The Effects of Omega 3 and Omega 6 Fatty Acids on Glucose Metabolism: An Updated Review
Abstract
1. Background
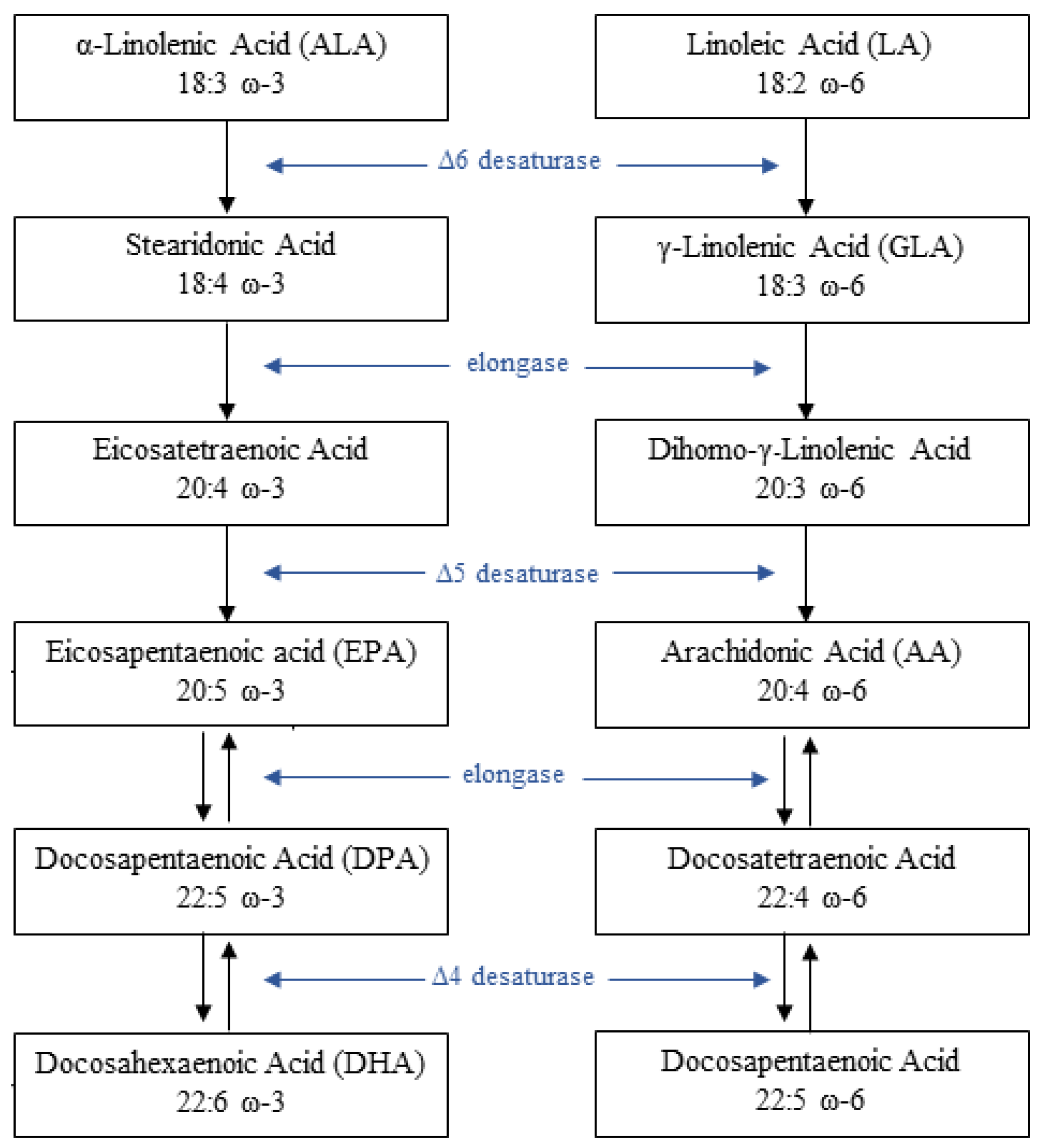
2. Pathophysiology of PUFAs and Glucose Metabolism Interaction
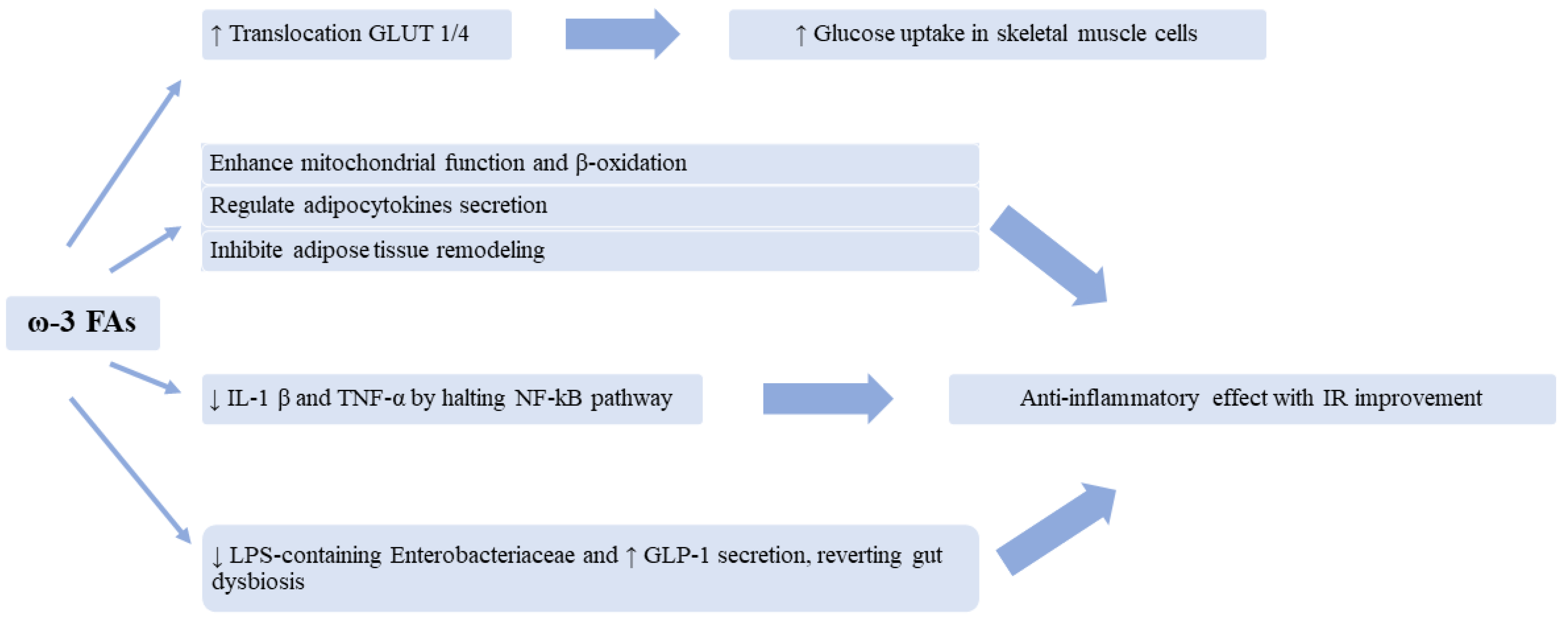
3. Evidence from the Literature
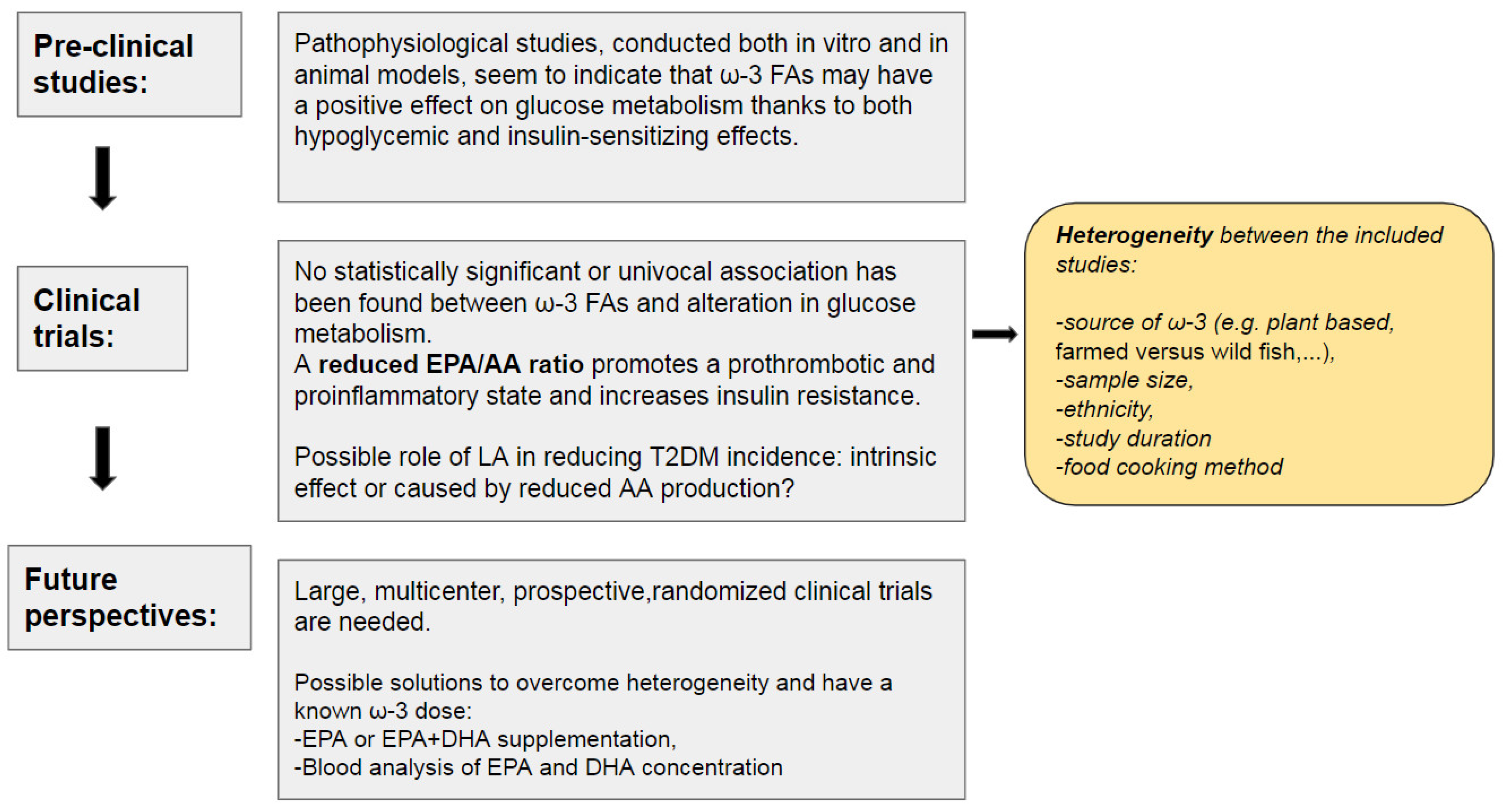
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mori, T.A. Marine OMEGA-3 Fatty Acids in the Prevention of Cardiovascular Disease. Fitoterapia 2017, 123, 51–58. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Yeom, W.W.; Kim, H.J.; Lee, K.-R.; Cho, H.S.; Kim, J.-Y.; Jung, H.W.; Oh, S.-W.; Jun, S.E.; Kim, H.U.; Chung, Y.-S. Increased Production of α-Linolenic Acid in Soybean Seeds by Overexpression of Lesquerella FAD3-1. Front. Plant. Sci. 2020, 10, 1812. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170148/nutrients (accessed on 25 May 2023).
- FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/174271/nutrients (accessed on 25 May 2023).
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of Plant Proteins for Improved Functionality: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 Fatty Acids in Health and Disease and in Growth and Development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef]
- De Gŏmez Dumm, I.N.; Brenner, R.R. Oxidative Desaturation of Alpha-Linoleic, Linoleic, and Stearic Acids by Human Liver Microsomes. Lipids 1975, 10, 315–317. [Google Scholar] [CrossRef]
- Adlof, R.O.; Duval, S.; Emken, E.A. Biosynthesis of Conjugated Linoleic Acid in Humans. Lipids 2000, 35, 131–135. [Google Scholar] [CrossRef]
- Van Dael, P. Role of N-3 Long-Chain Polyunsaturated Fatty Acids in Human Nutrition and Health: Review of Recent Studies and Recommendations. Nutr. Res. Pract. 2021, 15, 137–159. [Google Scholar] [CrossRef]
- Ian Givens, D.; Gibbs, R.A. Current Intakes of EPA and DHA in European Populations and the Potential of Animal-Derived Foods to Increase Them. Proc. Nutr. Soc. 2008, 67, 273–280. [Google Scholar] [CrossRef]
- Weintraub, H. Update on Marine Omega-3 Fatty Acids: Management of Dyslipidemia and Current Omega-3 Treatment Options. Atherosclerosis 2013, 230, 381–389. [Google Scholar] [CrossRef] [PubMed]
- FoodData Central. Available online: https://fdc.nal.usda.gov/index.html (accessed on 28 May 2023).
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary Linoleic Acid and Human Health: Focus on Cardiovascular and Cardiometabolic Effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X. The Importance of Omega-6/Omega-3 Fatty Acid Ratio in Cell Function. The Gene Transfer of Omega-3 Fatty Acid Desaturase. World Rev. Nutr. Diet. 2003, 92, 23–36. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary Aspects of Diet and Essential Fatty Acids. World Rev. Nutr. Diet. 2001, 88, 18–27. [Google Scholar] [CrossRef]
- Schulze, M.B.; Minihane, A.M.; Saleh, R.N.M.; Risérus, U. Intake and Metabolism of Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Nutritional Implications for Cardiometabolic Diseases. Lancet Diabetes Endocrinol. 2020, 8, 915–930. [Google Scholar] [CrossRef]
- Miyata, J.; Arita, M. Role of Omega-3 Fatty Acids and Their Metabolites in Asthma and Allergic Diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef]
- Raper, N.R.; Cronin, F.J.; Exler, J. Omega-3 Fatty Acid Content of the US Food Supply. J. Am. Coll. Nutr. 1992, 11, 304–308. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Salem, N. Egg Yolk as a Source of Long-Chain Polyunsaturated Fatty Acids in Infant Feeding. Am. J. Clin. Nutr. 1992, 55, 411–414. [Google Scholar] [CrossRef]
- van Vliet, T.; Katan, M.B. Lower Ratio of N-3 to n-6 Fatty Acids in Cultured than in Wild Fish. Am. J. Clin. Nutr. 1990, 51, 1–2. [Google Scholar] [CrossRef]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of Sustainable Feeds on Omega-3 Long-Chain Fatty Acid Levels in Farmed Atlantic Salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef]
- Farmed Salmon, vs. Wild Salmon. Available online: https://doh.wa.gov/community-and-environment/food/fish/farmed-salmon (accessed on 25 May 2023).
- Tornero, V.; Hanke, G. Chemical Contaminants Entering the Marine Environment from Sea-Based Sources: A Review with a Focus on European Seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Möllmann, C.; Müller-Karulis, B.; Kornilovs, G.; St John, M.A. Effects of Climate and Overfishing on Zooplankton Dynamics and Ecosystem Structure: Regime Shifts, Trophic Cascade, and Feedback Loops in a Simple Ecosystem. ICES J. Mar. Sci. 2008, 65, 302–310. [Google Scholar] [CrossRef]
- Cavan, E.L.; Hill, S.L. Commercial Fishery Disturbance of the Global Ocean Biological Carbon Sink. Glob. Chang. Biol. 2022, 28, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. Omega-3 Fatty Acids for Nutrition and Medicine: Considering Microalgae Oil as a Vegetarian Source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef]
- Haimeur, A.; Mimouni, V.; Ulmann, L.; Martineau, A.-S.; Messaouri, H.; Pineau-Vincent, F.; Tremblin, G.; Meskini, N. Fish Oil and Microalga Omega-3 as Dietary Supplements: A Comparative Study on Cardiovascular Risk Factors in High-Fat Fed Rats. Lipids 2016, 51, 1037–1049. [Google Scholar] [CrossRef]
- Kazir, M.; Livney, Y.D. Plant-Based Seafood Analogs. Molecules 2021, 26, 1559. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Salem, N. Purslane: A Terrestrial Source of Omega-3 Fatty Acids. N. Engl. J. Med. 1986, 315, 833. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary Aspects of Omega-3 Fatty Acids in the Food Supply. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 421–429. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic Variation and Evolutionary Aspects of Diet. In Antioxidant Status, Diet, Nutrition, and Health; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-0-367-81109-9. [Google Scholar]
- Eaton, S.B.; Eaton, S.B.; Sinclair, A.J.; Cordain, L.; Mann, N.J. Dietary Intake of Long-Chain Polyunsaturated Fatty Acids during the Paleolithic. World Rev. Nutr. Diet. 1998, 83, 12–23. [Google Scholar] [CrossRef]
- Crawford, M.A. Fatty-Acid Ratios in Free-Living and Domestic Animals. Possible Implications for Atheroma. Lancet 1968, 1, 1329–1333. [Google Scholar] [CrossRef]
- Crawford, M.A.; Gale, M.M.; Woodford, M.H. Linoleic Acid and Linolenic Acid Elongation Products in Muscle Tissue of Sncerus Caffer and Other Ruminant Species. Biochem. J. 1969, 115, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The Omega-6:Omega-3 Ratio: A Critical Appraisal and Possible Successor. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Dyerberg, J.; Bang, H.O. Hæmostatic function and platelet polyunsaturated fatty acids in eskimos. Lancet 1979, 314, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Kromann, N.; Green, A. Epidemiological Studies in the Upernavik District, Greenland. Incidence of Some Chronic Diseases 1950–1974. Acta Med. Scand. 1980, 208, 401–406. [Google Scholar] [CrossRef]
- Adler, A.I.; Boyko, E.J.; Schraer, C.D.; Murphy, N.J. Lower Prevalence of Impaired Glucose Tolerance and Diabetes Associated with Daily Seal Oil or Salmon Consumption among Alaska Natives. Diabetes Care 1994, 17, 1498–1501. [Google Scholar] [CrossRef]
- Schraer, C.D.; Risica, P.M.; Ebbesson, S.O.; Go, O.T.; Howard, B.V.; Mayer, A.M. Low Fasting Insulin Levels in Eskimos Compared to American Indians: Are Eskimos Less Insulin Resistant? Int. J. Circumpolar Health 1999, 58, 272–280. [Google Scholar]
- Vannice, G.; Rasmussen, H. Position of the Academy of Nutrition and Dietetics: Dietary Fatty Acids for Healthy Adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef]
- 2015–2020 Dietary Guidelines for Americans. Available online: https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 4 May 2023).
- Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation, 10–14 November 2008, Geneva; eng 10-14 N. 2008; FAO: Rome, Italy, 2010. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Capra, S. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; Commonwealth of Australia: Canberra, Australia, 2006. [Google Scholar]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 Fatty Acids for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2018, 7, CD003177. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine N-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of N-3 Polyunsaturated Fatty Acids in Patients with Chronic Heart Failure (the GISSI-HF Trial): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef]
- ASCEND Study Collaborative Group; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of N-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of Eicosapentaenoic Acid on Major Coronary Events in Hypercholesterolaemic Patients (JELIS): A Randomised Open-Label, Blinded Endpoint Analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Budoff, M.J.; Bhatt, D.L.; Kinninger, A.; Lakshmanan, S.; Muhlestein, J.B.; Le, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Roy, S.K.; et al. Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides on Statin Therapy: Final Results of the EVAPORATE Trial. Eur. Heart J. 2020, 41, 3925–3932. [Google Scholar] [CrossRef]
- Nishizaki, Y.; Miyauchi, K.; Iwata, H.; Inoue, T.; Hirayama, A.; Kimura, K.; Ozaki, Y.; Murohara, T.; Ueshima, K.; Kuwabara, Y.; et al. Study Protocol and Baseline Characteristics of Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy-Statin and Eicosapentaenoic Acid: RESPECT-EPA, the Combination of a Randomized Control Trial and an Observational Biomarker Study. Am. Heart J. 2023, 257, 1–8. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs. Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Bittner, D.O.; Goeller, M.; Dey, D.; Zopf, Y.; Achenbach, S.; Marwan, M. High Levels of Eicosapentaenoic Acid Are Associated with Lower Pericoronary Adipose Tissue Attenuation as Measured by Coronary CTA. Atherosclerosis 2021, 316, 73–78. [Google Scholar] [CrossRef]
- Tzolos, E.; Williams, M.C.; McElhinney, P.; Lin, A.; Grodecki, K.; Flores Tomasino, G.; Cadet, S.; Kwiecinski, J.; Doris, M.; Adamson, P.D.; et al. Pericoronary Adipose Tissue Attenuation, Low-Attenuation Plaque Burden, and 5-Year Risk of Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.R.; Raskin, S. The Eicosapentaenoic Acid:Arachidonic Acid Ratio and Its Clinical Utility in Cardiovascular Disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Mason, R.P. Eicosapentaenoic Acid Inhibits Oxidation of High Density Lipoprotein Particles in a Manner Distinct from Docosahexaenoic Acid. Biochem. Biophys. Res. Commun. 2018, 496, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Dawoud, H.; Bhatt, D.L.; Malinski, T.; Mason, R.P. Omega-3 and Omega-6 Fatty Acids Have Distinct Effects on Endothelial Fatty Acid Content and Nitric Oxide Bioavailability. Prostaglandins Leukot. Essent. Fatty Acids 2021, 173, 102337. [Google Scholar] [CrossRef]
- Burns, T.; Maciejewski, S.R.; Hamilton, W.R.; Zheng, M.; Mooss, A.N.; Hilleman, D.E. Effect of Omega-3 Fatty Acid Supplementation on the Arachidonic Acid:Eicosapentaenoic Acid Ratio. Pharmacotherapy 2007, 27, 633–638. [Google Scholar] [CrossRef]
- Chen, I.S.; Hotta, S.S.; Ikeda, I.; Cassidy, M.M.; Sheppard, A.J.; Vahouny, G.V. Digestion, Absorption and Effects on Cholesterol Absorption of Menhaden Oil, Fish Oil Concentrate and Corn Oil by Rats. J. Nutr. 1987, 117, 1676–1680. [Google Scholar] [CrossRef]
- Flaten, H.; Høstmark, A.T.; Kierulf, P.; Lystad, E.; Trygg, K.; Bjerkedal, T.; Osland, A. Fish-Oil Concentrate: Effects on Variables Related to Cardiovascular Disease. Am. J. Clin. Nutr. 1990, 52, 300–306. [Google Scholar] [CrossRef]
- Conquer, J.A.; Cheryk, L.A.; Chan, E.; Gentry, P.A.; Holub, B.J. Effect of Supplementation with Dietary Seal Oil on Selected Cardiovascular Risk Factors and Hemostatic Variables in Healthy Male Subjects. Thromb. Res. 1999, 96, 239–250. [Google Scholar] [CrossRef]
- Petrović-Oggiano, G.; Debeljak-Martačić, J.; Ranković, S.; Pokimica, B.; Mirić, A.; Glibetić, M.; Popović, T. The Effect of Walnut Consumption on N-3 Fatty Acid Profile of Healthy People Living in a Non-Mediterranean West Balkan Country, a Small Scale Randomized Study. Nutrients 2020, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Matsuo, R.; Matsumoto, N. A Longitudinal Study of the Association of the Eicosapentaenoic Acid/Arachidonic Acid Ratio Derived from Fish Consumption with the Serum Lipid Levels: A Pilot Study. Heart Vessel. 2019, 34, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Friesen, R.W.; Innis, S.M. Dietary Arachidonic Acid to EPA and DHA Balance Is Increased among Canadian Pregnant Women with Low Fish Intake. J. Nutr. 2009, 139, 2344–2350. [Google Scholar] [CrossRef]
- Ito, R.; Satoh-Asahara, N.; Yamakage, H.; Sasaki, Y.; Odori, S.; Kono, S.; Wada, H.; Suganami, T.; Ogawa, Y.; Hasegawa, K.; et al. An Increase in the EPA/AA Ratio Is Associated with Improved Arterial Stiffness in Obese Patients with Dyslipidemia. J. Atheroscler. Thromb. 2014, 21, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Nagao, K.; Kawauchi, K.; Yagi, T.; Atsumi, W.; Matsuo, R.; Hirayama, A. The Ratio of Eicosapentaenoic Acid (EPA) to Arachidonic Acid May Be a Residual Risk Marker in Stable Coronary Artery Disease Patients Receiving Treatment with Statin Following EPA Therapy. Am. J. Cardiovasc. Drugs 2017, 17, 409–420. [Google Scholar] [CrossRef]
- Inagaki, Y.; Arashi, H.; Yamaguchi, J.; Ogawa, H.; Hagiwara, N. Greater Change in the Eicosapentaenoic Acid to Arachidonic Acid Ratio Is Associated with Decreased Incidence of Cardiovascular Events in Acute Coronary Syndrome Patients with Elevated Triglyceride Levels. Circ. J. 2021, 85, 1746–1753. [Google Scholar] [CrossRef]
- Ishitobi, T.; Hyogo, H.; Kan, H.; Hiramatsu, A.; Arihiro, K.; Aikata, H.; Chayama, K. Eicosapentaenoic Acid/Arachidonic Acid Ratio as a Possible Link between Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease. Hepatol. Res. 2015, 45, 533–539. [Google Scholar] [CrossRef]
- Young, T.K.; Schraer, C.D.; Shubnikoff, E.V.; Szathmary, E.J.; Nikitin, Y.P. Prevalence of Diagnosed Diabetes in Circumpolar Indigenous Populations. Int. J. Epidemiol. 1992, 21, 730–736. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Z.-H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; Lyu, J. Trends in the Incidence of Diabetes Mellitus: Results from the Global Burden of Disease Study 2017 and Implications for Diabetes Mellitus Prevention. BMC Public Health 2020, 20, 1415. [Google Scholar] [CrossRef]
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of Diet on Type 2 Diabetes Mellitus: A Review. Int. J. Health Sci. Qassim 2017, 11, 65–71. [Google Scholar]
- Khatib, O. Noncommunicable Diseases: Risk Factors and Regional Strategies for Prevention and Care. East. Mediterr. Health J. 2004, 10, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kang, M.S.; Nam, M.; Kim, S.A.; Hwang, G.-S.; Kim, H.S. Eicosapentaenoic Acid (EPA) Modulates Glucose Metabolism by Targeting AMP-Activated Protein Kinase (AMPK) Pathway. Int. J. Mol. Sci. 2019, 20, 4751. [Google Scholar] [CrossRef]
- Jeromson, S.; Mackenzie, I.; Doherty, M.K.; Whitfield, P.D.; Bell, G.; Dick, J.; Shaw, A.; Rao, F.V.; Ashcroft, S.P.; Philp, A.; et al. Lipid Remodeling and an Altered Membrane-Associated Proteome May Drive the Differential Effects of EPA and DHA Treatment on Skeletal Muscle Glucose Uptake and Protein Accretion. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E605–E619. [Google Scholar] [CrossRef] [PubMed]
- Aas, V.; Rokling-Andersen, M.H.; Kase, E.T.; Thoresen, G.H.; Rustan, A.C. Eicosapentaenoic Acid (20:5 n-3) Increases Fatty Acid and Glucose Uptake in Cultured Human Skeletal Muscle Cells. J. Lipid Res. 2006, 47, 366–374. [Google Scholar] [CrossRef]
- Martins, A.R.; Crisma, A.R.; Masi, L.N.; Amaral, C.L.; Marzuca-Nassr, G.N.; Bomfim, L.H.M.; Teodoro, B.G.; Queiroz, A.L.; Serdan, T.D.A.; Torres, R.P.; et al. Attenuation of Obesity and Insulin Resistance by Fish Oil Supplementation Is Associated with Improved Skeletal Muscle Mitochondrial Function in Mice Fed a High-Fat Diet. J. Nutr. Biochem. 2018, 55, 76–88. [Google Scholar] [CrossRef]
- Pinel, A.; Pitois, E.; Rigaudiere, J.-P.; Jouve, C.; De Saint-Vincent, S.; Laillet, B.; Montaurier, C.; Huertas, A.; Morio, B.; Capel, F. EPA Prevents Fat Mass Expansion and Metabolic Disturbances in Mice Fed with a Western Diet. J. Lipid Res. 2016, 57, 1382–1397. [Google Scholar] [CrossRef]
- Bhaswant, M.; Poudyal, H.; Brown, L. Mechanisms of Enhanced Insulin Secretion and Sensitivity with N-3 Unsaturated Fatty Acids. J. Nutr. Biochem. 2015, 26, 571–584. [Google Scholar] [CrossRef]
- Huber, J.; Löffler, M.; Bilban, M.; Reimers, M.; Kadl, A.; Todoric, J.; Zeyda, M.; Geyeregger, R.; Schreiner, M.; Weichhart, T.; et al. Prevention of High-Fat Diet-Induced Adipose Tissue Remodeling in Obese Diabetic Mice by n-3 Polyunsaturated Fatty Acids. Int. J. Obes. 2007, 31, 1004–1013. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.J.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic Acid Prevents and Reverses Insulin Resistance in High-Fat Diet-Induced Obese Mice via Modulation of Adipose Tissue Inflammation. J. Nutr. 2010, 140, 1915–1922. [Google Scholar] [CrossRef]
- White, P.J.; Arita, M.; Taguchi, R.; Kang, J.X.; Marette, A. Transgenic Restoration of Long-Chain n-3 Fatty Acids in Insulin Target Tissues Improves Resolution Capacity and Alleviates Obesity-Linked Inflammation and Insulin Resistance in High-Fat-Fed Mice. Diabetes 2010, 59, 3066–3073. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of Plasma Polyunsaturated Fatty Acids to Circulating Inflammatory Markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Higgs, W.; Rotondo, D. Eicosapentaenoic Acid Suppression of Systemic Inflammatory Responses and Inverse Up-Regulation of 15-DeoxyΔ(12,14) Prostaglandin J2 Production. Br. J. Pharmacol. 2013, 169, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Buckley, J.D.; Howe, P.R.C. Anti-Obesity Effects of Long-Chain Omega-3 Polyunsaturated Fatty Acids. Obes. Rev. 2009, 10, 648–659. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 Polyunsaturated Fatty Acids, Inflammation, and Inflammatory Diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Tsukumo, D.M.L.; Carvalho-Filho, M.A.; Carvalheira, J.B.C.; Prada, P.O.; Hirabara, S.M.; Schenka, A.A.; Araújo, E.P.; Vassallo, J.; Curi, R.; Velloso, L.A.; et al. Loss-of-Function Mutation in Toll-like Receptor 4 Prevents Diet-Induced Obesity and Insulin Resistance. Diabetes 2007, 56, 1986–1998. [Google Scholar] [CrossRef]
- Zhuang, P.; Li, H.; Jia, W.; Shou, Q.; Zhu, Y.; Mao, L.; Wang, W.; Wu, F.; Chen, X.; Wan, X.; et al. Eicosapentaenoic and Docosahexaenoic Acids Attenuate Hyperglycemia through the Microbiome-Gut-Organs Axis in Db/Db Mice. Microbiome 2021, 9, 185. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Marklund, M.; Imamura, F.; Tintle, N.; Ardisson Korat, A.V.; de Goede, J.; Zhou, X.; Yang, W.-S.; de Oliveira Otto, M.C.; Kröger, J.; et al. Omega-6 Fatty Acid Biomarkers and Incident Type 2 Diabetes: Pooled Analysis of Individual-Level Data for 39–740 Adults from 20 Prospective Cohort Studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Jalilpiran, Y.; Karimi, E.; Aune, D.; Larijani, B.; Mozaffarian, D.; Willett, W.C.; Esmaillzadeh, A. Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Diabetes Care 2021, 44, 2173–2181. [Google Scholar] [CrossRef]
- Belury, M.A. Linoleic Acid, an Omega-6 Fatty Acid That Reduces Risk for Cardiometabolic Diseases: Premise, Promise and Practical Implications. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B. Dietary Linoleic Acid: Will Modifying Dietary Fat Quality Reduce the Risk of Type 2 Diabetes? Diabetes Care 2021, 44, 1913–1915. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Fang, Z.; Zhang, T.; Chen, Y. Polyunsaturated Fatty Acid Intake and Incidence of Type 2 Diabetes in Adults: A Dose Response Meta-Analysis of Cohort Studies. Diabetol. Metab. Syndr. 2022, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Di Giuseppe, D.; Orsini, N.; Patel, P.S.; Forouhi, N.G.; Wolk, A. Fish Consumption, Dietary Long-Chain n-3 Fatty Acids, and Risk of Type 2 Diabetes: Systematic Review and Meta-Analysis of Prospective Studies. Diabetes Care 2012, 35, 918–929. [Google Scholar] [CrossRef]
- Pastorino, S.; Bishop, T.; Sharp, S.J.; Pearce, M.; Akbaraly, T.; Barbieri, N.B.; Bes-Rastrollo, M.; Beulens, J.W.J.; Chen, Z.; Du, H.; et al. Heterogeneity of Associations between Total and Types of Fish Intake and the Incidence of Type 2 Diabetes: Federated Meta-Analysis of 28 Prospective Studies Including 956,122 Participants. Nutrients 2021, 13, 1223. [Google Scholar] [CrossRef]
- Rylander, C.; Sandanger, T.M.; Engeset, D.; Lund, E. Consumption of Lean Fish Reduces the Risk of Type 2 Diabetes Mellitus: A Prospective Population Based Cohort Study of Norwegian Women. PLoS ONE 2014, 9, e89845. [Google Scholar] [CrossRef]
- Villegas, R.; Xiang, Y.-B.; Elasy, T.; Li, H.-L.; Yang, G.; Cai, H.; Ye, F.; Gao, Y.-T.; Shyr, Y.; Zheng, W.; et al. Fish, Shellfish, and Long-Chain n-3 Fatty Acid Consumption and Risk of Incident Type 2 Diabetes in Middle-Aged Chinese Men and Women. Am. J. Clin. Nutr. 2011, 94, 543–551. [Google Scholar] [CrossRef]
- Patel, P.S.; Forouhi, N.G.; Kuijsten, A.; Schulze, M.B.; van Woudenbergh, G.J.; Ardanaz, E.; Amiano, P.; Arriola, L.; Balkau, B.; Barricarte, A.; et al. The Prospective Association between Total and Type of Fish Intake and Type 2 Diabetes in 8 European Countries: EPIC-InterAct Study. Am. J. Clin. Nutr. 2012, 95, 1445–1453. [Google Scholar] [CrossRef]
- Djoussé, L.; Gaziano, J.M.; Buring, J.E.; Lee, I.-M. Dietary Omega-3 Fatty Acids and Fish Consumption and Risk of Type 2 Diabetes. Am. J. Clin. Nutr. 2011, 93, 143–150. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, C.; Jia, C. Association of Fish and N-3 Fatty Acid Intake with the Risk of Type 2 Diabetes: A Meta-Analysis of Prospective Studies. Br. J. Nutr. 2012, 108, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.S.; Galano, J.-M.; Durand, T.; Lee, J.C.-Y. Profiling of Omega-Polyunsaturated Fatty Acids and Their Oxidized Products in Salmon after Different Cooking Methods. Antioxidants 2018, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Flaskerud, K.; Bukowski, M.; Golovko, M.; Johnson, L.; Brose, S.; Ali, A.; Cleveland, B.; Picklo, M.; Raatz, S. Effects of Cooking Techniques on Fatty Acid and Oxylipin Content of Farmed Rainbow Trout (Oncorhynchus Mykiss). Food Sci. Nutr. 2017, 5, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Demirchieva, S.M.; Kalachova, G.S. Effect of Way of Cooking on Content of Essential Polyunsaturated Fatty Acids in Muscle Tissue of Humpback Salmon (Oncorhynchus Gorbuscha). Food Chem. 2006, 96, 446–451. [Google Scholar] [CrossRef]
- Al Rijjal, D.; Liu, Y.; Lai, M.; Song, Y.; Danaei, Z.; Wu, A.; Mohan, H.; Wei, L.; Schopfer, F.J.; Dai, F.F.; et al. Vascepa Protects against High-Fat Diet-Induced Glucose Intolerance, Insulin Resistance, and Impaired β-Cell Function. iScience 2021, 24, 102909. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Tsubata, H.; Hashimoto, N.; Takabe, M.; Miyata, T.; Aoki, K.; Yamashita, S.; Oishi, S.; Osue, T.; Yokoi, K.; et al. Effects of 6-Month Eicosapentaenoic Acid Treatment on Postprandial Hyperglycemia, Hyperlipidemia, Insulin Secretion Ability, and Concomitant Endothelial Dysfunction among Newly-Diagnosed Impaired Glucose Metabolism Patients with Coronary Artery Disease. An Open Label, Single Blinded, Prospective Randomized Controlled Trial. Cardiovasc. Diabetol. 2016, 15, 121. [Google Scholar] [CrossRef]
- Ricordi, C.; Garcia-Contreras, M.; Farnetti, S. Diet and Inflammation: Possible Effects on Immunity, Chronic Diseases, and Life Span. J. Am. Coll. Nutr. 2015, 34 (Suppl. S1), 10–13. [Google Scholar] [CrossRef]
- Soldavini, C.M.; Piuri, G.; Rossi, G.; Corsetto, P.A.; Benzoni, L.; Maggi, V.; Privitera, G.; Spadafranca, A.; Rizzo, A.M.; Ferrazzi, E. Maternal AA/EPA Ratio and Triglycerides as Potential Biomarkers of Patients at Major Risk for Pharmacological Therapy in Gestational Diabetes. Nutrients 2022, 14, 2502. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Alsughayyir, J.; Basudan, A.; Alfaifi, M.; Awan, Z.A.; Algethami, M.R.; Al-Sheikh, Y.A. Blood Indices of Omega-3 and Omega-6 Polyunsaturated Fatty Acids Are Altered in Hyperglycemia. Saudi J. Biol. Sci. 2023, 30, 103577. [Google Scholar] [CrossRef]
- Takahashi, M.; Ando, J.; Shimada, K.; Nishizaki, Y.; Tani, S.; Ogawa, T.; Yamamoto, M.; Nagao, K.; Hirayama, A.; Yoshimura, M.; et al. The Ratio of Serum N-3 to n-6 Polyunsaturated Fatty Acids Is Associated with Diabetes Mellitus in Patients with Prior Myocardial Infarction: A Multicenter Cross-Sectional Study. BMC Cardiovasc. Disord. 2017, 17, 41. [Google Scholar] [CrossRef]
- Poreba, M.; Rostoff, P.; Siniarski, A.; Mostowik, M.; Golebiowska-Wiatrak, R.; Nessler, J.; Undas, A.; Gajos, G. Relationship between Polyunsaturated Fatty Acid Composition in Serum Phospholipids, Systemic Low-Grade Inflammation, and Glycemic Control in Patients with Type 2 Diabetes and Atherosclerotic Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Savastio, S.; Pozzi, E.; Mancioppi, V.; Boggio Sola, V.; Carrera, D.; Antoniotti, V.; Corsetto, P.A.; Montorfano, G.; Rizzo, A.M.; Bagnati, M.; et al. Vitamin D Repletion and AA/EPA Intake in Children with Type 1 Diabetes: Influences on Metabolic Status. Nutrients 2022, 14, 4603. [Google Scholar] [CrossRef] [PubMed]

| ALA (18:3 ω-3) | LA (18:2 ω-6) | |
|---|---|---|
| Chia seeds | 17.8 | 5.9 |
| Flaxseeds | 19.4 | 5.3 |
| Hemp seeds | 8.7 | 27.5 |
| Walnuts | 9.1 | 38.1 |
| Soybean oil | 6.8 | 51 |
| Corn oil | 1.0 | 51.9 |
| Sunflower oil | 0.2 | 20.5 |
| Avocado oil | 0.9 | 12.5 |
| Extra virgin olive oil | 0.7 | 8.4 |
| Peanut oil | 0.3 | 19.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egalini, F.; Guardamagna, O.; Gaggero, G.; Varaldo, E.; Giannone, B.; Beccuti, G.; Benso, A.; Broglio, F. The Effects of Omega 3 and Omega 6 Fatty Acids on Glucose Metabolism: An Updated Review. Nutrients 2023, 15, 2672. https://doi.org/10.3390/nu15122672
Egalini F, Guardamagna O, Gaggero G, Varaldo E, Giannone B, Beccuti G, Benso A, Broglio F. The Effects of Omega 3 and Omega 6 Fatty Acids on Glucose Metabolism: An Updated Review. Nutrients. 2023; 15(12):2672. https://doi.org/10.3390/nu15122672
Chicago/Turabian StyleEgalini, Filippo, Ornella Guardamagna, Giulia Gaggero, Emanuele Varaldo, Beatrice Giannone, Guglielmo Beccuti, Andrea Benso, and Fabio Broglio. 2023. "The Effects of Omega 3 and Omega 6 Fatty Acids on Glucose Metabolism: An Updated Review" Nutrients 15, no. 12: 2672. https://doi.org/10.3390/nu15122672
APA StyleEgalini, F., Guardamagna, O., Gaggero, G., Varaldo, E., Giannone, B., Beccuti, G., Benso, A., & Broglio, F. (2023). The Effects of Omega 3 and Omega 6 Fatty Acids on Glucose Metabolism: An Updated Review. Nutrients, 15(12), 2672. https://doi.org/10.3390/nu15122672






