Understanding Type 2 Diabetes Mellitus Risk Parameters through Intermittent Fasting: A Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Intermittent Fasting Interventions
2.2. Preparing and Pre-Processing the Data
2.2.1. Selecting the Features
2.2.2. Selecting Individuals
2.2.3. Calculating HOMA-IR
2.3. Constructing the Datasets
2.3.1. Dataset to Predict Whether a Specific Intervention Can Improve HOMA-IR
2.3.2. Continuous Target Column: Improving Fasting Glucose
2.3.3. Excluding the Interventions’ Feature
2.3.4. Increasing the Threshold for Improvement in HOMA-IR or Fasting Glucose
2.4. Machine Learning Classifiers
2.5. Testing Approach
3. Results
3.1. Predicting Whether a Certain Intervention Can Improve HOMA-IR
3.2. Can We Predict Improvement in HOMA-IR without Knowing the Intervention?
3.3. Predicting Whether a Specific Intervention Can Improve Fasting Glucose Only
3.4. Comparison of Different Interventions in Improving T2DM Risk Parameters Using Continuous Difference
3.5. Random Testing
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Organization 2023. Available online: https://www.who.int/data/stories/world-health-statistics-2023-a-visual-summary/ (accessed on 5 August 2023).
- International Diabetes Federation. Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2022. [Google Scholar]
- Mourouti, N.; Mavrogianni, C.; Mouratidou, T.; Liatis, S.; Valve, P.; Rurik, I.; Torzsa, P.; Cardon, G.; Bazdarska, Y.; Iotova, V.; et al. The Association of Lifestyle Patterns with Prediabetes in Adults from Families at High Risk for Type 2 Diabetes in Europe: The Feel4Diabetes Study. Nutrients 2023, 15, 3155. [Google Scholar] [CrossRef]
- Shin, J.; Zhou, X.; Tan, J.T.M.; Hyppönen, E.; Benyamin, B.; Lee, S.H. Lifestyle Modifies the Diabetes-Related Metabolic Risk, Conditional on Individual Genetic Differences. Front. Genet. 2022, 13, 759309. [Google Scholar] [CrossRef]
- Swift, D.L.; Johannsen, N.M.; Lavie, C.J.; Earnest, C.P.; Church, T.S. The role of exercise and physical activity in weight loss and maintenance. Prog. Cardiovasc. Dis. 2014, 56, 441–447. [Google Scholar] [CrossRef]
- Vieira-Lara, M.A.; Reijne, A.C.; Koshian, S.; Ciapaite, J.; Abegaz, F.; Talarovicova, A.; van Dijk, T.H.; Versloot, C.J.; Bandsma, R.H.J.; Wolters, J.C.; et al. Age and Diet Modulate the Insulin-Sensitizing Effects of Exercise: A Tracer-Based Oral Glucose Tolerance Test. Diabetes 2023, 72, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Al-Sulaiti, H.; Diboun, I.; Agha, M.V.; Mohamed, F.F.S.; Atkin, S.; Dömling, A.S.; Elrayess, M.A.; Mazloum, N.A. Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J. Transl. Med. 2019, 17, 348. [Google Scholar] [CrossRef]
- Rastogi, A.; Weir, M.R. Multimodal efforts to slow the progression of chronic kidney disease in patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2023, 37, 108515. [Google Scholar] [CrossRef] [PubMed]
- Pfuetzner, A.; Manessis, A.; Do, L.; Hanna, M. De-Escalation Treatment (DET)—A personalized pharmacological intervention to stop disease progression in patients with type 2 diabetes. Diabetes 2020, 69, 1077-P. [Google Scholar] [CrossRef]
- Grajower, M.M.; Horne, B.D. Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 2019, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Marshe, V.S.; Pira, S.; Mantere, O.; Bosche, B.; Looper, K.J.; Herrmann, N.; Müller, D.J.; Rej, S. C-reactive protein and cardiovascular risk in bipolar disorder patients: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 442–451. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef]
- Wibowo, R.A.; Nurámalia, R.; Nurrahma, H.A.; Oktariani, E.; Setiawan, J.; Icanervilia, A.V.; Agustiningsih, D. The Effect of Yoga on Health-Related Fitness among Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4199. [Google Scholar] [CrossRef] [PubMed]
- Khadanga, S.; Barrett, K.; Sheahan, K.H.; Savage, P.D. Novel Therapeutics for Type 2 Diabetes, Obesity, and Heart Failure: A review and practical recommendations for cardiac rehabilitation. J. Cardiopulm. Rehabil. Prev. 2023, 43, 1–7. [Google Scholar] [CrossRef]
- Grams, J.; Garvey, W.T. Weight Loss and the Prevention and Treatment of Type 2 Diabetes Using Lifestyle Therapy, Pharmacotherapy, and Bariatric Surgery: Mechanisms of Action. Curr. Obes. Rep. 2015, 4, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kriska, A.M.; Devaraj, S.M.; Kramer, K.; Napoleone, J.M.; Rockette-Wagner, B.; Eaglehouse, Y.; Arena, V.C.; Miller, R.G. The Likely Underestimated Impact of Lifestyle Intervention: Diabetes Prevention Program Translation Examples. Am. J. Prev. Med. 2022, 62, e248–e254. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Ciotola, M.; Maiorino, M.I.; Giugliano, D. Lifestyle approach for type 2 diabetes and metabolic syndrome. Curr. Atheroscler Rep. 2008, 10, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Czaja, K. Can Circadian Eating Pattern Adjustments Reduce Risk or Prevent Development of T2D? Nutrients 2023, 15, 1762. [Google Scholar] [CrossRef]
- Joaquim, L.; Faria, A.; Loureiro, H.; Matafome, P. Benefits, mechanisms, and risks of intermittent fasting in metabolic syndrome and type 2 diabetes. J. Physiol. Biochem. 2022, 78, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Khalfallah, M.; Elnagar, B.; Soliman, S.S.; Eissa, A.; Allaithy, A. The Value of Intermittent Fasting and Low Carbohydrate Diet in Prediabetic Patients for the Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2023, 120, e20220606. [Google Scholar] [CrossRef]
- Pelc, M.A. The effects of intermittent fasting and a low-carbohydrate diet on type 2 diabetes. JAAPA 2023, 36, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Mudgal, S.K.; Kalra, S.; Gaur, R.; Thakur, K.; Agarwal, R. Effect of Intermittent Fasting on Glycaemic Control in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Randomized Controlled Trials. touchREV Endocrinol. 2023, 19, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.I.; Direito, M.; Pinto-Ribeiro, F.; Ludovico, P.; Sampaio-Marques, B. Effects of Intermittent Fasting on Regulation of Metabolic Homeostasis: A Systematic Review and Meta-Analysis in Health and Metabolic-Related Disorders. J. Clin. Med. 2023, 12, 3699. [Google Scholar] [CrossRef]
- Silver, D.T.; Pekari, T.B. A Review of Intermittent Fasting as a Treatment for Type 2 Diabetes Mellitus. Med. J. 2023, 65–71. [Google Scholar]
- Ojo, T.K.; Joshua, O.O.; Ogedegbe, O.J.; Oluwole, O.; Ademidun, A.; Jesuyajolu, D. Role of Intermittent Fasting in the Managementof Prediabetes and Type 2 Diabetes Mellitus. Cureus 2022, 14, e28800. [Google Scholar] [CrossRef]
- van den Burg, E.L.; van Peet, P.G.; Schoonakker, M.P.; van de Haar, D.E.; Numans, M.E.; Pijl, H. Metabolic impact of intermittent energy restriction and periodic fasting in patients with type 2 diabetes: A systematic review. Nutr. Rev. 2023, nuad015. [Google Scholar] [CrossRef] [PubMed]
- Borgundvaag, E. Metabolic Impact of Intermittent Fastingin Patients with Type2 Diabetes Mellitus A Systematic Review and Meta analysis of Interventional Studies. J. Clin. Metab. 2021, 106, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Lee, S.; Sun, Y.; Zhang, C.; Hill, M.A.; Li, Y.; Zhang, H. Alternate Day Fasting Improves Endothelial Function in Type 2 Diabetic Mice: Role of Adipose-Derived Hormones. Front. Cardiovasc. Med. 2022, 9, 925080. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Alternate-Day Intermittent Fasting Improves Diabetes and Protects Beta-Cell Function in Polygenic Mouse Models of T2DM. Diabetes 2022, 71, 1354-P. [Google Scholar] [CrossRef]
- Barua, S.; Bruno, J.; Nasserifar, S.; Vanegas, S.M.; Popp, C.; Walker, J.M.; Aleman, J.O. Early Time-Restricted Feeding Reduces Time in Elevated Glucose Range in Adults with Prediabetes. Diabetes 2023, 72, 114-LB. [Google Scholar] [CrossRef]
- de Toro-Martín, J.; Arsenault, B.J.; Després, J.P.; Vohl, M.C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef]
- Gharipour, M.; Nezafati, P.; Sadeghian, L.; Eftekhari, A.; Rothenberg, I.; Jahanfar, S. Precision medicine and metabolic syndrome. ARYA Atheroscler. 2022, 18, 1–10. [Google Scholar] [CrossRef]
- Ling, C.; Bacos, K.; Rönn, T. Epigenetics of type 2 diabetes mellitus and weight change—A tool for precision medicine? Nat. Rev. Endocrinol. 2022, 18, 433–448. [Google Scholar] [CrossRef]
- Harreiter, J.; Kautzky-Willer, A. Sex and Gender Differences in Prevention of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 220. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Corapi, S.; Gabel, K.; Ezpeleta, M.; Kalam, F.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of Intermittent Fasting on Reproductive Hormone Levels in Females and Males: A Review of Human Trials. Nutrients 2022, 14, 2343. [Google Scholar] [CrossRef]
- Khatami, F.; Mohajeri-Tehrani, M.R.; Tavangar, S.M. The Importance of Precision Medicine in Type 2 Diabetes Mellitus (T2DM): From Pharmacogenetic and Pharmacoepigenetic Aspects. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 719–731. [Google Scholar] [CrossRef]
- Peter, P.R.; Lupsa, B.C. Personalized Management of Type 2 Diabetes. Curr. Diab. Rep. 2019, 19, 115. [Google Scholar] [CrossRef]
- Pilla, S.J.; Mathioudakis, N.N.; Maruthur, N.M. Trialing precision medicine for type 2 diabetes. Nat. Med. 2023, 29, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Jones, H.; Stephens, J.W. Personalized Type 2 Diabetes Management: An Update on Recent Advances and Recommendations. Diabetes Metab. Syndr. Obes. 2022, 15, 281–295. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Chowdhury, E.A.; Richardson, J.D.; Holman, G.D.; Tsintzas, K.; Thompson, D.; Betts, J.A. The causal role of breakfast in energy balance and health: A randomized controlled trial in obese adults. Am. J. Clin. Nutr. 2016, 103, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M.; Noakes, M.; Keogh, J.B. Very low-fat (12%) and high monounsaturated fat (35%) diets do not differentially affect abdominal fat loss in overweight, nondiabetic women. J. Nutr. 2004, 134, 1741–1745. [Google Scholar] [CrossRef][Green Version]
- Hutchison, A.T.; Liu, B.; Wood, R.E.; Vincent, A.D.; Thompson, C.H.; O’Callaghan, N.J.; Wittert, G.A.; Heilbronn, L.K. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity 2019, 27, 50–58. [Google Scholar] [CrossRef]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Su, W.; Yu, S.; Yin, Y.; Li, B.; Xue, J.; Wang, J.; Gu, Y.; Zhang, H.; Lyu, Z.; Mu, Y.; et al. Diabetic microenvironment preconditioning of adipose tissue-derived mesenchymal stem cells enhances their anti-diabetic, anti-long-term complications, and anti-inflammatory effects in type 2 diabetic rats. Stem. Cell. Res. Ther. 2022, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cheng, Y.; Zhang, L.; Yin, Y.; Xue, J.; Li, B.; Gong, Z.; Gao, J.; Mu, Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem. Cell. Res. Ther. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Päth, G.; Perakakis, N.; Mantzoros, C.S.; Seufert, J. Stem cells in the treatment of diabetes mellitus—Focus on mesenchymal stem cells. Metabolism 2019, 90, 1–15. [Google Scholar] [CrossRef]
- Molcanyi, M.; Bosche, B.; Kraitsy, K.; Patz, S.; Zivcak, J.; Riess, P.; El Majdoub, F.; Hescheler, J.; Goldbrunner, R.; Schäfer, U. Pitfalls and fallacies interfering with correct identification of embryonic stem cells implanted into the brain after experimental traumatic injury. J. Neurosci. Methods 2013, 215, 60–70. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.; Li, M. The promise of CRISPR/Cas9 technology in diabetes mellitus therapy: How gene editing is revolutionizing diabetes research and treatment. J. Diabetes Complicat. 2023, 37, 108524. [Google Scholar] [CrossRef]
- Bora, J.; Dey, A.; Lyngdoh, A.R.; Dhasmana, A.; Ranjan, A.; Kishore, S.; Rustagi, S.; Tuli, H.S.; Chauhan, A.; Rath, P.; et al. A critical review on therapeutic approaches of CRISPR-Cas9 in diabetes mellitus. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 1–23. [Google Scholar] [CrossRef]
- Chen, L.; Tian, F.Y.; Hu, X.H.; Wu, J.W.; Xu, W.D.; Huang, Q. Intermittent fasting in type 2 diabetes: From fundamental science to clinical applications. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Shula, S. A Machine Learning Approach to select the Type of Intermittent Fasting in Order to Improve Health by Effects on Type 2 Diabetes. In Proceedings of the 11th International Conference on Bioinformatics Models, Methods and Algorithms, Valletta, Malta, 24–26 February 2020. [Google Scholar] [CrossRef]
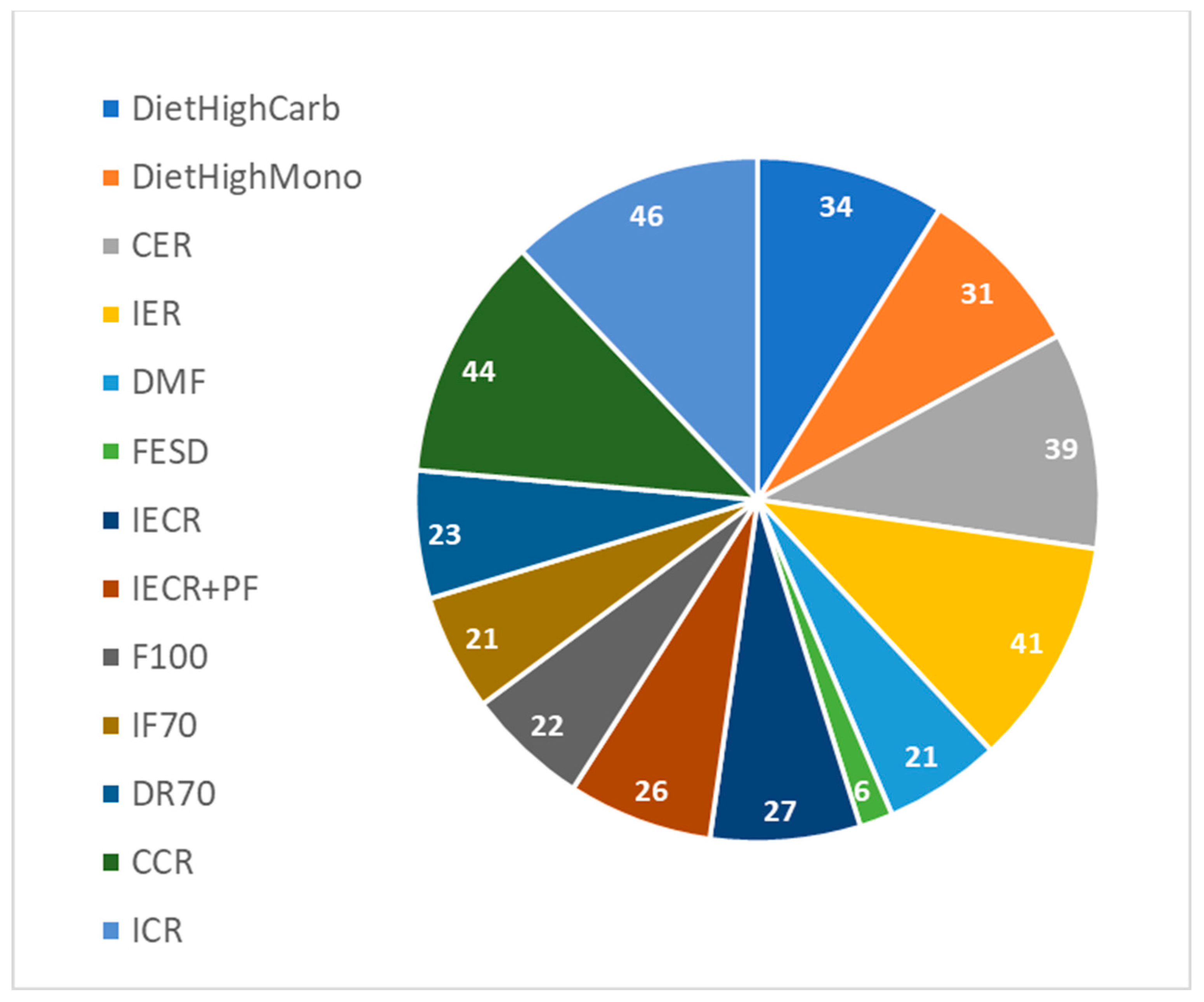


| Intervention Name | Details | Reference |
|---|---|---|
| CER | Continuous energy restriction—7-days-a-week trial; eating restricted calories every day. | Harvie et al., 2011 [40] |
| IER | Intermittent energy restriction, 2-day-a-week trial; eating restricted calories only two days a week. | Harvie et al., 2011 [40] |
| DMF | Daily morning fasting; start eating at noon and finish at 20:00. | Chowdhury et al., 2016 [41] |
| FESD | Fasting every second day; eating only four days a week. | Halberg et al., 2005 [42] |
| IECR | Intermittent energy and carbohydrate restriction; eating restricted calories only two days a week. | Harvie et al., 2013 [43] |
| IECR + PF | Intermittent energy and carbohydrate restriction + free protein and fat; eating restricted calories only two days a week. | Harvie et al., 2013 [43] |
| High Carb | High carbohydrate weight loss diet; eating restricted calories every day. | Clifton et al., 2004 [44] |
| High Mono | High monounsaturated weight loss diet; eating restricted calories every day. | Clifton et al., 2004 [44] |
| IF100 | Fasting three non-consecutive days per week. | Hutchison et al., 2019 [45] |
| IF70 | Fasting three non-consecutive days per week and on eating days have 70% energy. | Hutchison et al., 2019 [45] |
| DR70 | Seven days a week with 70% energy. | Hutchison et al., 2019 [45] |
| CCR | Daily energy deficit ∼20%. | Ruth Schübel et al., 2018 [46] |
| ICR | Fasting two non-consecutive days per week and on eating days have 75% energy. | Ruth Schübel et al., 2018 [46] |
| Fasting Glucose | HOMA-IR | ||||
|---|---|---|---|---|---|
| With Control | No Control | With Control | No Control | ||
| Discrete difference | J48 | 0.66 65% | 0.67 67% | 0.68 70% | 0.65 68% |
| LMT | 0.72 67% | 0.73 66% | 0.60 72% | 0.70 73% | |
| Random forest | 0.71 68% | 0.70 65% | 0.68 70% | 0.71 71% | |
| Logistic | 0.72 68% | 0.73 66% | 0.70 71% | 0.70 74% | |
| Discrete difference. No interventions | J48 | 0.61 63% | 0.63 65% | 0.57 68% | 0.54 68% |
| LMT | 0.70 64% | 0.72 66% | 0.65 70% | 0.62 70% | |
| Random forest | 0.68 63% | 0.68 64% | 0.60 69% | 0.63 72% | |
| Logistic | 0.71 65% | 0.71 66% | 0.65 70% | 0.64 71% | |
| Discrete difference above 15%. | J48 | 0.79 93% | 0.82 96% | 0.74 74% | 0.73 74% |
| LMT | 0.90 94% | 0.91 96% | 0.83 75% | 0.89 82% | |
| Random forest | 0.90 95% | 0.93 96% | 0.82 76% | 0.87 79% | |
| Logistic | 0.82 95% | 0.82 96% | 0.83 76% | 0.88 82% | |
| Discrete difference above 15%. No interventions | J48 | 0.64 93% | 0.73 94% | 0.75 75% | 0.74 73% |
| LMT | 0.91 95% | 0.90 95% | 0.82 74% | 0.82 76% | |
| Random forest | 0.91 95% | 0.92 95% | 0.82 76% | 0.82 75% | |
| Logistic | 0.90 95% | 0.94 95% | 0.83 77% | 0.82 78% | |
| Continuous difference | Random forest | 0.51 | 0.51 | 0.36 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shazman, S. Understanding Type 2 Diabetes Mellitus Risk Parameters through Intermittent Fasting: A Machine Learning Approach. Nutrients 2023, 15, 3926. https://doi.org/10.3390/nu15183926
Shazman S. Understanding Type 2 Diabetes Mellitus Risk Parameters through Intermittent Fasting: A Machine Learning Approach. Nutrients. 2023; 15(18):3926. https://doi.org/10.3390/nu15183926
Chicago/Turabian StyleShazman, Shula. 2023. "Understanding Type 2 Diabetes Mellitus Risk Parameters through Intermittent Fasting: A Machine Learning Approach" Nutrients 15, no. 18: 3926. https://doi.org/10.3390/nu15183926
APA StyleShazman, S. (2023). Understanding Type 2 Diabetes Mellitus Risk Parameters through Intermittent Fasting: A Machine Learning Approach. Nutrients, 15(18), 3926. https://doi.org/10.3390/nu15183926





