1. Introduction
Nowadays, animal-derived proteins account for about 45% of human total protein consumption in developed countries [
1]. The growing standards of living in developing nations and the increase in population are the primary factors driving even greater demand for animal-derived protein [
2,
3,
4]. However, the current production of animal-derived protein, even after intensification, cannot keep up with this high demand [
4] and is putting pressure on the planet’s resources. Therefore, there is a need to transition towards diets that include fewer animal-derived proteins and more sustainable alternatives. This transition will offer benefits for the environment, animal welfare, and human health [
3].
Regarding sustainability, reducing food loss and waste is of significant importance. Currently, around one-third of the global food production intended for human consumption goes uneaten [
5]. Food loss, as defined by the Food and Agriculture Organization (FAO) of the United Nations, refers to products meant for human consumption that are either not consumed by people or have experienced diminished quality in terms of their nutritional value, economic value, or food safety [
6]. A considerable amount of the food produced is lost before it even reaches the end users, occurring during agricultural production (21–33%) or during product manufacturing, storage, distribution, and processing (21–25%) [
5,
7].
In the brewing industry, the most commonly used grain for the production of beer-type beverages, often in combination with adjunct grains such as rice and corn, is barley [
8]. The annual global production of brewing waste in this industry is 39 million tons [
9]. With the rapid development of the brewing industry, these numbers are likely to increase even further. Brewer’s spent grain (BSG) constitutes approximately 85% of the total brewing waste [
10]. Until now, BSG has primarily been utilized as animal feed or, to a lesser extent, discarded directly, leading to resource waste and environmental pollution [
11]. Given that BSG is the most voluminous by-product of the brewing industry, the valorization and utilization of spent grain protein is of great interest in terms of sustainability [
8].
Upcycling side streams that are suitable for human consumption presents an excellent opportunity to meet the increasing demand for sustainable protein sources while simultaneously reducing food loss. Utilizing BSG would allow producers to repurpose protein-rich and nutrient-rich barley [
12]. In addition to technical feasibility, assessing the protein quality is important before implementation in potential upcycling processes. Consequently, there is a growing interest in evaluating the protein quality of emerging sustainable protein sources. The hydrolysis of dietary protein into smaller compounds (tripeptides, dipeptides, or single amino acids (AA)) by proteases and peptidases in the small intestinal lumen, followed by their absorption into the bloodstream, is crucial for their nutritional value. Numerous plant proteins have been previously tested, with some commonly used in products as substitutes for dairy and/or meat products. Notably, a significant number of these market-available products incorporate pea protein isolates [
13].
BSG is rich in cellulose and non-cellulosic polysaccharides, lignin, and proteins [
14], making it a valuable resource for human nutrition and health. On average, BSG consists of 20% protein in terms of dry matter [
8], with approximately 30% being essential amino acids (EAA) [
15]. The BSG protein fraction consists mainly of hordeins (30–50%) and a mix of albumins, globulins, and glutelin [
8]. In general, cereal products are low in the EAA lysine. Within this category of cereals, however, compared to grains like wheat and rice, BSG has a relatively high content of lysine [
9,
16]. Additionally, BSG is rich in bioactive compounds and micronutrients [
17,
18]. Due to its high nutritional value, BSG can be applied in human food products for fortification purposes [
9]. Upcycled barley/rice protein (BRP) is enzymatically extracted from the BSG and purified via downstream processes, including filtration steps. The resulting BRP isolate can be applied in a range of products to enhance their protein content (e.g., plant-based milk, coffees, or smoothies) [
19]. A significant degree of protein degradation occurs during the brewing process, resulting in a BSG protein isolate with a substantial high density of small peptides of all molecular weights [
19]. This potentially aids in the digestibility of upcycled barley protein obtained from the beer brewing industry. The objective of the current study was to evaluate the postprandial AA uptake kinetics of BRP in a healthy adult population, aiming to assess its protein quality for human consumption. Furthermore, the study aimed to compare these outcomes with those of pea protein isolate (PP), which is frequently used in the plant protein market, as well as with the widely consumed benchmark, whey protein isolate (WP). As protein intake can also affect insulin responses, this study also investigated postprandial plasma glucose and insulin responses.
4. Discussion
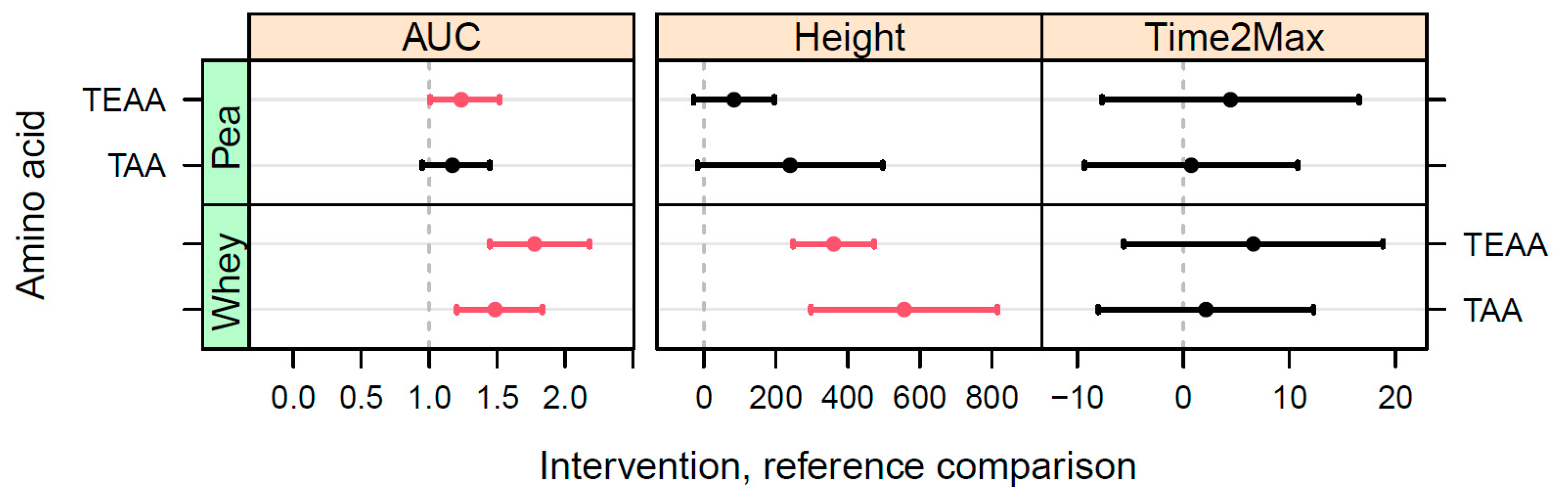
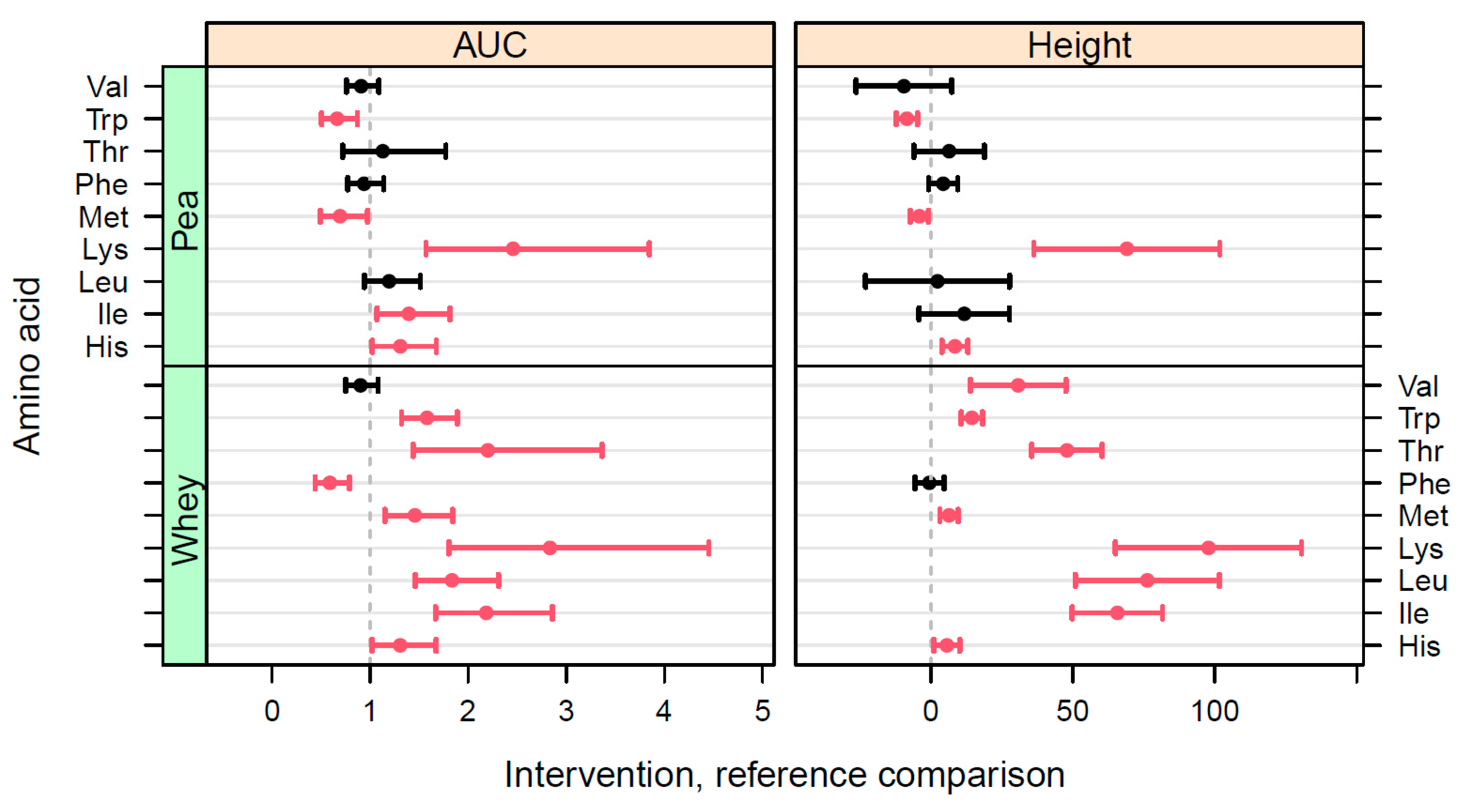
We evaluated, in a double-blind, cross-over intervention trial, the postprandial AA uptake of protein from BRP and compared this response to PP and the benchmark protein WP. Differences in postprandial plasma TAA kinetics were observed between BRP, PP, and WP, with WP giving the highest response, followed by the two other protein sources. When looking at postprandial plasma TEAA confidence intervals, the response after BRP shake consumption was slightly lower when compared to PP. The time to reach the maximum values was similar between the three protein sources. Seven out of nine EAA showed higher postprandial responses after WP shake consumption when compared to BRP. BRP shake consumption did result in a higher uptake of the Phe when compared to WP. When comparing individual EAA responses between BRP and PP, we observed higher responses in methionine (Met) and tryptophane (TRP) and lower responses in lysine (Lys), histidine (His), and isoleucine (Ile) after BRP shake consumption when compared to PP.
The estimated TAA uptake of BRP, as determined by comparing the calculated iAUC, is 69% compared to the benchmark WP. The EAA uptake of BRP is somewhat lower, with an estimated relative uptake of 58% compared to WP. Plant proteins exhibit reduced digestibility due to the presence of indigestible fractions within their sequence as well as the presence of anti-nutritional factors [
23]. Hence, a higher uptake of WP was expected. We included a whey intervention as a benchmark since this protein is known for its optimal absorption and well-balanced EAA composition [
24,
25]. Since several other studies also include a whey intervention arm in their design, it allows us to calculate a relative uptake and estimate and compare effect sizes across studies that used a similar approach.
Previously, we conducted a comparable study on postprandial AA uptake investigating Lemna protein concentrate. In that publication, we also reported an overview of the available literature data on iAUC values of EAA uptake from other protein sources in a human cross-over design with whey protein as the benchmark [
20]. From that overview, it is apparent that in today’s literature, postprandial AA uptake studies in humans comparing whey with plant proteins and or protein blends are still relatively scarce. A human postprandial study described by Lui et al. [
26] reported a relative AA uptake of pea protein compared with whey protein that was somewhat lower, around 70%, than the relative TAA uptake we found in the current study, around 80%. The iAUC results for BRP fall within the range of those previously reported for casein, with studies reporting a relative uptake of TEAA ranging from 49% to 60% compared to whey [
27,
28,
29,
30]. Interestingly, it appears that BRP exhibits a higher estimated range of EAA uptake compared to other cereal proteins reported in the literature, which might be explained only partially by the rice component [
16]. Gorissen et al. [
28] evaluated postprandial plasma AA concentrations of wheat protein hydrolysate and compared them to casein and whey and reported an estimated TEAA uptake of 45% for wheat protein hydrolysate compared to whey. The brewing process, in combination with the processing methods applied to isolate BRP, may potentially impact its protein bioavailability [
19]. It is, however, important to exercise caution when interpreting these comparisons, as direct comparisons between wheat, casein, and barley/rice were not made in our study. Furthermore, certain aspects, such as protein quality, processing steps, purity, or amount of protein ingested, could also differ between studies.
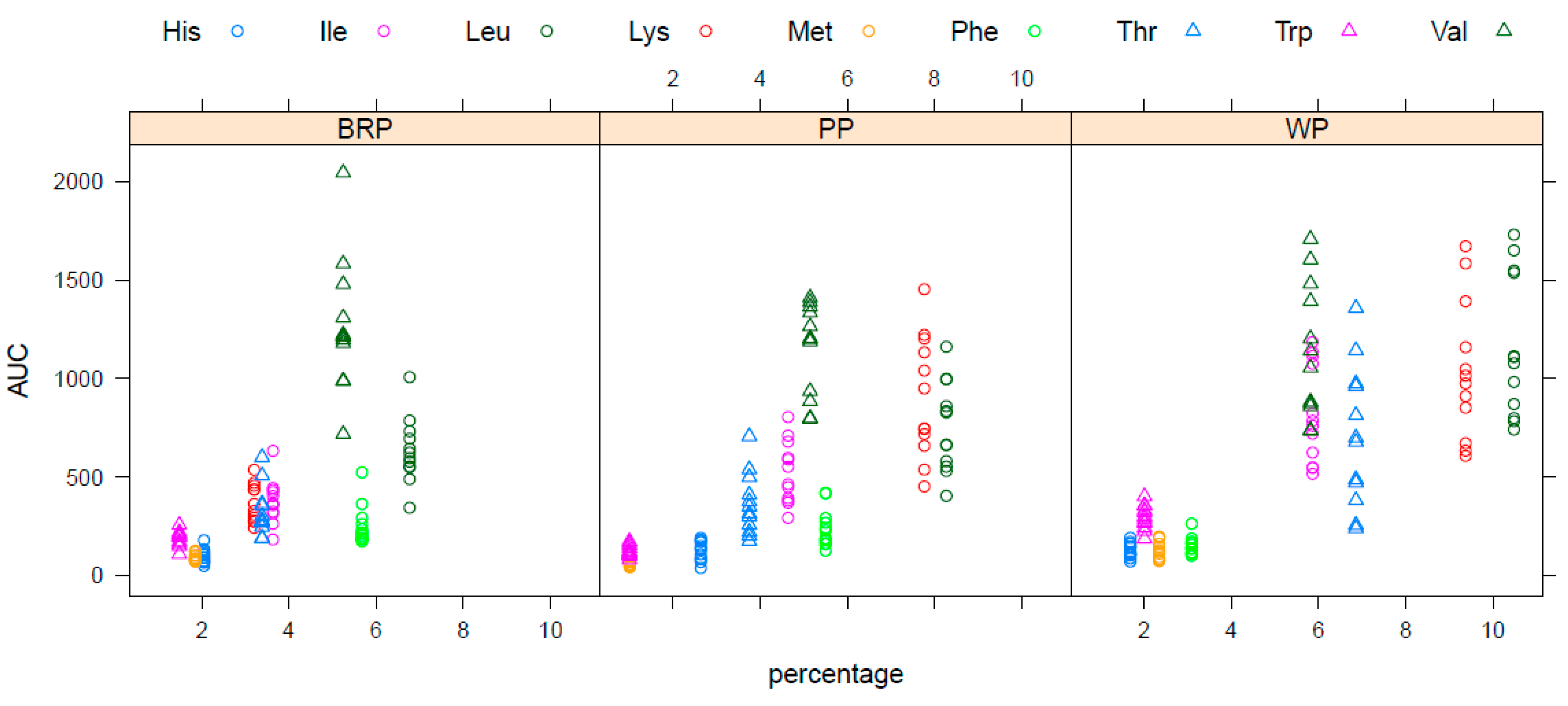
Statistically significant differences were not observed when examining the iAUC of the TAA for the two plant proteins investigated in this study. We did, however, observe a slightly greater overall response in TEAA following the consumption of the PP shake compared to the BRP shake. Even though the iAUC of TEAA for BRP was approximately 82% in comparison to PP, certain individual EAA clearly exhibited higher responses for BRP. Furthermore, no significant differences in the peak heights of TAA and TEAA were observed between BRP and PP. BRP has been shown to have substantially higher solubility in comparison to pea protein, with on average 102% (expressed as total protein percentage remaining in a 1% solution) and 22%, respectively, making BRP nearly five times more soluble than PP [
19]. Due to its high solubility, it would be possible to administer higher BRP quantities in similar product applications, allowing to compensate for potential lower uptake.
Next to the lower overall uptake rates of plant proteins, the absorption speed of plant proteins may also be delayed. For instance, higher time-2-max values of the TAA and TEAA responses were observed in a previous study with corn protein isolate (manuscript in preparation) and Lemna protein concentrate [
20] when compared to whey as a benchmark protein source. Here, we demonstrate that the speed of BRP uptake, determined by the time needed to reach the maximal postprandial blood AA levels, was comparable to and in a similar range of PP and even in the same range of WP. Whey is considered a fast-acting protein, and its absorption rate has been estimated at ~10 g per hour [
31]. These results indicate that BRP also has a relatively fast absorption rate.
This study provides valuable insights into the potential absorption rates of individual EAA. When a particular EAA is limited in availability in the diet, the synthesis of protein is hampered [
32]. Therefore, estimations regarding the proportion of EAA taken up into the bloodstream may provide us with valuable information. Still, these outcomes on individual AA responses should be interpreted with caution since we did not correct for multiple testing. The effect sizes obtained in this study, however, provide valuable insights for future studies. For example, our findings showed substantial inter-individual variation in the percentage of individual EAA taken up into the blood after consumption of the specific protein sources. This implies that not only the rate-limiting EAA may differ between protein sources, but it may also vary between individuals.
Since whey is considered a protein source with an optimal EAA composition and easy to digest [
24], it is not surprising that 7 out of 9 EAA had higher postprandial responses after WP shake consumption compared to BRP. However, BRP shake consumption resulted in a higher postprandial uptake of Phe, an EAA which serves as a precursor for the synthesis of the catecholamines (dopamine, norepinephrine, and epinephrine). The synthesis and release rates of catecholamines are directly influenced by the availability of their precursor from blood. Therefore, Phe demonstrates potential for brain function, manifested through various effects such as analgesic and antidepressant properties [
33]. Furthermore, since this EAA cannot be oxidized by the muscles, its uptake and incorporation into muscle are assumed to serve as accurate indicators of muscle protein synthesis [
34]. We consider the finding on higher postprandial levels of Phe a potential interesting lead to study further.
Uptake scores of individual EAA of BRP, estimated by comparing the iAUC, were in a higher (Met, Trp), similar (Val, Thr, Phe, Leu), or lower (Lys, His, Ile) range compared to PP. The higher postprandial uptake levels of Met and lower levels of Lys after consumption of the BRP shake were expected. This is because the AA composition of pea, in general, is characterized by a limited content of Met [
35], whereas barley and other cereals such as wheat and rice, as mentioned earlier, are known to be low in Lys [
16,
36]. This makes them interesting complementary protein sources [
16,
32]. Previously conducted research indicates that Met serves as an important cellular antioxidant, stabilizes the structure of proteins, participates in the sequence-independent recognition of protein surfaces, and can act as a regulatory switch through reversible oxidation and reduction [
37]. Higher levels of tryptophan (Trp) may also be beneficial as this AA and its metabolites, such as serotonin, play a key signaling role in the gut-brain axis, thereby modulating health effects [
38,
39].
Despite the significantly higher Glu content in BRP compared to PP and WP, as well as in relation to other AA, there was no significant difference observed in the postprandial iAUC of Glu among the three protein shakes. Additionally, the iAUC of Glu in relation to its presence in the product was generally low compared to other AA. One plausible explanation for the low concentration of Glu in the bloodstream is the high energy demand of the small intestine epithelium, particularly in the postprandial state. Consequently, Glu is either directly utilized or taken up from the bloodstream for extensive oxidation within the cells of the small intestine epithelium [
40]. Moreover, Glu serves as a precursor for several other AA, including alanine, aspartate, ornithine, and proline, thus playing a role in interorgan metabolic processes [
40].
In the current study, participants assessed their general health and reported their experiences of bloating, nausea, occurrence of belching, flatulence, diarrhea, and constipation using an online questionnaire after each test day. The occurrence of these gastrointestinal symptoms was minimal and did not significantly differ among the three protein shakes. This indicates that the consumption of 20 g BRP per day was well-tolerated.
Despite having an equivalent carbohydrate composition, differences were observed in the postprandial insulin and glucose responses among the protein shakes. Consumption of the BRP shake, although not significant, led to a reduced peak in insulin levels, while the peak in glucose levels was comparable to that of the PP shake. This may be partly explained by differences in the type of carbohydrates, as WP and PP contained added maltodextrin to match the higher carbohydrate content of BRP. The breakdown of proteins into AA triggers an insulin response, and indeed, a rapid surge in insulin was observed shortly after protein ingestion. The purpose of this insulin surge is to facilitate the transportation of AA into the muscles, thereby stimulating muscle protein synthesis [
41]. However, the specific nature of the insulin response may vary depending on the source of the protein, as emerging evidence suggests that substituting animal protein with plant protein yields better glycemic control [
42]. The endocrine hormones GIP and GLP-1, whose secretion can be stimulated by certain dietary proteins, peptides, and AA, may potentially contribute to this process [
43]. However, it is important to note that these hormones were not evaluated in the present study.
The evaluation of protein digestibility and nutritional characteristics of emerging sustainable protein sources poses challenges, particularly when avoiding animal experiments. The gold standard methodologies for assessing digestibility, namely the protein digestibility-corrected AA score, PDCAAS, and the more recent digestible indispensable AA score, DIAAS [
6], rely solely on animal experiments and lack the ability to capture variations in response among individuals. Although in vitro alternatives do exist, they are not sufficient as it has been proven difficult to accurately measure the bioavailable fraction. Accurately determining true AA digestibility in humans is methodologically complex and requires costly stable isotope techniques. Consequently, the reported AA digestibility in humans remains limited for a select range of proteins. In this study, we measured postprandial blood AA concentrations to estimate protein quality, recognizing that the appearance of free AA in the blood only partially reflects protein digestibility. The AA profile in the blood following a meal is influenced by factors such as splanchnic tissue metabolism, intestinal passage speed, protein uptake/breakdown, and endogenous synthesis. The latter not being relevant for the EAA, which are of more importance for human health. Comparing the postprandial response to a reference and measuring it over time allows us to minimize the impact of confounding factors. Postprandial AA kinetics, therefore, serve as a valuable characteristic for assessing the potential nutritional impact of a protein, particularly for novel protein sources. Compared to stable isotope techniques, this method is relatively straightforward to perform, making it an important tool for evaluating the protein quality of emerging protein sources. It is, however, important to note that this method does not provide a quantitative protein digestibility score.