Biological Properties and Antimicrobial Potential of Cocoa and Its Effects on Systemic and Oral Health
Abstract
:1. Introduction
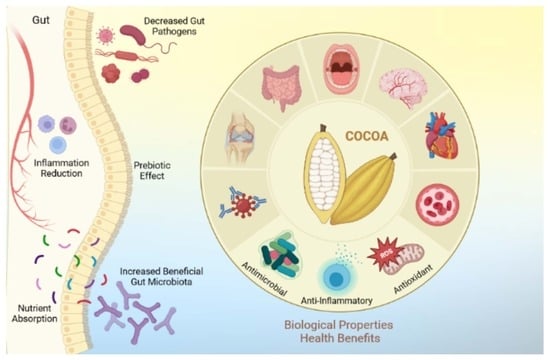
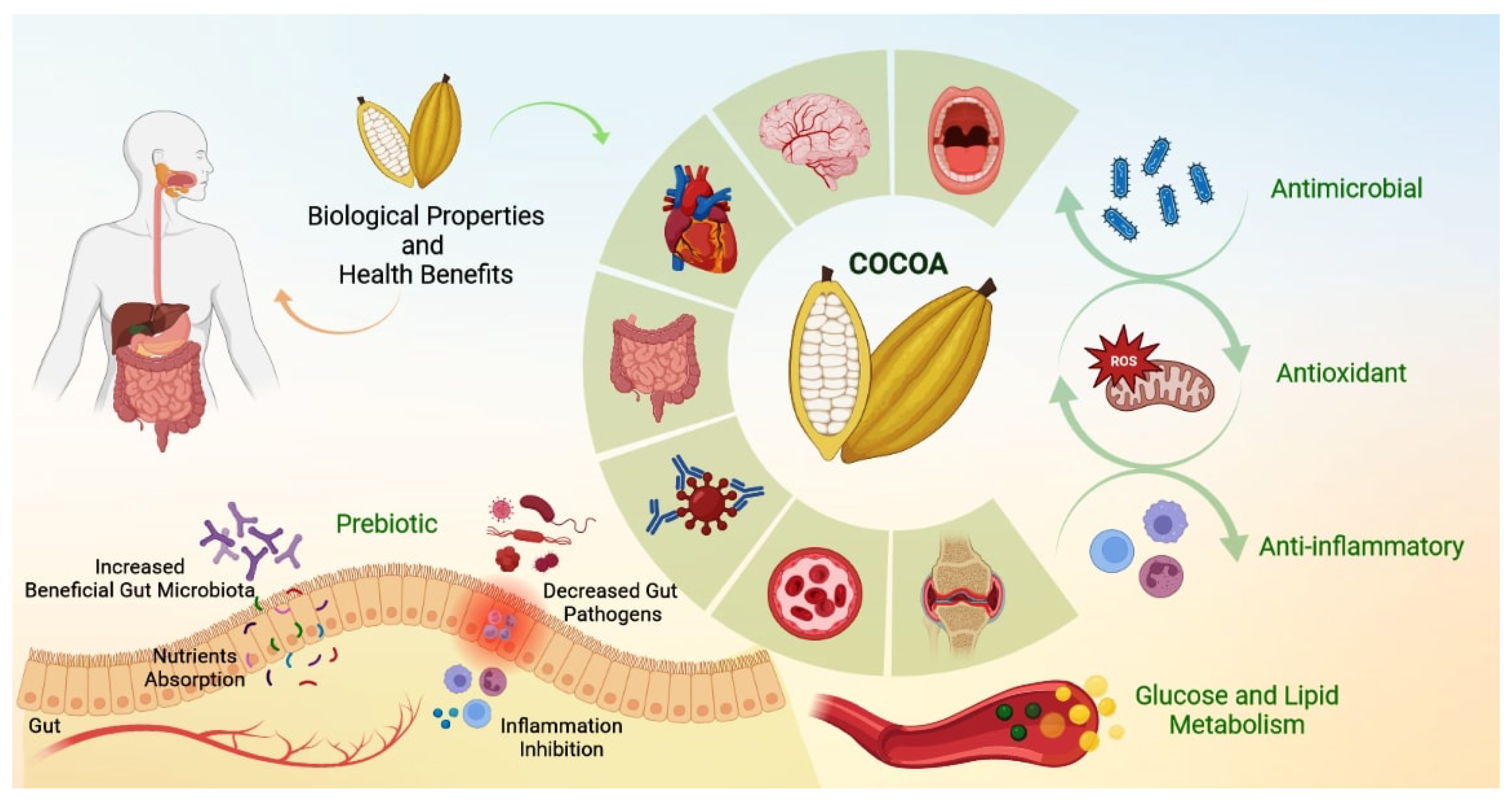
2. Health Benefits of Cocoa
3. Biological Properties of Cocoa and Oral Health
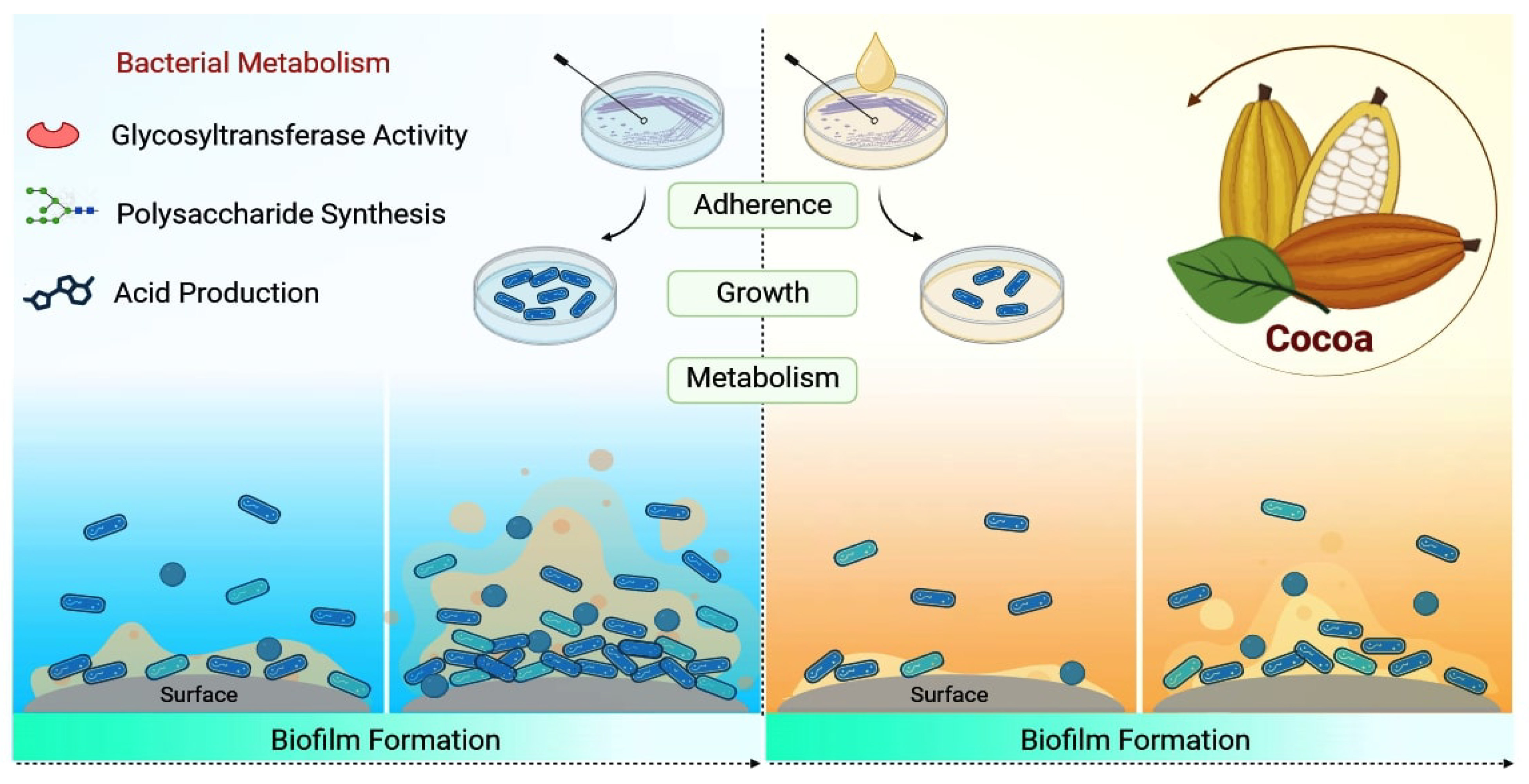
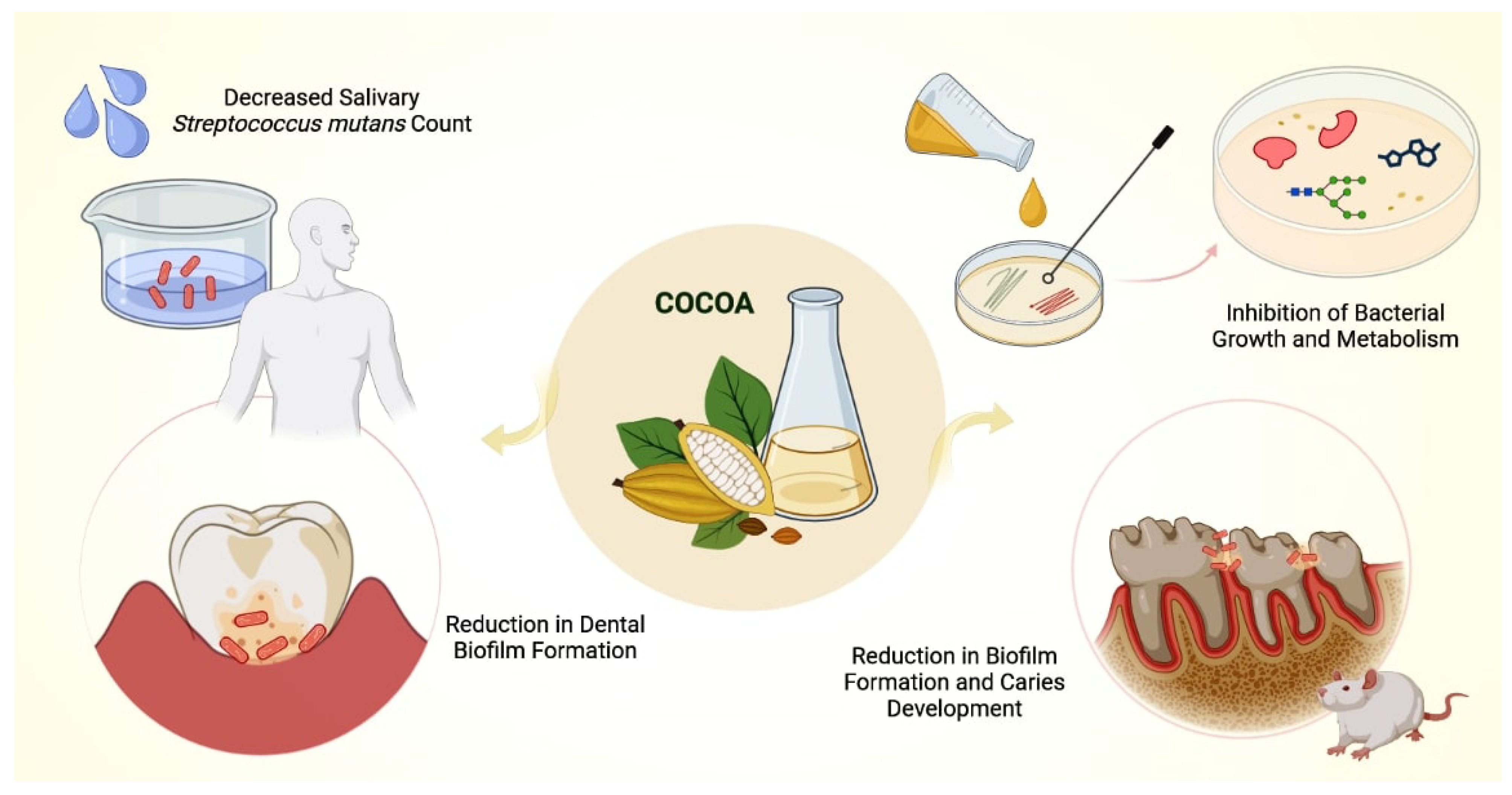
Antimicrobial Potential of Cocoa
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellam, S.; Williamson, G. Cocoa and human health. Annu. Rev. Nutr. 2013, 33, 105–128. [Google Scholar] [CrossRef]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and Chocolate in Human Health and Disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef]
- Martin, M.A.; Ramos, S. Impact of cocoa flavanols on human health. Rev. Food Chem. Toxicol. 2021, 151, 112121. [Google Scholar] [CrossRef]
- Badrie, N.; Bekele, F.; Sikora, E.; Sikora, M. Cocoa agronomy, quality, nutritional, and health aspects. Crit. Rev. Food Sci. Nutr. 2015, 55, 620–659. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant Structure-Activity Relationship Analysis of Five Dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Jeffery, E. Flavonoids. Adv. Nutr. 2013, 4, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Balentić, J.P.; Ačkar, D.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; de Natale, A.; Pollio, A. Anti-cariogenic effects of polyphenols from plant stimulant beverages (cocoa, coffee, tea). Fitoterapia 2009, 80, 255–262. [Google Scholar] [CrossRef]
- Hwang, S.L.; Yen, G.C. Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J. Agric. Food Chem. 2008, 56, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Park, J.H.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Won, M.H.; Lee, C.H.; Hwang, I.K.; Kim, J.D.; et al. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regen. Res. 2017, 12, 220–227. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Crascì, L.; Basile, L.; Panico, A.; Puglia, C.; Bonina, F.P.; Basile, P.M.; Rizza, L.; Guccione, S. Correlating In Vitro Target-Oriented Screening and Docking: Inhibition of Matrix Metalloproteinases Activities by Flavonoids. Planta Med. 2017, 83, 901–911. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Annabi, B.; Tahanian, E.; Sanchez, L.A.; Shiao, T.C.; Roy, R. Flavonoids targeting of IkappaB phosphorylation abrogates carcinogen-induced MMP-9 and COX-2 expression in human brain endothelial cells. Drug Des. Dev. Ther. 2011, 5, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharm. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; D’Onofrio, G.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Neuroprotective Effects of Quercetin: From Chemistry to Medicine. CNS Neurol. Disord. Drug Targets 2016, 15, 964–975. [Google Scholar] [CrossRef]
- Logar, D.B.; Komadina, R.; Preželj, J.; Ostanek, B.; Trošt, Z.; Marc, J. Expression of bone resorption genes in osteoarthritis and in osteoporosis. J. Bone Miner. Metab. 2007, 25, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Hardcastle, A.C. The Effects of Flavonoids on Bone. Curr. Osteoporos. Rep. 2014, 12, 205–210. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid Intake and Bone Health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef]
- Preethi Soundarya, S.; Sanjay, V.; Haritha Menon, A.; Dhivya, S.; Selvamurugan, N. Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Puig, E.; Castell, M. Cocoa: Antioxidant and immunomodulator. Br. J. Nutr. 2009, 101, 931–940. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Devine, A.; Burke, V.; Dick, I.M.; Prince, R.L. Chocolate consumption and bone density in older women. Am. J. Clin. Nutr. 2008, 87, 175–180. [Google Scholar] [CrossRef]
- Clough, B.H.; Ylostalo, J.; Browder, E.; McNeill, E.P.; Bartosh, T.J.; Rawls, H.R.; Nakamoto, T.; Gregory, C.A. Theobromine Upregulates Osteogenesis by Human Mesenchymal Stem Cells In Vitro and Accelerates Bone Development in Rats. Calcif. Tissue Int. 2017, 100, 298–310. [Google Scholar] [CrossRef]
- Tomofuji, T.; Ekuni, D.; Irie, K.; Azuma, T.; Endo, Y.; Tamaki, N.; Sanbe, T.; Murakami, J.; Yamamoto, T.; Morita, M. Preventive effects of a cocoa-enriched diet on gingival oxidative stress in experimental periodontitis. J. Periodontol. 2009, 80, 1799–1808. [Google Scholar] [CrossRef]
- Helbock, H.J.; Beckman, K.B.; Ames, B.N. 8-Hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of oxidative DNA damage. Methods Enzymol. 1999, 300, 156–166. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ishiwa, J.; Sato, T.; Mimaki, Y.; Sashida, Y. The citrus flavonoid nobiletin suppresses the production and gene expression of matrix metalloproteinases-9/gelatinase B in rabbit synovial cells. Ann. N. Y. Acad. Sci. 1999, 878, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, F.; Zhang, L.; Yu, H.; Yu, F.; Chen, J. The effect of active components from citrus fruits on dentin MMPs. Arch. Oral Biol. 2017, 83, 111–117. [Google Scholar] [CrossRef]
- van Strijp, A.J.; Takatsuka, T.; Sono, R.; Iijima, Y. Inhibition of dentine collagen degradation by hesperidin: An in situ study. Eur. J. Oral Sci. 2015, 123, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Hannas, A.R.; Pereira, J.C.; Granjeiro, J.; Tjäderhane, L. The role of matrix metalloproteinases in the oral environment. Acta Odontol. Scand. 2007, 65, 1–13. [Google Scholar] [CrossRef]
- Longhi, M.; Cerroni, L.; Condò, S.G.; Ariano, V.; Pasquantonio, G. The effects of host derived metalloproteinases on dentin Bond and the role of MMPs inhibitors on dentin matrix degradation. Oral Implant. 2015, 7, 71–79. [Google Scholar]
- Hiraishi, N.; Sono, R.; Islam, M.S.; Otsuki, M.; Tagami, J.; Takatsuka, T. Effect of hesperidin in vitro on root dentine collagen and demineralization. J. Dent. 2011, 39, 391–396. [Google Scholar] [CrossRef]
- Islam, M.S.; Hiraishi, N.; Nassar, M.; Sono, R.; Otsuki, M.; Takatsura, T.; Yiu, C.; Tagami, J. In vitro effect of hesperidin on root dentin collagen and de/re-mineralization. Dent. Mater. J. 2012, 31, 362–367. [Google Scholar] [CrossRef]
- Palenik, C.J.; Park, K.; Katz, S.; Stookey, G.K. Effect of water soluble components derived from cocoa on plaque formation. J. Dent. Res. 1979, 58, 1749. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Miyazaki, K.; Shimura, S.; Okuda, J.; Matsumoto, M.; Ooshima, T. Identification of cariostatic substances in the cacao bean husk: Their anti-glucosyltransferase and antibacterial activities. J. Dent. Res. 2001, 80, 2000–2004. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tsuji, M.; Okuda, J.; Sasaki, H.; Nakano, K.; Osawa, K.; Shimura, S.; Ooshima, T. Inhibitory effects of cacao bean husk extract on plaque formation in vitro and in vivo. Eur. J. Oral Sci. 2004, 112, 249–252. [Google Scholar] [CrossRef]
- Ooshima, T.; Osaka, Y.; Sasaki, H.; Osawa, K.; Yasuda, H.; Matsumura, M.; Sobue, S.; Matsumoto, M. Caries inhibitory activity of cacao bean husk extract in in-vitro and animal experiments. Arch. Oral Biol. 2000, 45, 639–645. [Google Scholar] [CrossRef]
- Smullen, J.; Koutsou, G.A.; Foster, H.A.; Zumbé, A.; Storey, D.M. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007, 41, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Percival, R.S.; Devine, D.A.; Duggal, M.S.; Chartron, S.; Marsh, P.D. The effect of cocoa polyphenols on the growth, metabolism, and biofilm formation by Streptococcus mutans and Streptococcus sanguinis. Eur. J. Oral Sci. 2006, 114, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Lagha, A.B.; Huacho, P.M.; Grenier, D. A cocoa (Theobroma cacao L.) extract impairs the growth, virulence properties, and inflammatory potential of Fusobacterium nucleatum and improves oral epithelial barrier function. PLoS ONE 2021, 16, e0252029. [Google Scholar] [CrossRef]
- Lakshmi, A.; Vishnurekha, C.; Baghkomeh, P.N. Effect of theobromine in antimicrobial activity: An in vitro study. Dent. Res. J. 2019, 16, 76–80. [Google Scholar] [CrossRef]
- Demir, S.; Keskin, G.; Akal, N.; Zer, Y. Antimicrobial effect of natural kinds of toothpaste on oral pathogenic bactéria. J. Infect. Dev. Ctries. 2021, 15, 1436–1442. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Ribeiro, S.O.; Anton-Sales, C.; Keymeulen, F.; Barbosa-Pereira, L.; Delporte, C.; Zeppa, G.; Stévigny, C. Evaluation of Cocoa Bean Shell Antimicrobial Activity: A Tentative Assay Using a Metabolomic Approach for Active Compound Identification. Planta Med. 2021, 87, 841–849. [Google Scholar] [CrossRef]
- Srikanth, R.K.; Shashikiran, N.D.; Subba Reddy, V.V. Chocolate mouth rinse: Effect on plaque accumulation and mutans streptococci counts when used by children. J. Indian Soc. Pedod. Prev. Dent. 2008, 26, 67–70. [Google Scholar] [CrossRef]
- Fajriani; Mustamin, A.W.; Asmawati. The role of cacao extract in reduction of the number of mutans streptococci colonies in the saliva of 12–14 year-old-children. J. Indian Soc. Pedod. Prev. Dent. 2016, 34, 120–123. [Google Scholar] [CrossRef]
- Venkatesh Babu, N.S.; Vivek, D.K.; Ambika, G. Comparative evaluation of chlorhexidine mouthrinse versus cacao bean husk extract mouthrinse as antimicrobial agents in children. Eur. Arch. Paediatr. Dent. 2011, 12, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Shrimathi, S.; Kemparaj, U.; Umesh, S.; Karuppaiah, M.; Pandian, P.; Krishnaveni, A. Comparative Evaluation of Cocoa Bean Husk, Ginger and Chlorhexidine Mouth Washes in the Reduction of Steptococcus Mutans and Lactobacillus Count in Saliva: A Randomized Controlled Trial. Cureus 2019, 11, e4968. [Google Scholar] [CrossRef]
- Kibriya, S.; Srinivasan, I.; Setty, J.V.; Anu, S.; Khan, B.S. Characterization of Cocoa Bean Husk Extract Particles and its Comparison as a Mouthrinse with Different Vehicles in Children aged 7–12 Years. Int. J. Clin. Pediatr. Dent. 2023, 16, 54–59. [Google Scholar] [CrossRef] [PubMed]
| References | Participants | Intervention | Main Outcomes |
|---|---|---|---|
| Babu et al. (2011) [53] | 50 children (6–10 years) | Use of mouthwash containing 0.1% cocoa bean husk extract or mouthwash containing 0.2% chlorhexidine for up to 2 months. | Mouthwashes containing 0.2% chlorhexidine (23.2%) or 0.1% cocoa bean husk extract (22.4%) significantly reduced S. mutans counts (CFU) in saliva, with no statistical differences between them. |
| Matsumoto et al. (2014) [43] | 28 participants (19–29 years) | Use of mouthwash containing cocoa bean husk extract or vehicle for 4 days, without oral hygiene procedures. | Mouthwash containing cocoa bean husk extract significantly reduced dental biofilm accumulation and S. mutans counts in saliva, however, without expressive alterations in the total count of salivary streptococci (CFU).No adverse or undesirable effects were reported. |
| Fajriani et al. (2016) [52] | 30 children (12–14 years) | A single rinse with mouthwash containing 0.1% cocoa extract. | Analysis of saliva collected 15 and 30 min after rinsing showed that cocoa extract significantly reduced the S. mutans count (CFU) in relation to baseline. |
| Srikanth et al. (2019) [51] | 32 children (10–14 years) | Use of mouthwash containing 0.1% cocoa bean husk extract or a placebo mouthwash (control) for 4 days, without oral hygiene procedures. | Mouthwash containing 0.1% cocoa bean husk extract reduced S. mutans count (CFU) and plaque index scores (modified Quigley and Hein Index) by 20.9% and 49.6%, respectively, differing statistically from the control. |
| Shrimathi et al. (2019) [54] | 75 participants (18–25 years) | Use of mouthwashes containing cocoa bean husk extract (0.5%), chlorhexidine (0.2%) or ginger (12.5%) for 7 days. | Mouthwashes containing cocoa extract or chlorhexidine significantly reduced the population of S. mutans, while the mouthwash containing ginger resulted in a significant reduction in Lactobacillus levels (CFU). |
| Kibriya et al. (2023) [55] | 80 children (7–12 years) | Use of mouthwashes containing 0.1% cocoa bean husk extract (tested with different vehicles, such as distilled water, Ringer’s lactate or saline solution), 0.12% chlorhexidine or 0.05% sodium fluoride (NaF) for up to 14 days. | The groups treated with mouthwash containing cocoa bean husk extract, in distilled water or Ringer’s lactate, or mouthwash containing chlorhexidine showed the lowest plaque index scores (Simplified Oral Hygiene Index). Considering the S. mutans count (CFU) in saliva, the mouthwash containing cocoa husk extract in saline solution showed the best antibacterial effect. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fideles, S.O.M.; Ortiz, A.d.C.; Reis, C.H.B.; Buchaim, D.V.; Buchaim, R.L. Biological Properties and Antimicrobial Potential of Cocoa and Its Effects on Systemic and Oral Health. Nutrients 2023, 15, 3927. https://doi.org/10.3390/nu15183927
Fideles SOM, Ortiz AdC, Reis CHB, Buchaim DV, Buchaim RL. Biological Properties and Antimicrobial Potential of Cocoa and Its Effects on Systemic and Oral Health. Nutrients. 2023; 15(18):3927. https://doi.org/10.3390/nu15183927
Chicago/Turabian StyleFideles, Simone Ortiz Moura, Adriana de Cássia Ortiz, Carlos Henrique Bertoni Reis, Daniela Vieira Buchaim, and Rogério Leone Buchaim. 2023. "Biological Properties and Antimicrobial Potential of Cocoa and Its Effects on Systemic and Oral Health" Nutrients 15, no. 18: 3927. https://doi.org/10.3390/nu15183927
APA StyleFideles, S. O. M., Ortiz, A. d. C., Reis, C. H. B., Buchaim, D. V., & Buchaim, R. L. (2023). Biological Properties and Antimicrobial Potential of Cocoa and Its Effects on Systemic and Oral Health. Nutrients, 15(18), 3927. https://doi.org/10.3390/nu15183927