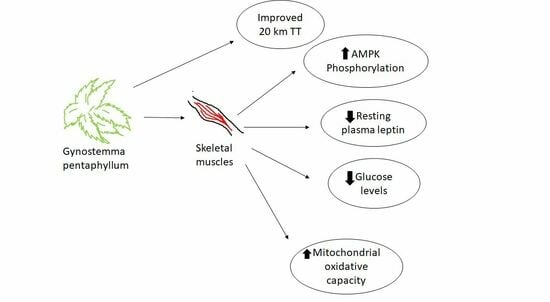
Gynostemma Pentaphyllum Increases Exercise Performance and Alters Mitochondrial Respiration and AMPK in Healthy Males
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Graded Exercise Test (GXT) and Familiarization
2.3. Supplementation
2.4. Testing Sessions
2.5. Muscle Analyses
2.6. Blood Analyses
2.7. RNA Isolation and Reverse Transcription
2.8. Protein Analyses
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lokman, E.F.; Gu, H.F.; Wan Mohamud, W.N.; Ostenson, C.G. Evaluation of Antidiabetic Effects of the Traditional Medicinal Plant Gynostemma pentaphyllum and the Possible Mechanisms of Insulin Release. Evid. Based Complement. Altern. Med. 2015, 2015, 120572. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, W.; Huang, J.; Xie, Y.; Ma, W. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chin. Med. 2016, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Chiranthanut, N.; Teekachunhatean, S.; Panthong, A.; Khonsung, P.; Kanjanapothi, D.; Lertprasertsuk, N. Toxicity evaluation of standardized extract of Gynostemma pentaphyllum Makino. J. Ethnopharmacol. 2013, 149, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Razmovski-Naumovski, V.; Salam, N.K.; Duke, R.K.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. A novel LXR-alpha activator identified from the natural product Gynostemma pentaphyllum. Biochem. Pharmacol. 2005, 70, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Huh, T.L.; Kim, S.Y.; Oh, M.R.; Tirupathi Pichiah, P.B.; Chae, S.W.; Cha, Y.S. Antiobesity effect of Gynostemma pentaphyllum extract (actiponin): A randomized, double-blind, placebo-controlled trial. Obesity 2014, 22, 63–71. [Google Scholar] [CrossRef]
- Takemoto, T.; Arihara, S.; Nakajima, T.; Okuhira, M. Studies on the constituents of fructus Momordicae. III. Structure of mogrosides. Yakugaku Zasshi 1983, 103, 1167–1173. [Google Scholar] [CrossRef]
- Hung, T.M.; Thu, C.V.; Cuong, T.D.; Hung, N.P.; Kwack, S.J.; Huh, J.I.; Min, B.S.; Choi, J.S.; Lee, H.K.; Bae, K. Dammarane-type glycosides from Gynostemma pentaphyllum and their effects on IL-4-induced eotaxin expression in human bronchial epithelial cells. J. Nat. Prod. 2010, 73, 192–196. [Google Scholar] [CrossRef]
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef]
- Rao, A.; Clayton, P.; Briskey, D. The effect of an orally-dosed Gynostemma pentaphyllum extract (ActivAMP(R)) on body composition in overweight, adult men and women: A double-blind, randomised, placebo-controlled study. J. Hum. Nutr. Diet. 2022, 35, 583–589. [Google Scholar] [CrossRef]
- Xie, P.; Guo, M.; Xie, J.B.; Xiao, M.Y.; Qi, Y.S.; Duan, Y.; Li, F.F.; Piao, X.L. Effects of heat-processed Gynostemma pentaphyllum on high-fat diet-fed mice of obesity and functional analysis on network pharmacology and molecular docking strategy. J. Ethnopharmacol. 2022, 294, 115335. [Google Scholar] [CrossRef]
- Lee, H.S.; Lim, S.M.; Jung, J.I.; Kim, S.M.; Lee, J.K.; Kim, Y.H.; Cha, K.M.; Oh, T.K.; Moon, J.M.; Kim, T.Y.; et al. Gynostemma pentaphyllum Extract Ameliorates High-Fat Diet-Induced Obesity in C57BL/6N Mice by Upregulating SIRT1. Nutrients 2019, 11, 2475. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Gauhar, R.; Hwang, S.L.; Dao, T.T.; Park, D.C.; Kim, J.E.; Song, H.; Huh, T.L.; Oh, W.K. New dammarane-type glucosides as potential activators of AMP-activated protein kinase (AMPK) from Gynostemma pentaphyllum. Bioorg Med. Chem. 2011, 19, 6254–6260. [Google Scholar] [CrossRef] [PubMed]
- Gauhar, R.; Hwang, S.-L.; Jeong, S.-S.; Kim, J.-E.; Song, H.; Park, D.C.; Song, K.-S.; Kim, T.Y.; Oh, W.K.; Huh, T.-L. Heat-processed Gynostemma pentaphyllum extract improves obesity in ob/ob mice by activating AMP-activated protein kinase. Biotechnol. Lett. 2012, 34, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef]
- Wu, S.B.; Wu, Y.T.; Wu, T.P.; Wei, Y.H. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim. Biophys. Acta 2014, 1840, 1331–1344. [Google Scholar] [CrossRef]
- Bergeron, R.; Ren, J.M.; Cadman, K.S.; Moore, I.K.; Perret, P.; Pypaert, M.; Young, L.H.; Semenkovich, C.F.; Shulman, G.I. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1340–E1346. [Google Scholar] [CrossRef]
- Slavin, M.B.; Memme, J.M.; Oliveira, A.N.; Moradi, N.; Hood, D.A. Regulatory networks coordinating mitochondrial quality control in skeletal muscle. Am. J. Physiol.-Cell Physiol. 2022, 322, C913–C926. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Blazev, R.; Carl, C.S.; Ng, Y.K.; Molendijk, J.; Voldstedlund, C.T.; Zhao, Y.; Xiao, D.; Kueh, A.J.; Miotto, P.M.; Haynes, V.R.; et al. Phosphoproteomics of three exercise modalities identifies canonical signaling and C18ORF25 as an AMPK substrate regulating skeletal muscle function. Cell Metab. 2022, 34, 1561–1577.e1569. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare (AIHW). The Active Australia Survey: A Guide and Manual for Implementation, Analysis and Reporting. 2003. Available online: http://www.aihw.gov.au (accessed on 30 September 2023).
- Laursen, P.B.; Shing, C.M.; Jenkins, D.G. Reproducibility of a laboratory-based 40-km cycle time-trial on a stationary wind-trainer in highly trained cyclists. Int. J. Sports Med. 2003, 24, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress-Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Moon, J.M.; Kyong, S.H.; Kim, Y.H. Preparation Method of Gynostemma pentaphyllum Leaves Extract for Increasing Small Molecular Effective Saponin Contents and Decreasing Benzopyrene. U.S. Patent 10,639,343, 5 May 2020. [Google Scholar]
- Yan, X.; Eynon, N.; Papadimitriou, I.D.; Kuang, J.; Munson, F.; Tirosh, O.; O’Keefe, L.; Griffiths, L.R.; Ashton, K.J.; Byrne, N.; et al. The gene SMART study: Method, study design, and preliminary findings. BMC Genom. 2017, 18, 821. [Google Scholar] [CrossRef] [PubMed]
- Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Murray, T.F.; Hostler, D.P.; Crill, M.T.; Ragg, K.E.; Toma, K. Fiber type composition of the vastus lateralis muscle of young men and women. J. Histochem. Cytochem. 2000, 48, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Timpani, C.A.; Trewin, A.J.; Stojanovska, V.; Robinson, A.; Goodman, C.A.; Nurgali, K.; Betik, A.C.; Stepto, N.; Hayes, A.; McConell, G.K.; et al. Attempting to Compensate for Reduced Neuronal Nitric Oxide Synthase Protein with Nitrate Supplementation Cannot Overcome Metabolic Dysfunction but Rather Has Detrimental Effects in Dystrophin-Deficient mdx Muscle. Neurotherapeutics 2017, 14, 429–446. [Google Scholar] [CrossRef]
- McAinch, A.J.; Steinberg, G.R.; Mollica, J.; O’Brien, P.E.; Dixon, J.B.; Macaulay, S.L.; Kemp, B.E.; Cameron-Smith, D. Differential regulation of adiponectin receptor gene expression by adiponectin and leptin in myotubes derived from obese and diabetic individuals. Obesity 2006, 14, 1898–1904. [Google Scholar] [CrossRef]
- Chen, M.B.; McAinch, A.J.; Macaulay, S.L.; Castelli, L.A.; O’Brien, P.E.; Dixon, J.B.; Cameron-Smith, D.; Kemp, B.E.; Steinberg, G.R. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J. Clin. Endocrinol. Metab. 2005, 90, 3665–3672. [Google Scholar] [CrossRef]
- Simcocks, A.C.; O’Keefe, L.; Jenkin, K.A.; Cornall, L.M.; Grinfeld, E.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. The Role of Atypical Cannabinoid Ligands O-1602 and O-1918 on Skeletal Muscle Homeostasis with a Focus on Obesity. Int. J. Mol. Sci. 2020, 21, 5922. [Google Scholar] [CrossRef]
- Godfrey, T.E.; Kim, S.H.; Chavira, M.; Ruff, D.W.; Warren, R.S.; Gray, J.W.; Jensen, R.H. Quantitative mRNA expression analysis from formalin-fixed, paraffin-embedded tissues using 5’ nuclease quantitative reverse transcription-polymerase chain reaction. J. Mol. Diagn. 2000, 2, 84–91. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gilda, J.E.; Gomes, A.V. Stain-Free total protein staining is a superior loading control to β-actin for Western blots. Anal. Biochem. 2013, 440, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.A.; McDonald, K.; Kohn, J.E.; Posch, A. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Gavrieli, A.; Mantzoros, C.S. Leptin applications in 2015: What have we learned about leptin and obesity? Curr Opin Endocrinol. Diabetes Obes. 2015, 22, 353–359. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef]
- Landecho, M.F.; Tuero, C.; Valenti, V.; Bilbao, I.; de la Higuera, M.; Fruhbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci. 2006, 9, 281–291. [Google Scholar]
- Huyen, V.T.; Phan, D.V.; Thang, P.; Ky, P.T.; Hoa, N.K.; Ostenson, C.G. Antidiabetic Effects of Add-On Gynostemma pentaphyllum Extract Therapy with Sulfonylureas in Type 2 Diabetic Patients. Evid. Based Complement. Altern. Med. 2012, 2012, 452313. [Google Scholar] [CrossRef]
- Huyen, V.T.; Phan, D.V.; Thang, P.; Hoa, N.K.; Ostenson, C.G. Antidiabetic effect of Gynostemma pentaphyllum tea in randomly assigned type 2 diabetic patients. Horm. Metab. Res. 2010, 42, 353–357. [Google Scholar] [CrossRef]
- Huyen, V.T.; Phan, D.V.; Thang, P.; Hoa, N.K.; Ostenson, C.G. Gynostemma pentaphyllum Tea Improves Insulin Sensitivity in Type 2 Diabetic Patients. J. Nutr. Metab. 2013, 2013, 765383. [Google Scholar] [CrossRef]
- Chi, A.; Tang, L.; Zhang, J.; Zhang, K. Chemical composition of three polysaccharides from Gynostemma pentaphyllum and their antioxidant activity in skeletal muscle of exercised mice. Int. J. Sport. Nutr. Exerc. Metab. 2012, 22, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jung, J.I.; Jeon, Y.E.; Kim, S.M.; Hong, S.H.; Kim, T.Y.; Kim, E.J. Gynostemma pentaphyllum extract and its active component gypenoside L improve the exercise performance of treadmill-trained mice. Nutr. Res. Pract. 2022, 16, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Lin-Na, S.; Yong-Xiu, S. Effects of polysaccharides from Gynostemma pentaphyllum (Thunb.), Makino on physical fatigue. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Pokrywka, A.; Cholbinski, P.; Kaliszewski, P.; Kowalczyk, K.; Konczak, D.; Zembron-Lacny, A. Metabolic modulators of the exercise response: Doping control analysis of an agonist of the peroxisome proliferator-activated receptor delta (GW501516) and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR). J. Physiol. Pharmacol. 2014, 65, 469–476. [Google Scholar] [PubMed]
- Winder, W.W.; Holmes, B.F. Insulin stimulation of glucose uptake fails to decrease palmitate oxidation in muscle if AMPK is activated. J. Appl. Physiol. 2000, 89, 2430–2437. [Google Scholar] [CrossRef]
- McConell, G.K.; Ng, G.P.; Phillips, M.; Ruan, Z.; Macaulay, S.L.; Wadley, G.D. Central role of nitric oxide synthase in AICAR and caffeine-induced mitochondrial biogenesis in L6 myocytes. J. Appl. Physiol. 2010, 108, 589–595. [Google Scholar] [CrossRef]
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [CrossRef]
- Granata, C.; Oliveira, R.S.; Little, J.P.; Renner, K.; Bishop, D.J. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 3413–3423. [Google Scholar] [CrossRef]
- Broome, S.C.; Braakhuis, A.J.; Mitchell, C.J.; Merry, T.L. Mitochondria-targeted antioxidant supplementation improves 8 km time trial performance in middle-aged trained male cyclists. J. Int. Soc. Sports Nutr. 2021, 18, 58. [Google Scholar] [CrossRef]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Chang, E.; Park, D.H.; Kang, J.H.; Seo, D.Y.; Han, J.; Jung, S.J.; Hwangbo, K.; et al. Effects of a single bout of exercise on mitochondria-mediated apoptotic signaling in rat cardiac and skeletal muscles. J. Exerc. Rehabil. 2019, 15, 512–517. [Google Scholar] [CrossRef]
- Trewin, A.J.; Parker, L.; Shaw, C.S.; Hiam, D.S.; Garnham, A.; Levinger, I.; McConell, G.K.; Stepto, N.K. Acute HIIE elicits similar changes in human skeletal muscle mitochondrial H2O2 release, respiration, and cell signaling as endurance exercise even with less work. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1003–R1016. [Google Scholar] [CrossRef] [PubMed]
- Layec, G.; Blain, G.M.; Rossman, M.J.; Park, S.Y.; Hart, C.R.; Trinity, J.D.; Gifford, J.R.; Sidhu, S.K.; Weavil, J.C.; Hureau, T.J.; et al. Acute High-Intensity Exercise Impairs Skeletal Muscle Respiratory Capacity. Med. Sci. Sports Exerc. 2018, 50, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Hirano, R.; Igarashi, O.; Kondo, K.; Itakura, H.; Matsumoto, A. Regulation by long-chain fatty acids of the expression of cholesteryl ester transfer protein in HepG2 cells. Lipids 2001, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]
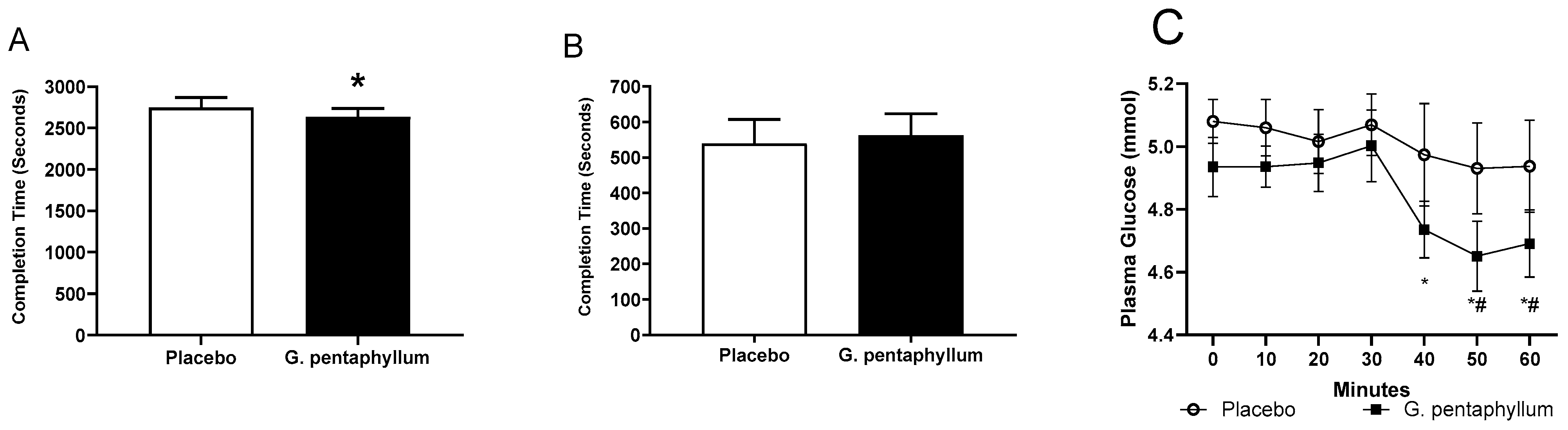
 n = 15) compared to placebo (
n = 15) compared to placebo ( n = 15 and 60 min of steady state exercise. Data were expressed as mean ± SEM. * p < 0.05 compared to 0 min of the respective treatment. ^ p < 0.05 comparing treatments at the respective time point. # p < 0.05 time effect of the exercise irrespective of treatment.
n = 15 and 60 min of steady state exercise. Data were expressed as mean ± SEM. * p < 0.05 compared to 0 min of the respective treatment. ^ p < 0.05 comparing treatments at the respective time point. # p < 0.05 time effect of the exercise irrespective of treatment.
 n = 15) compared to placebo (
n = 15) compared to placebo ( n = 15 and 60 min of steady state exercise. Data were expressed as mean ± SEM. * p < 0.05 compared to 0 min of the respective treatment. ^ p < 0.05 comparing treatments at the respective time point. # p < 0.05 time effect of the exercise irrespective of treatment.
n = 15 and 60 min of steady state exercise. Data were expressed as mean ± SEM. * p < 0.05 compared to 0 min of the respective treatment. ^ p < 0.05 comparing treatments at the respective time point. # p < 0.05 time effect of the exercise irrespective of treatment.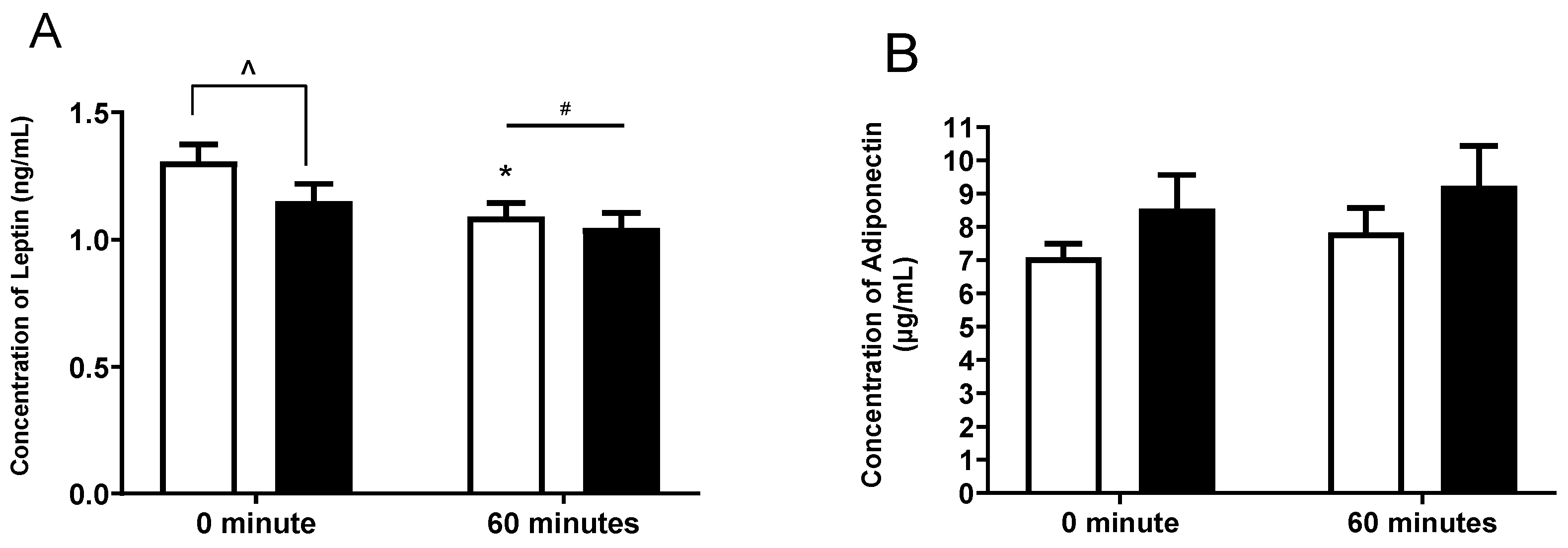
 n = 14–15) and G. pentaphyllum (
n = 14–15) and G. pentaphyllum ( n = 14–15) with 0, 30, and 60 min exercise. All genes were normalized to the average of two housekeeping genes (cyclophilin and glyceraldehydes-3-phosphate dehydrogenase (GAPDH)). Data were expressed as mean ± SEM. * p < 0.05 compared to 0 min of the respective treatment. # p < 0.05 time effect of the exercise irrespective of treatment. ^ p < 0.05 comparing treatments at the same timepoint.
n = 14–15) with 0, 30, and 60 min exercise. All genes were normalized to the average of two housekeeping genes (cyclophilin and glyceraldehydes-3-phosphate dehydrogenase (GAPDH)). Data were expressed as mean ± SEM. * p < 0.05 compared to 0 min of the respective treatment. # p < 0.05 time effect of the exercise irrespective of treatment. ^ p < 0.05 comparing treatments at the same timepoint.
 n = 14–15) and G. pentaphyllum (
n = 14–15) and G. pentaphyllum ( n = 14–15) with 0, 30, and 60 min exercise. All genes were normalized to the average of two housekeeping genes (cyclophilin and glyceraldehydes-3-phosphate dehydrogenase (GAPDH)). Data were expressed as mean ± SEM. * p < 0.05 compared to 0 min of the respective treatment. # p < 0.05 time effect of the exercise irrespective of treatment. ^ p < 0.05 comparing treatments at the same timepoint.
n = 14–15) with 0, 30, and 60 min exercise. All genes were normalized to the average of two housekeeping genes (cyclophilin and glyceraldehydes-3-phosphate dehydrogenase (GAPDH)). Data were expressed as mean ± SEM. * p < 0.05 compared to 0 min of the respective treatment. # p < 0.05 time effect of the exercise irrespective of treatment. ^ p < 0.05 comparing treatments at the same timepoint.
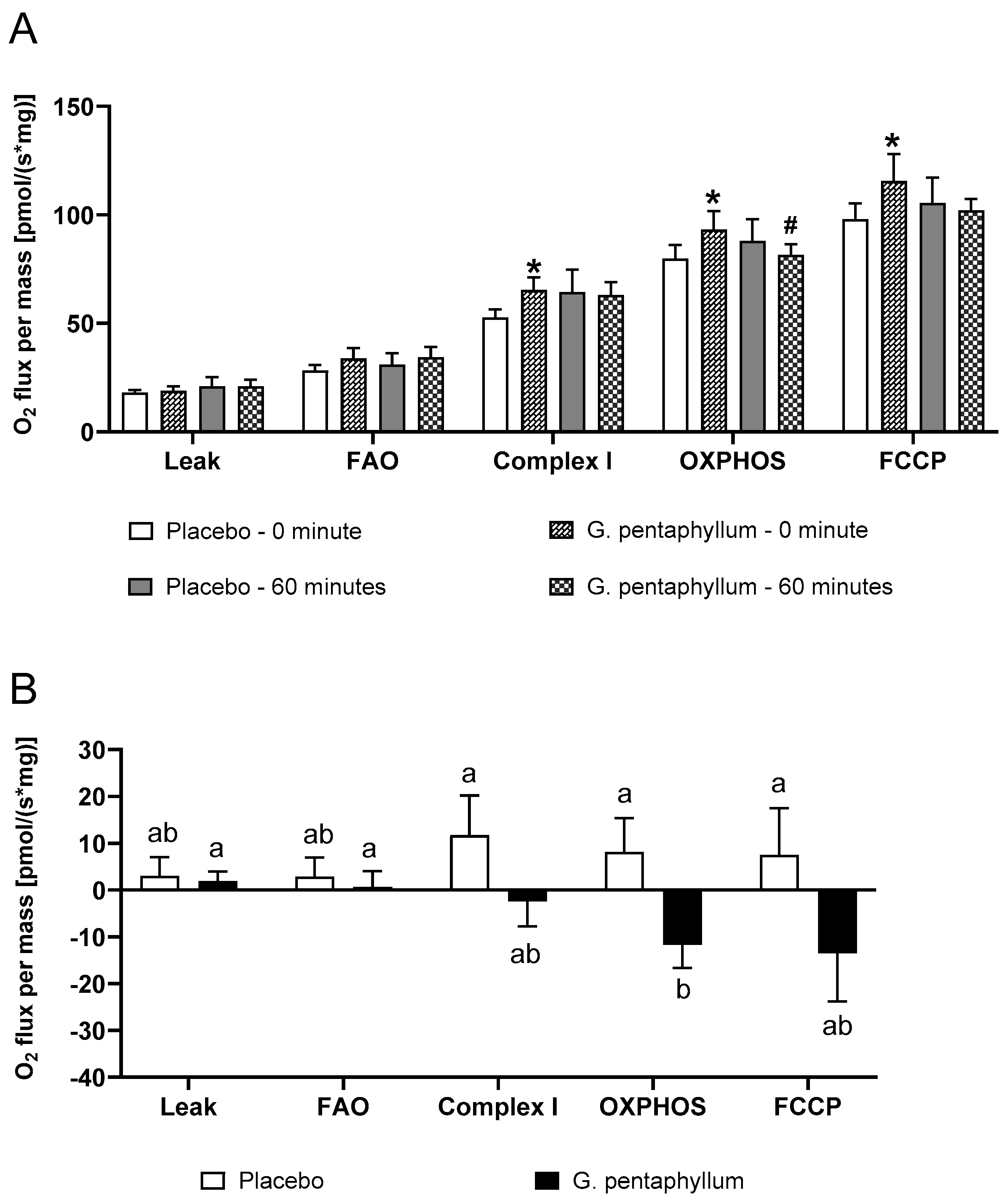
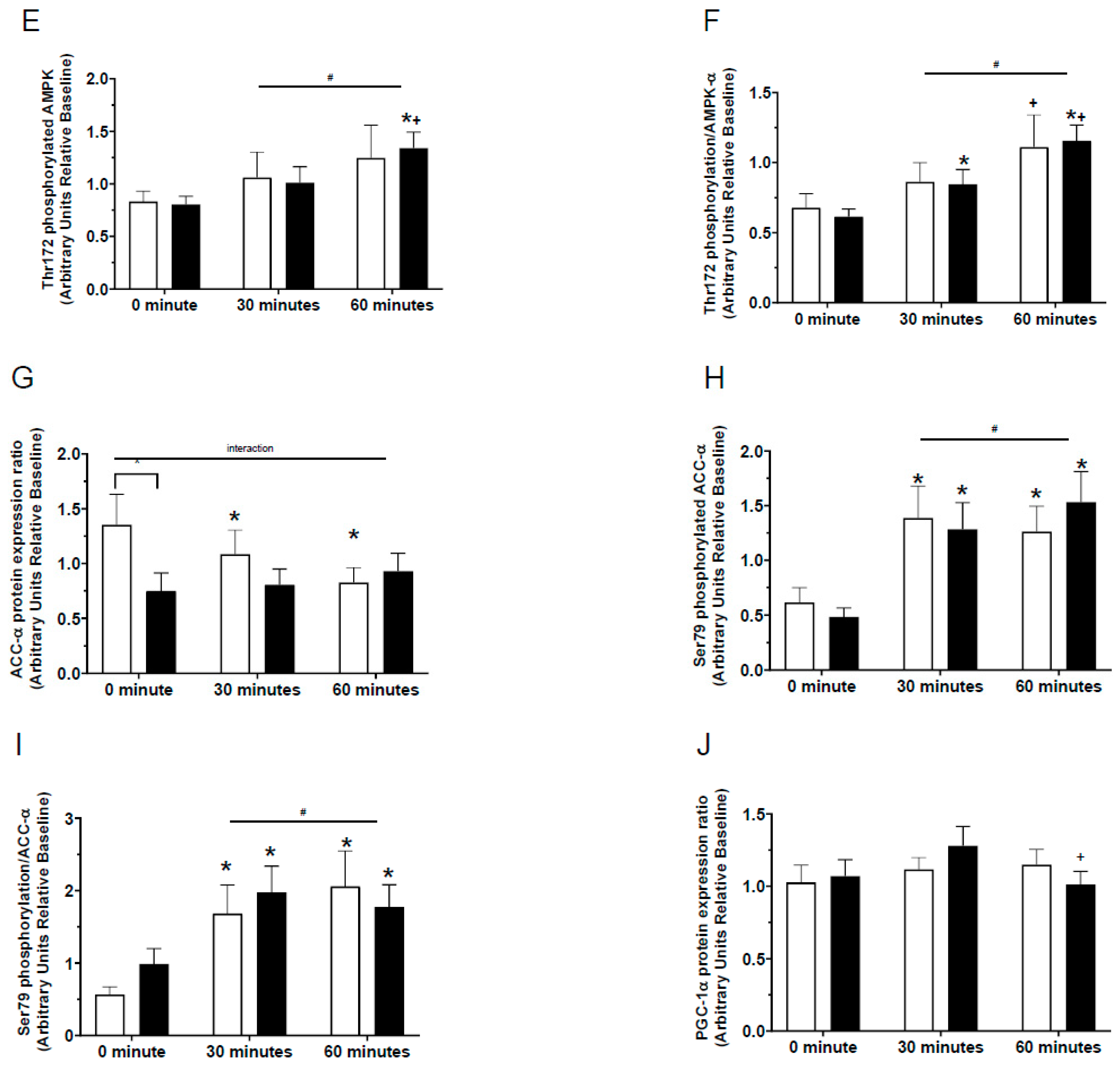
 n = 13–14) and G. pentaphyllum supplementation (
n = 13–14) and G. pentaphyllum supplementation ( n = 13–14) with 30 and 60 munities exercise. Error bars represent the means ± SEM; * p < 0.05 compared to 0 min of the respective supplement. ^ p < 0.05 comparing treatments at the respective time. + p < 0.05 compared to 30 min of the respective supplement. # p < 0.05 time effect of the exercise irrespective of supplementation.
n = 13–14) with 30 and 60 munities exercise. Error bars represent the means ± SEM; * p < 0.05 compared to 0 min of the respective supplement. ^ p < 0.05 comparing treatments at the respective time. + p < 0.05 compared to 30 min of the respective supplement. # p < 0.05 time effect of the exercise irrespective of supplementation.
 n = 13–14) and G. pentaphyllum supplementation (
n = 13–14) and G. pentaphyllum supplementation ( n = 13–14) with 30 and 60 munities exercise. Error bars represent the means ± SEM; * p < 0.05 compared to 0 min of the respective supplement. ^ p < 0.05 comparing treatments at the respective time. + p < 0.05 compared to 30 min of the respective supplement. # p < 0.05 time effect of the exercise irrespective of supplementation.
n = 13–14) with 30 and 60 munities exercise. Error bars represent the means ± SEM; * p < 0.05 compared to 0 min of the respective supplement. ^ p < 0.05 comparing treatments at the respective time. + p < 0.05 compared to 30 min of the respective supplement. # p < 0.05 time effect of the exercise irrespective of supplementation.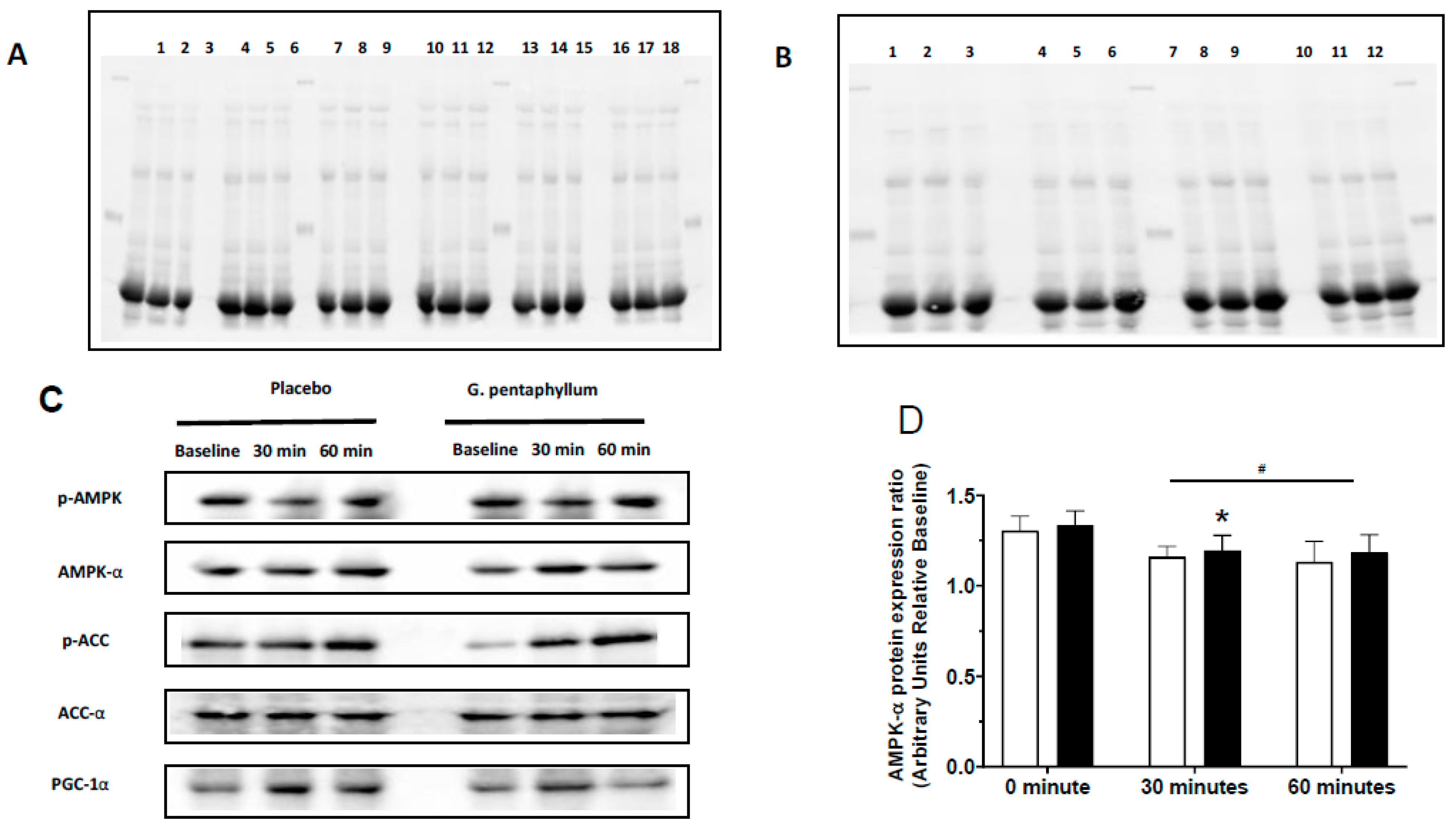
| Gene | Accession Number | Sequence |
|---|---|---|
| ACC-α | NM_198836.2 | Forward (5′–3′) CTGGAGCCCTCAACAAAGTC Reverse (5′–3′) CCAGTGCAGGACAGTGAAAA |
| AMPK-α | XM_016919269 | Forward (5′–3′) AACTGCAGAGAGCCATTCACTTT Reverse (5′–3′) GGTGAAACTGAAGACAATGTGCTT |
| Cyclophilin | XM_024747056 | Forward (5′–3′) CATCTGCACTGCCAAGACTGA Reverse (5′–3′) TTCATGCCTTCTTTCACTTTGC |
| FoxO1 | XM_522749 | Forward (5′–3′) TCATGGATGGAGATACATTGGATT Reverse (5′–3′) TCCTGCTGTCAGACAATCTGAAG |
| GAPDH | XM_024733357 | Forward (5′–3′) CAA CGA CCA CTT TGT CAA GC Reverse (5′–3′) TTA CTC CTT GGA GGC CAT GT |
| IRS-1 | NM_000208 | Forward (5′–3′) GTTTCCAGAAGCAGCCAGAG Reverse (5′–3′) TGAAATGGATGCATCGTACC |
| IRS-2 | NM_003749.2 | Forward (5′–3′) ACGCCAGCATTGACTTCTTGT Reverse (5′–3′) TGACATGTGACATCCTGGTGATAA |
| PGC1-α | NM_013261.4 | Forward (5′–3′) CAAGCCAAACCAACAACTTTATCTCT Reverse (5′–3′) CACACTTAAGGTGCGTTCAATAGTC |
| PI3K | NM_181504.3 | Forward (5′–3′) GGAAGCAGCAACCGAAACAA Reverse (5′–3′) TTCGCCGTCCACCACTACA |
| PPAR-α | XM_024452253 | Forward (5′–3′) GAAGCTGTCCTGGCTCAGAT Reverse (5′–3′) GGGGACCACAGGATAAGTCA |
| Participants | ||
|---|---|---|
| Placebo | G. pentaphyllum | |
| Age (years) | 23.3 ± 1.1 | |
| Height (cm) | 174.4 ± 1.6 | |
| Body Mass (kg) | 69.0 ± 1.9 | 68.7 ± 1.8 |
| BMI (kg/m2) | 22.6 ± 0.5 | 22.6 ± 0.5 |
| VO2 peak (mL·kg−1·min−1) | 40.8 ± 1.7 | |
| Peak power (Watts) | 214.3 ± 11.3 | |
| TtE power output (Watts) | 161.2 ± 9.2 | 160.9 ± 9.3 |
| SS power output (Watts) | 103.6 ± 6.5 | 105.2 ± 7.2 |
| Plasma fasting glucose (mmol/L) | 5.15 ± 0.11 | 4.86 ± 0.07 * |
| Total cholesterol (mmol/L) | 3.71 ± 0.18 | 3.76 ± 0.14 |
| HDL (mmol/L) | 1.24 ± 0.04 | 1.3 ± 0.05 * |
| LDL (mmol/L) | 2.05 ± 0.18 | 1.98 ± 0.12 |
| LDL/HDL | 1.69 ± 0.16 | 1.56 ± 0.13 |
| Total cholesterol/HDLC | 3.04 ± 0.16 | 2.90 ± 0.16 |
| Total triglycerides (mmol/L) | 0.98 ± 0.11 | 1.04 ± 0.16 |
| Adiponectin (µg/mL) | 7.09 ± 0.42 | 8.55 ± 1.02 |
| Leptin (ng/mL) | 1.31 ± 0.07 | 1.15 ± 0.07 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayyar, D.; Yan, X.; Xu, G.; Shi, M.; Garnham, A.P.; Mathai, M.L.; McAinch, A.J. Gynostemma Pentaphyllum Increases Exercise Performance and Alters Mitochondrial Respiration and AMPK in Healthy Males. Nutrients 2023, 15, 4721. https://doi.org/10.3390/nu15224721
Nayyar D, Yan X, Xu G, Shi M, Garnham AP, Mathai ML, McAinch AJ. Gynostemma Pentaphyllum Increases Exercise Performance and Alters Mitochondrial Respiration and AMPK in Healthy Males. Nutrients. 2023; 15(22):4721. https://doi.org/10.3390/nu15224721
Chicago/Turabian StyleNayyar, Deepti, Xu Yan, Guoqin Xu, Min Shi, Andrew P. Garnham, Michael L. Mathai, and Andrew J. McAinch. 2023. "Gynostemma Pentaphyllum Increases Exercise Performance and Alters Mitochondrial Respiration and AMPK in Healthy Males" Nutrients 15, no. 22: 4721. https://doi.org/10.3390/nu15224721
APA StyleNayyar, D., Yan, X., Xu, G., Shi, M., Garnham, A. P., Mathai, M. L., & McAinch, A. J. (2023). Gynostemma Pentaphyllum Increases Exercise Performance and Alters Mitochondrial Respiration and AMPK in Healthy Males. Nutrients, 15(22), 4721. https://doi.org/10.3390/nu15224721