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Pimentel, M.; Lembo, A. Microbiome and Its Role in Irritable Bowel Syndrome. Dig. Dis. Sci. 2020, 65, 829–839. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Aroniadis, O.C.; Brandt, L.J.; Oneto, C.; Feuerstadt, P.; Sherman, A.; Wolkoff, A.W.; Kassam, Z.; Sadovsky, R.G.; Elliott, R.J.; Budree, S.; et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: A double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, C.J.; de Wit, N.J.; Muris, J.W.M.; Whorwell, P.J.; A Knottnerus, J.; Hoes, A.W. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009, 339, b3154. [Google Scholar] [CrossRef]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.A.; Stimpson, J.; Wang, D.; Plummer, S.; Garaiova, I.; Barker, M.E.; Corfe, B.M. Clinical trial: A multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2009, 29, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4. [Google Scholar] [CrossRef]
- Yin, L.; Wan, Y.-D.; Pan, X.-T.; Zhou, C.-Y.; Lin, N.; Ma, C.-T.; Yao, J.; Su, Z.; Wan, C.; Yu, Y.-W.; et al. Association between gut bacterial diversity and mortality in septic shock patients: A cohort study. Experiment 2019, 25, 7376–7382. [Google Scholar] [CrossRef]
- Li, K.; Bihan, M.; Yooseph, S.; Methé, B.A. Analyses of the microbial diversity across the human microbiome. PLoS ONE 2012, 7, e32118. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Mansi, C.; Dulbecco, P.; Savarino, V. Role of partially hydrolyzed guar gum in the treatment of ir-ritable bowel syndrome. Nutrition 2006, 22, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.P.; Quartarone, G. Role of guar fiber in improving digestive health and function. Nutrition 2018, 59, 158–169. [Google Scholar] [CrossRef]
- Parisi, G.; Bottona, E.; Carrara, M.; Cardin, F.; Faedo, A.; Goldin, D.; Marino, M.; Pantalena, M.; Tafner, G.; Verdianelli, G.; et al. Treatment effects of partially hydrolyzed guar gum on symptoms and quality of life of patients with irritable bowel syndrome. a multicenter randomized open trial. Dig. Dis. Sci. 2005, 50, 1107–1112. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response toPrevotellabacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Noh, C.-K.; Lee, K.J. Fecal microbiota alterations and small intestinal bacterial overgrowth in functional abdominal bloating/distention. J. Neurogastroenterol. Motil. 2020, 26, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2017, 65, 57–62. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Etxeberria, U.; Hijona, E.; Aguirre, L.; Milagro, F.I.; Bujanda, L.; Rimando, A.M.; Martínez, J.A.; Portillo, M.P. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol. Nutr. Food Res. 2016, 61, 1500906. [Google Scholar] [CrossRef]
- Lima, S.F.; Gogokhia, L.; Viladomiu, M.; Chou, L.; Putzel, G.; Jin, W.B.; Pires, S.; Guo, C.J.; Gerardin, Y.; Crawford, C.V.; et al. Transferable immunoglobulin A–Coated Odoribacter splanchnicus in responders to fecal microbiota trans-plantation for ulcerative colitis limits colonic inflammation. Gastroenterology 2022, 162, 166–178. [Google Scholar] [CrossRef]
- Price, C.E.; O’toole, G.A. The Gut-Lung Axis in Cystic Fibrosis. J. Bacteriol. 2021, 203, e0031121. [Google Scholar] [CrossRef]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Suutarinen, M.; Kainulainen, V.; Bowers, J.R.; Lemmer, D.; Engelthaler, D.M.; Eklund, K.K.; et al. Novel Odoribacter splanchnicus strain and its outer membrane vesicles exert im-munoregulatory effects in vitro. Front. Microbiol. 2020, 11, 575455. [Google Scholar] [CrossRef]
- Huber-Ruano, I.; Calvo, E.; Mayneris-Perxachs, J.; Rodríguez-Peña, M.-M.; Ceperuelo-Mallafré, V.; Cedó, L.; Núñez-Roa, C.; Miro-Blanch, J.; Arnoriaga-Rodríguez, M.; Balvay, A.; et al. Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate. Microbiome 2022, 10, 1–22. [Google Scholar] [CrossRef]
- Camilleri, M. Management of the irritable bowel syndrome. Gastroenterology 2001, 120, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Koloski, N.A.; Talley, N.J.; Boyce, P.M. The impact of functional gastrointestinal disorders on quality of life. Am. J. Gastroenterol. 2000, 95, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, W.E.; Burnett, C.K.; Cook, E.W.; Taub, E. Impact of irritable bowel syndrome on quality of life. Dig. Dis. Sci. 1996, 41, 2248–2253. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Hays, R.D.; Kilbourne, A.; Naliboff, B.; Mayer, E.A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000, 119, 654–660. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome—A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef]
- Akehurst, R.; Kaltenthaler, E. Treatment of irritable bowel syndrome: A review of randomised controlled trials. Gut 2001, 48, 272–282. [Google Scholar] [CrossRef]
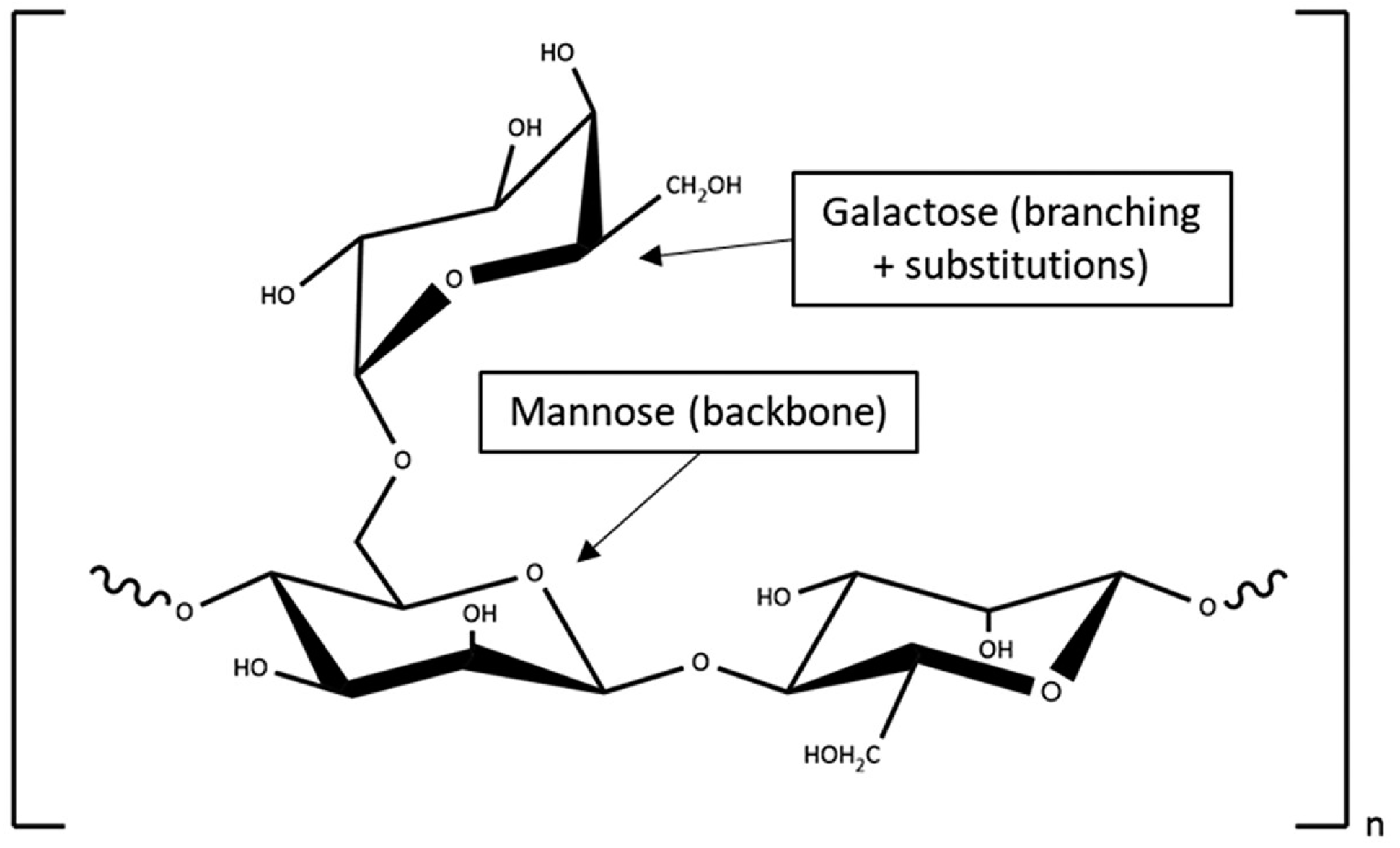

| Characteristics | Normal Diversity (n = 14) | Low Diversity (n = 26) | p Value # |
|---|---|---|---|
| Female gender n (%) | 12 (79%) | 20 (77%) | |
| Age, mean years ± SD | 52.0 ± 17.8 | 49.0 ± 16.5 | 0.677 |
| BMI, mean ± SD | 32.0 ± 6.7 | 27.4 ± 6.1 | 0.102 |
| Diagnosed IBS subtype n (%) | |||
| Constipation Predominant | 10 (71%) | 6 (23%) | |
| Diarrhea Predominant | 0 | 7 (27%) | |
| Alternating/Mixed | 4 (29%) | 13 (50%) | |
| Baseline daily servings of fruits and vegetables, mean ± SD | 2.9 ± 1.8 | 3.0 ± 1.8 | 0.859 |
| Baseline microbiota diversity (Shannon index ± SD) | |||
| Baseline (Day 0) | 3.2 ± 0.1 | 2.3 ± 0.6 | >0.001 |
| Intervention (Days 1–30) | 2.8 ± 0.7 | 2.5 ± 0.4 | 0.353 |
| Intervention (Day 31–90) | 2.8 ± 0.4 | 2.5 ± 0.5 | 0.295 |
| Follow-up (Days 91–120) | 2.8 ± 0.4 | 2.8 ± 0.4 * | 0.984 |
| Normal Diversity (n = 14) | Low Diversity (n = 26) | p Value # | |
|---|---|---|---|
| IBS-SSS | |||
| Baseline (Day 0) ± SD | 240 ± 44 | 258 ± 98 | 0.080 |
| Intervention (Days 1–30) ± SD | 155 ± 57 ** | 219 ± 85 | 0.042 |
| Intervention (Day 31–90) ± SD | 128 ± 56 ** | 194 ± 108 | 0.064 |
| Follow-up (Days 91–120) ± SD | 153 ± 79 * | 227 ± 123 | 0.127 |
| Response rate ^ | |||
| Intervention (Days 1–30) | 10 (71%) | 9 (35%) | |
| Intervention (Day 31–90) | 12 (86%) | 15 (57%) | |
| Follow-up (Days 91–120) | 10 (71%) | 11(42%) |
| Phylum | Bacteria | Normal Diversity (% ± SD) | Low Diversity (% ± SD) | p Value * | FDR | LDA Score |
|---|---|---|---|---|---|---|
| Actinobacteria | 1.43 ± 2.32 | 0.18 ± 0.23 | <0.001 | <0.001 | 4.82 | |
| Firmicutes | Oscillospira | 5.07 ± 4.56 | 1.23 ± 0.81 | <0.001 | 0.002 | 5.28 |
| Bacteroidetes | Odoribacter | 2.05 ± 3.59 | 0.16 ± 0.13 | <0.001 | 0.007 | 4.98 |
| Firmicutes | F. prausnitzii | 7.17 ± 5.98 | 19.46 ± 15.14 | 0.0033 | 0.0357 | −5.79 |
| Bacteroidetes | P. copri | 0.07 ± 0.13 | 13.80 ± 28.13 | 0.0034 | 0.0422 | −5.84 |
| Firmicutes | Faecalibacterium | 8.78 ± 6.17 | 20.12 ± 15.82 | 0.0182 | 0.0153 | −5.11 |
| Bacteroidetes | Prevotella | 0.09 ± 0.19 | 15.81 ± 27.61 | 0.0035 | 0.0421 | −5.90 |
| Normal Diversity Group (n = 14) | ||||
|---|---|---|---|---|
| Subscale | Day 0 ± SD | Day 30 ± SD | Day 90 ± SD | Day 120 ± SD |
| Physical Function | 72.1 ± 21.1 | 83.2 ± 21.5 | 92.8 ± 19.7 * | 90.1 ± 21.4 * |
| Role–Physical | 68.7 ± 22.6 | 72.0 ± 26.9 | 87.2 ± 27.2 * | 82.8 ± 23.3 * |
| Role–Emotional | 71.6 ± 22.1 | 82.8 ± 25.8 * | 86.3 ± 21.3 * | 88.2 ± 14.3 * |
| Vitality | 45.8 ± 21.8 | 51.5 ± 23.1 | 62.6 ± 21.1 | 61.9 ± 23.1 |
| Mental Health | 74.1 ± 14.8 | 85.8 ± 17.9 * | 87.3 ± 18.4 * | 81.6 ± 13.4 * |
| Social Functioning | 71.7 ± 23.7 | 88.1 ± 18.2 * | 92.2 ± 17.6 * | 84.7 ± 25.1 |
| Bodily Pain | 64.2 ± 16.5 | 76.5 ± 27.9 | 79.8 ± 22.2 * | 76.7 ± 23.6 |
| General Health | 52.1 ± 21.8 | 67.3 ± 19.9 * | 74.4 ± 21.2 * | 73.1 ± 22.0 * |
| Low Diversity Group (n = 26) | ||||
| Subscale | Day 0 ± SD | Day 30 ± SD | Day 90 ± SD | Day 120 ± SD |
| Physical Function | 73.3 ± 23.7 | 77.7 ± 25.4 | 75.3 ± 29.1 | 76.6 ± 26.3 |
| Role–Physical | 66.4 ± 21.8 | 62.5 ± 21.5 | 76.8 ± 20.1 * | 62.5 ± 22.9 |
| Role–Emotional | 72.3 ± 17.3 | 57.1 ± 10.9 | 67.6 ± 19.0 | 63.3 ± 18.3 |
| Vitality | 47.9 ± 22.3 | 46.1 ± 25.4 | 48.7 ± 22.1 | 42.7 ± 28.1 |
| Mental Health | 75.5 ± 28.5 | 74.8 ± 22.5 | 77.2 ± 27.1 | 75.0 ± 26.9 |
| Social Functioning | 69.0 ± 29.0 | 71.8 ± 28.1 | 78.8 ± 24.1 | 72.7 ± 25.9 |
| Bodily Pain | 61.2 ± 17.3 | 61.4 ± 20.7 | 67.4 ± 21.2 | 59.1 ± 22.3 |
| General Health | 55.8 ± 19.8 | 57.1 ± 22.9 | 53.0 ± 18.3 | 57.5 ± 23.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Ho, V. Role of Baseline Gut Microbiota on Response to Fiber Intervention in Individuals with Irritable Bowel Syndrome. Nutrients 2023, 15, 4786. https://doi.org/10.3390/nu15224786
Zhou J, Ho V. Role of Baseline Gut Microbiota on Response to Fiber Intervention in Individuals with Irritable Bowel Syndrome. Nutrients. 2023; 15(22):4786. https://doi.org/10.3390/nu15224786
Chicago/Turabian StyleZhou, Jerry, and Vincent Ho. 2023. "Role of Baseline Gut Microbiota on Response to Fiber Intervention in Individuals with Irritable Bowel Syndrome" Nutrients 15, no. 22: 4786. https://doi.org/10.3390/nu15224786
APA StyleZhou, J., & Ho, V. (2023). Role of Baseline Gut Microbiota on Response to Fiber Intervention in Individuals with Irritable Bowel Syndrome. Nutrients, 15(22), 4786. https://doi.org/10.3390/nu15224786







