Low-Calorie Ketogenic Diet: Potential Application in the Treatment of Polycystic Ovary Syndrome in Adolescents
Abstract
1. Introduction
2. Methods
3. Polycystic Ovary Syndrome in Adolescents
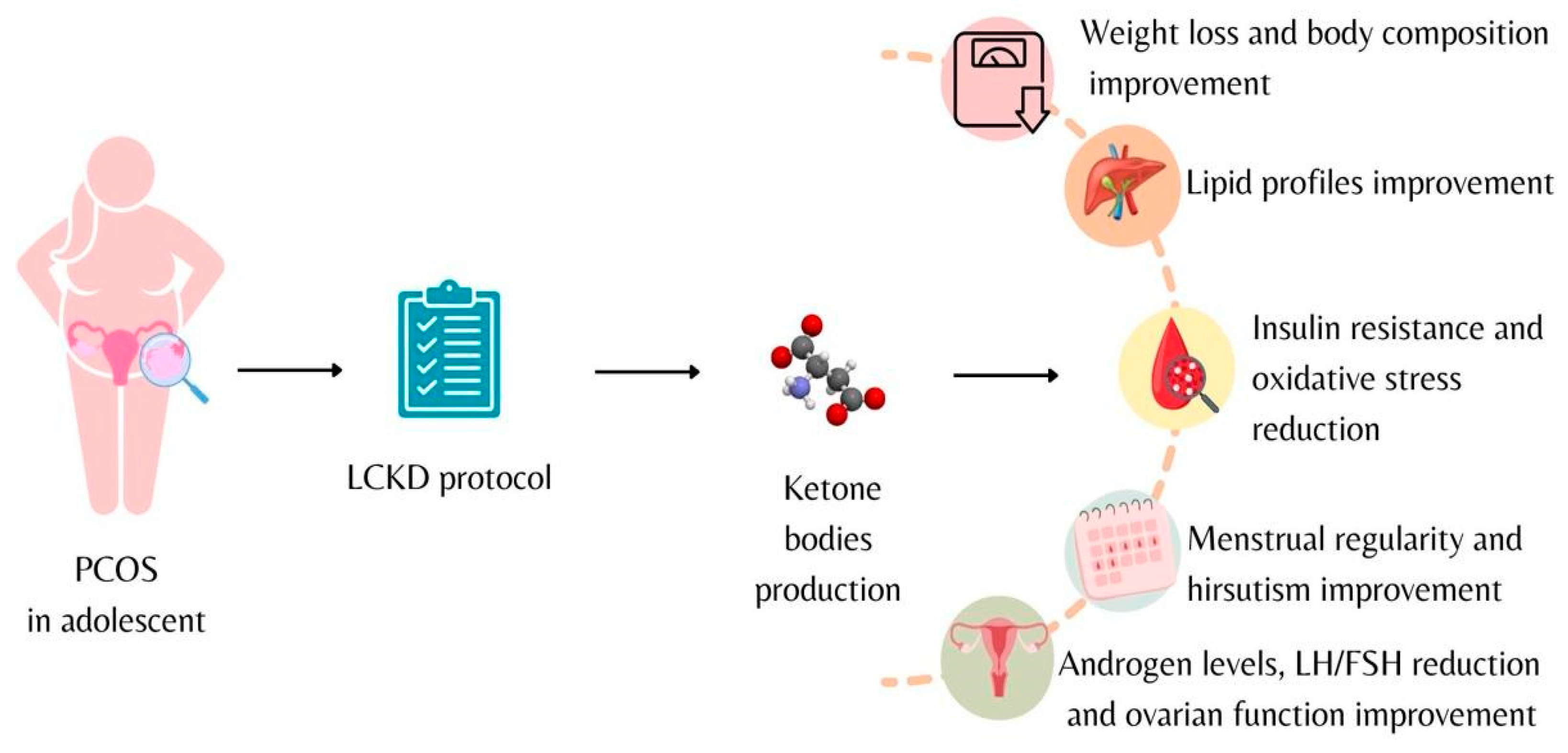
4. Ketogenic Diet
- –
- Physical examination (anthropometric measurements, blood pressure, heart rate, etc.);
- –
- Laboratory analysis (complete blood count, creatinine, uric acid, glucose, lipid profile, sodium, potassium, calcium, magnesium, inorganic phosphate, thyroid stimulating hormone (TSH), free- thyroxine (FT4), 25-Hydroxyvitamin D, complete urinalysis and microalbuminuria) [52].
5. Ketogenic Diet and Polycystic Ovary Syndrome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Cena, H.; Magenes, V.C.; Todisco, C.F.; Tenuta, E.; Gregorio, C.; De Giuseppe, R.; Bosetti, A.; Di Profio, E.; et al. Polycystic Ovary Syndrome in Insulin-Resistant Adolescents with Obesity: The Role of Nutrition Therapy and Food Supplements as a Strategy to Protect Fertility. Nutrients 2021, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- Gołąbek, K.D.; Regulska-Ilow, B. Dietary Support in Insulin Resistance: An Overview of Current Scientific Reports. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2019, 28, 1577–1585. [Google Scholar] [CrossRef]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic Dysfunction in Polycystic Ovary Syndrome: Pathogenic Role of Androgen Excess and Potential Therapeutic Strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public. Health 2022, 19, 10429. [Google Scholar] [CrossRef]
- Cox, N.; Gibas, S.; Salisbury, M.; Gomer, J.; Gibas, K. Ketogenic Diets Potentially Reverse Type II Diabetes and Ameliorate Clinical Depression: A Case Study. Diabetes Metab. Syndr. 2019, 13, 1475–1479. [Google Scholar] [CrossRef]
- Goday, A.; Bellido, D.; Sajoux, I.; Crujeiras, A.B.; Burguera, B.; García-Luna, P.P.; Oleaga, A.; Moreno, B.; Casanueva, F.F. Short-Term Safety, Tolerability and Efficacy of a Very Low-Calorie-Ketogenic Diet Interventional Weight Loss Program versus Hypocaloric Diet in Patients with Type 2 Diabetes Mellitus. Nutr. Diabetes 2016, 6, e230. [Google Scholar] [CrossRef]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, S.; Siejka, A. The Pathogenesis and Treatment of Polycystic Ovary Syndrome: What’s New? Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2017, 26, 359–367. [Google Scholar] [CrossRef]
- Tay, C.T.; Hart, R.J.; Hickey, M.; Moran, L.J.; Earnest, A.; Doherty, D.A.; Teede, H.J.; Joham, A.E. Updated Adolescent Diagnostic Criteria for Polycystic Ovary Syndrome: Impact on Prevalence and Longitudinal Body Mass Index Trajectories from Birth to Adulthood. BMC Med. 2020, 18, 389. [Google Scholar] [CrossRef]
- Hickey, M.; Doherty, D.A.; Atkinson, H.; Sloboda, D.M.; Franks, S.; Norman, R.J.; Hart, R. Clinical, Ultrasound and Biochemical Features of Polycystic Ovary Syndrome in Adolescents: Implications for Diagnosis. Hum. Reprod. Oxf. Engl. 2011, 26, 1469–1477. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [PubMed]
- Kiconco, S.; Earnest, A.; Enticott, J.; Hart, R.; Mori, T.A.; Hickey, M.; Teede, H.J.; Joham, A.E. Normative Cut-Offs for Polycystic Ovary Syndrome Diagnostic Features in Adolescents Using Cluster Analysis. Eur. J. Endocrinol. 2023, 188, 494–502. [Google Scholar] [CrossRef]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of Polycystic Ovary Syndrome in a Dutch Twin-Family Study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Gragnoli, C. Genome-Wide Linkage and Association Study Identifies Novel Genes and Pathways Implicated in Polycystic Ovarian Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3719–3732. [Google Scholar] [CrossRef] [PubMed]
- Govind, A.; Obhrai, M.S.; Clayton, R.N. Polycystic Ovaries Are Inherited as an Autosomal Dominant Trait: Analysis of 29 Polycystic Ovary Syndrome and 10 Control Families. J. Clin. Endocrinol. Metab. 1999, 84, 38–43. [Google Scholar] [CrossRef]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.-P.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal Androgen Exposure and Transgenerational Susceptibility to Polycystic Ovary Syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.-L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundström-Poromaa, I.; Piltonen, T.T.; et al. Elevated Prenatal Anti-Müllerian Hormone Reprograms the Fetus and Induces Polycystic Ovary Syndrome in Adulthood. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef]
- Sir-Petermann, T.; Codner, E.; Pérez, V.; Echiburú, B.; Maliqueo, M.; Ladrón de Guevara, A.; Preisler, J.; Crisosto, N.; Sánchez, F.; Cassorla, F.; et al. Metabolic and Reproductive Features before and during Puberty in Daughters of Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 1923–1930. [Google Scholar] [CrossRef]
- Crisosto, N.; Echiburú, B.; Maliqueo, M.; Luchsinger, M.; Rojas, P.; Recabarren, S.; Sir-Petermann, T. Reproductive and Metabolic Features during Puberty in Sons of Women with Polycystic Ovary Syndrome. Endocr. Connect. 2017, 6, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; de Zegher, F. Adolescent PCOS: A Postpubertal Central Obesity Syndrome. Trends Mol. Med. 2023, 29, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Genazzani, A.R. Polycystic Ovary Syndrome as Metabolic Disease: New Insights on Insulin Resistance. TouchREVIEWS Endocrinol. 2023, 19, 71–77. [Google Scholar] [CrossRef]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef]
- Glueck, C.J.; Goldenberg, N. Characteristics of Obesity in Polycystic Ovary Syndrome: Etiology, Treatment, and Genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xie, Y.-J.; Liu, Y.-T.; Long, S.-L.; Mo, Z.-C. Polycystic Ovarian Syndrome: Correlation between Hyperandrogenism, Insulin Resistance and Obesity. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 502, 214–221. [Google Scholar] [CrossRef]
- Corbould, A. Effects of Androgens on Insulin Action in Women: Is Androgen Excess a Component of Female Metabolic Syndrome? Diabetes Metab. Res. Rev. 2008, 24, 520–532. [Google Scholar] [CrossRef]
- Zhang, H.; Butoyi, C.; Yuan, G.; Jia, J. Exploring the Role of Gut Microbiota in Obesity and PCOS: Current Updates and Future Prospects. Diabetes Res. Clin. Pract. 2023, 202, 110781. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-Disrupting Chemicals: Implications for Human Health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Borruel, S.; Fernández-Durán, E.; Alpañés, M.; Martí, D.; Alvarez-Blasco, F.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Global Adiposity and Thickness of Intraperitoneal and Mesenteric Adipose Tissue Depots Are Increased in Women with Polycystic Ovary Syndrome (PCOS). J. Clin. Endocrinol. Metab. 2013, 98, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Allard, C.; Morford, J.J.; Xu, W.; Liu, S.; Molinas, A.J.; Butcher, S.M.; Fine, N.H.; Blandino-Rosano, M.; Sure, V.N.; et al. Androgen Excess in Pancreatic β Cells and Neurons Predisposes Female Mice to Type 2 Diabetes. JCI Insight 2018, 3, e98607. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.M.; Wiegel, R.E.; Jansen, P.W.; Laven, J.S.E.; Sinclair, K.D. Polycystic Ovary Syndrome: A Brain Disorder Characterized by Eating Problems Originating during Puberty and Adolescence. Int. J. Mol. Sci. 2020, 21, 8211. [Google Scholar] [CrossRef] [PubMed]
- Yurtdaş, G.; Akdevelioğlu, Y. A New Approach to Polycystic Ovary Syndrome: The Gut Microbiota. J. Am. Coll. Nutr. 2020, 39, 371–382. [Google Scholar] [CrossRef]
- La Marca, A.; Artensio, A.C.; Stabile, G.; Volpe, A. Metformin Treatment of PCOS during Adolescence and the Reproductive Period. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 121, 3–7. [Google Scholar] [CrossRef]
- Reiser, E.; Lanbach, J.; Böttcher, B.; Toth, B. Non-Hormonal Treatment Options for Regulation of Menstrual Cycle in Adolescents with PCOS. J. Clin. Med. 2022, 12, 67. [Google Scholar] [CrossRef]
- Al Khalifah, R.A.; Florez, I.D.; Zoratti, M.J.; Dennis, B.; Thabane, L.; Bassilious, E. Efficacy of Treatments for Polycystic Ovarian Syndrome Management in Adolescents. J. Endocr. Soc. 2021, 5, bvaa155. [Google Scholar] [CrossRef]
- Ladson, G.; Dodson, W.C.; Sweet, S.D.; Archibong, A.E.; Kunselman, A.R.; Demers, L.M.; Lee, P.A.; Williams, N.I.; Coney, P.; Legro, R.S. Effects of Metformin in Adolescents with Polycystic Ovary Syndrome Undertaking Lifestyle Therapy: A Pilot Randomized Double-Blind Study. Fertil. Steril. 2011, 95, 2595–2598.e6. [Google Scholar] [CrossRef]
- Ganie, M.A.; Khurana, M.L.; Eunice, M.; Gupta, N.; Gulati, M.; Dwivedi, S.N.; Ammini, A.C. Comparison of Efficacy of Spironolactone with Metformin in the Management of Polycystic Ovary Syndrome: An Open-Labeled Study. J. Clin. Endocrinol. Metab. 2004, 89, 2756–2762. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Huang, X.; Lin, K.; Liu, Y.; Li, Z.; Wei, T.; Song, L.; Hua, Y.; Wang, X. Oral Contraceptives (OCs) in Combination with Metformin versus OCs Alone on Metabolism in Nonobese Polycystic Ovary Syndrome: A Meta-Analysis and Systematic Review of Randomized Controlled Trials. Clin. Endocrinol. 2023, 99, 3–16. [Google Scholar] [CrossRef]
- Butt, M.S.; Saleem, J.; Zakar, R.; Aiman, S.; Khan, M.Z.; Fischer, F. Benefits of Physical Activity on Reproductive Health Functions among Polycystic Ovarian Syndrome Women: A Systematic Review. BMC Public Health 2023, 23, 882. [Google Scholar] [CrossRef]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, dgaa425. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22 (Suppl. S3), 1–203. [Google Scholar] [CrossRef]
- Moore, J.M.; Waldrop, S.W.; Cree-Green, M. Weight Management in Adolescents with Polycystic Ovary Syndrome. Curr. Obes. Rep. 2021, 10, 311–321. [Google Scholar] [CrossRef]
- DiVall, S.A. Practical Considerations for Diagnosis and Treatment of Polycystic Ovary Syndrome in Adolescence—Distilling Guidelines into Clinical Practice. Curr. Opin. Pediatr. 2023, 35, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Chen, Z.; Liu, M.; Mo, Z. Dietary Interventions: A Promising Treatment for Polycystic Ovary Syndrome. Ann. Nutr. Metab. 2021, 77, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Cacciapuoti, N.; Lonardo, M.S.; Nasti, G.; Gautiero, C.; Belfiore, A.; Guida, B.; Chiurazzi, M. Pathophysiology and Nutritional Approaches in Polycystic Ovary Syndrome (PCOS): A Comprehensive Review. Curr. Nutr. Rep. 2023. [Google Scholar] [CrossRef]
- Günalan, E.; Yaba, A.; Yılmaz, B. The Effect of Nutrient Supplementation in the Management of Polycystic Ovary Syndrome-Associated Metabolic Dysfunctions: A Critical Review. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 220–232. [Google Scholar] [CrossRef]
- Frigerio, F.; Poggiogalle, E.; Donini, L.M. Definizione di dieta chetogena: Creatività o confusione? L’Endocrinologo 2022, 23, 587–591. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-Low-Calorie Ketogenic Diet (VLCKD) in the Management of Metabolic Diseases: Systematic Review and Consensus Statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- McGaugh, E.; Barthel, B. A Review of Ketogenic Diet and Lifestyle. Mo. Med. 2022, 119, 84–88. [Google Scholar] [PubMed]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The Management of Very Low-Calorie Ketogenic Diet in Obesity Outpatient Clinic: A Practical Guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L.; Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO). European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12, e9639. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.-M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a Ketogenic Diet on Hepatic Steatosis and Hepatic Mitochondrial Metabolism in Nonalcoholic Fatty Liver Disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef]
- Kim, J.Y. Optimal Diet Strategies for Weight Loss and Weight Loss Maintenance. J. Obes. Metab. Syndr. 2021, 30, 20–31. [Google Scholar] [CrossRef]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. The Polycystic Ovary Syndrome: A Position Statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, P1–P29. [Google Scholar] [CrossRef]
- Marzouk, T.M.; Sayed Ahmed, W.A. Effect of Dietary Weight Loss on Menstrual Regularity in Obese Young Adult Women with Polycystic Ovary Syndrome. J. Pediatr. Adolesc. Gynecol. 2015, 28, 457–461. [Google Scholar] [CrossRef]
- Gower, B.A.; Chandler-Laney, P.C.; Ovalle, F.; Goree, L.L.; Azziz, R.; Desmond, R.A.; Granger, W.M.; Goss, A.M.; Bates, G.W. Favourable Metabolic Effects of a Eucaloric Lower-Carbohydrate Diet in Women with PCOS. Clin. Endocrinol. 2013, 79, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, H.H.; Salehpour, S.; Amiri, Z.; Farahani, S.J.; Meyer, B.J.; Tahbaz, F. Beneficial Effects of a High-Protein, Low-Glycemic-Load Hypocaloric Diet in Overweight and Obese Women with Polycystic Ovary Syndrome: A Randomized Controlled Intervention Study. J. Am. Coll. Nutr. 2012, 31, 117–125. [Google Scholar] [CrossRef]
- Porchia, L.M.; Hernandez-Garcia, S.C.; Gonzalez-Mejia, M.E.; López-Bayghen, E. Diets with Lower Carbohydrate Concentrations Improve Insulin Sensitivity in Women with Polycystic Ovary Syndrome: A Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Marzullo, P.; Muscogiuri, G.; Di Somma, C.; Scacchi, M.; Orio, F.; Aimaretti, G.; Colao, A.; Savastano, S. Source and Amount of Carbohydrate in the Diet and Inflammation in Women with Polycystic Ovary Syndrome. Nutr. Res. Rev. 2018, 31, 291–301. [Google Scholar] [CrossRef]
- Mei, S.; Ding, J.; Wang, K.; Ni, Z.; Yu, J. Mediterranean Diet Combined With a Low-Carbohydrate Dietary Pattern in the Treatment of Overweight Polycystic Ovary Syndrome Patients. Front. Nutr. 2022, 9, 876620. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond Weight Loss: A Review of the Therapeutic Uses of Very-Low-Carbohydrate (Ketogenic) Diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef]
- Mavropoulos, J.C.; Yancy, W.S.; Hepburn, J.; Westman, E.C. The Effects of a Low-Carbohydrate, Ketogenic Diet on the Polycystic Ovary Syndrome: A Pilot Study. Nutr. Metab. 2005, 2, 35. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a Ketogenic Diet in Overweight Women with Polycystic Ovary Syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef]
- Cincione, R.I.; Losavio, F.; Ciolli, F.; Valenzano, A.; Cibelli, G.; Messina, G.; Polito, R. Effects of Mixed of a Ketogenic Diet in Overweight and Obese Women with Polycystic Ovary Syndrome. Int. J. Environ. Res. Public. Health 2021, 18, 12490. [Google Scholar] [CrossRef]
- Magagnini, M.C.; Condorelli, R.A.; Cimino, L.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Does the Ketogenic Diet Improve the Quality of Ovarian Function in Obese Women? Nutrients 2022, 14, 4147. [Google Scholar] [CrossRef]
- Pandurevic, S.; Mancini, I.; Mitselman, D.; Magagnoli, M.; Teglia, R.; Fazzeri, R.; Dionese, P.; Cecchetti, C.; Caprio, M.; Moretti, C.; et al. Efficacy of Very Low-Calorie Ketogenic Diet with the Pronokal® Method in Obese Women with Polycystic Ovary Syndrome: A 16-Week Randomized Controlled Trial. Endocr. Connect. 2023, 12, e220536. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Verde, L.; Camajani, E.; Cernea, S.; Frias-Toral, E.; Lamabadusuriya, D.; Ceriani, F.; Savastano, S.; Colao, A.; Muscogiuri, G. Ketogenic Diet as Medical Prescription in Women with Polycystic Ovary Syndrome (PCOS). Curr. Nutr. Rep. 2023, 12, 56–64. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year of Publication | Title | Study Type | Population | Intervention | Results |
|---|---|---|---|---|---|
| Paoli et al., 2020 [61] | Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. | Single-arm study (interventional) | 24 overweight women with PCOS Age: 18–45 yo | Participants followed a ketogenic diet (KD) for 12 weeks | KD as a possible therapeutic aid in PCOS |
| Cincione et al., 2021 [62] | Effects of Mixed of a Ketogenic Diet in Overweight and Obese Women with Polycystic Ovary Syndrome. | Interventional study | 17 overweight and obese women with PCOS Age: 18–45 yo | Participants were treated for 45 days with a modified KD protocol, defined as “mixed ketogenic” | KD improves the anthropometric and many biochemical parameters (LH, FSH, SHBG, insulin sensitivity and HOMA index) and reduces androgenic production |
| Magagnini et al., 2022 [63] | Does the Ketogenic Diet Improve the Quality of Ovarian Function in Obese Women? | Retrospective study | 25 women with PCOS and first-degree obesity Age: ≥18 yo | Participants followed a VLCKD protocol for 12 weeks | Metabolic and ovulatory improvement is achieved in a relatively short time |
| Pandurevic et al., 2023 [64] | Efficacy of very-low-calorie ketogenic diet with the Pronokal® method in obese women with polycystic ovary syndrome: a 16-week randomized controlled trial | Randomized controlled trial | 32 childbearing age women with PCOS, BMI 28–40 kg/m2 Age: 18–45 yo | Experimental group (n = 15): VLCKD for 8 weeks then LCD for 8 weeks, according to the Pronokal® method; Control group (n = 15): Mediterranean LCD for 16 weeks | In obese PCOS patients, 16 weeks of VLCKD protocol with the Pronokal® method was more effective than Mediterranean LCD in reducing total and visceral fat, and in ameliorating hyperandrogenism and ovulatory dysfunction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Cena, H.; Sottotetti, F.; Hruby, C.; Madini, N.; Zelaschi, N.; Zuccotti, G. Low-Calorie Ketogenic Diet: Potential Application in the Treatment of Polycystic Ovary Syndrome in Adolescents. Nutrients 2023, 15, 3582. https://doi.org/10.3390/nu15163582
Calcaterra V, Cena H, Sottotetti F, Hruby C, Madini N, Zelaschi N, Zuccotti G. Low-Calorie Ketogenic Diet: Potential Application in the Treatment of Polycystic Ovary Syndrome in Adolescents. Nutrients. 2023; 15(16):3582. https://doi.org/10.3390/nu15163582
Chicago/Turabian StyleCalcaterra, Valeria, Hellas Cena, Francesca Sottotetti, Chiara Hruby, Nagaia Madini, Noemi Zelaschi, and Gianvincenzo Zuccotti. 2023. "Low-Calorie Ketogenic Diet: Potential Application in the Treatment of Polycystic Ovary Syndrome in Adolescents" Nutrients 15, no. 16: 3582. https://doi.org/10.3390/nu15163582
APA StyleCalcaterra, V., Cena, H., Sottotetti, F., Hruby, C., Madini, N., Zelaschi, N., & Zuccotti, G. (2023). Low-Calorie Ketogenic Diet: Potential Application in the Treatment of Polycystic Ovary Syndrome in Adolescents. Nutrients, 15(16), 3582. https://doi.org/10.3390/nu15163582







