Effects of Endurance Exercise and Vitamin D Supplementation on Insulin Resistance and Plasma Lipidome in Middle-Aged Adults with Type 2 Diabetes
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Intervention
2.2.1. Vitamin D Intervention
2.2.2. Exercise Intervention
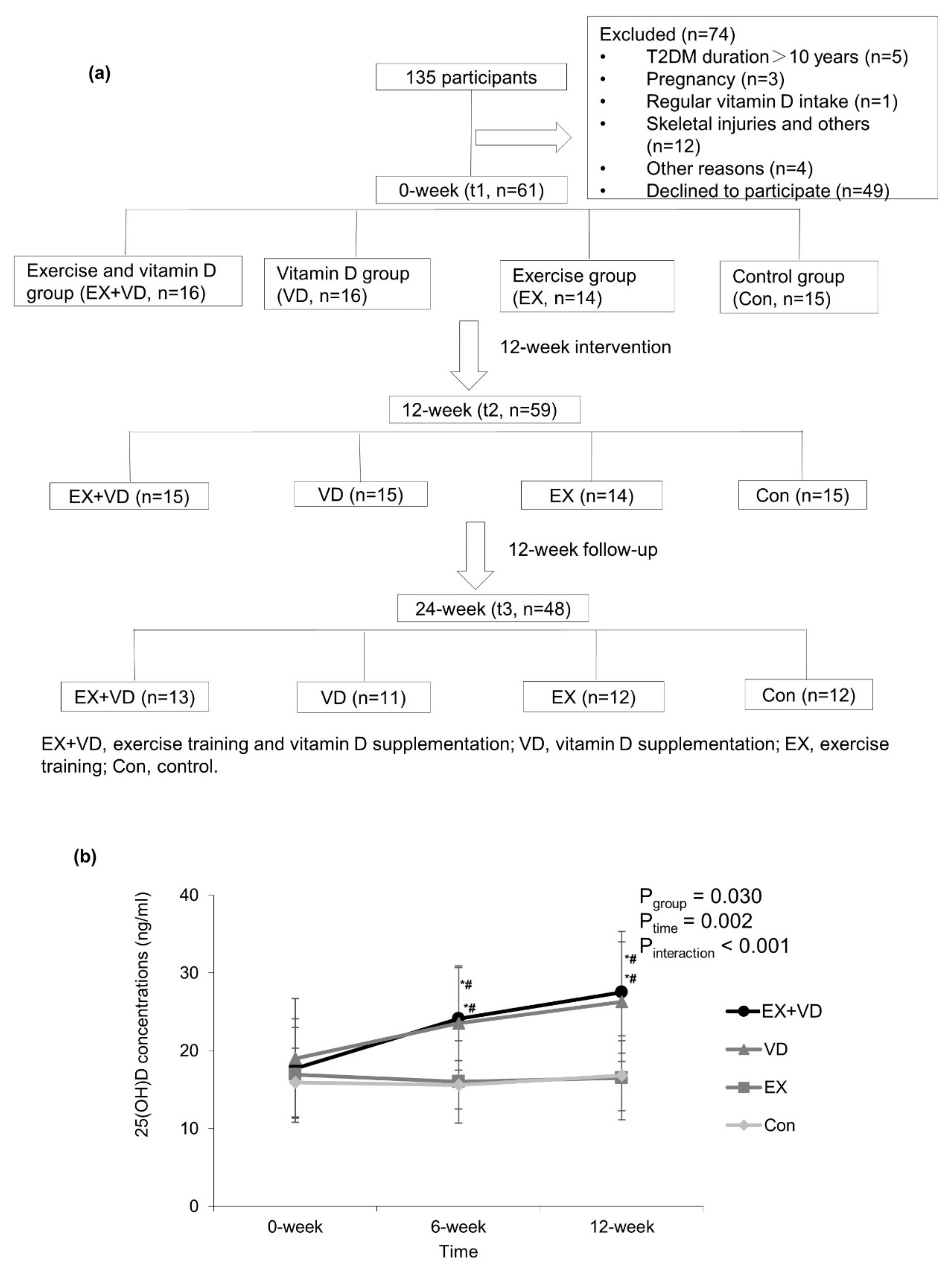
2.3. Data Collection
2.3.1. Anthropometric Measurements and Questionnaires
2.3.2. Glycemic Control Indicators
2.3.3. Plasma Lipidomic Profiling
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Effects of Exercise and Vitamin D on Serum 25(OH)D Concentrations and Glycemic Control Indicators
3.3. Effects of Exercise and Vitamin D on Plasma Lipidome
| Variables | EX + VD n = 15 | VD n = 15 | EX n = 14 | Con n = 15 | p baseline | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1 | t2 | Δ% | p | t1 | t2 | Δ% | p | t1 | t2 | Δ% | p | t1 | t2 | Δ% | p | ||
| Weight | 68.9 ± 13.1 | 68.6 ± 13.8 | −0.4% | 0.560 | 71.4 ± 13.1 | 71.8 ± 12.6 | 0.6% | 0.599 | 74.7 ± 15.1 | 73.6 ± 14.6 | −1.5% | 0.019 | 76.9 ± 10.9 | 76.7 ± 11.2 | −0.3% | 0.684 | 0.364 |
| BMI (kg/m2) | 25.1 ± 3.0 | 25.8 ± 4.1 | 2.8% | 0.365 | 25.3 ± 3.3 | 25.4 ± 3.0 | 0.4% | 0.668 | 26.1 ± 4.8 | 25.8 ± 4.6 | −1.1% | 0.036 | 27.2 ± 3.2 | 27.2 ± 3.3 | 0.0% | 0.745 | 0.363 |
| Body fat (%) a | 32.0 ± 6.1 | 31.0 ± 5.7 | −3.1% | 0.093 | 27.9 ± 9.2 | 28.4 ± 9.9 | 1.8% | 0.438 | 30.9 ± 8.6 | 29.8 ± 8.7 | −3.6% | 0.020 | 30.3 ± 6.6 | 29.8 ± 6.0 | −1.7% | 0.174 | 0.532 |
| Physical activity (MET-min/Week) | 3164 ± 2413 | 4971 ± 4070 | 57.1% | 0.138 | 4065 ± 4583 | 4225 ± 2722 | 3.9% | 0.896 | 4334 ± 4286 | 4092 ± 5709 | −5.6% | 0.831 | 2801 ± 1976 | 3311 ± 1959 | 18.2% | 0.221 | 0.596 |
| Sun exposure weekly score | 16.5 ± 8.3 | 16.4 ± 11.9 | −0.6% | 0.966 | 13.6 ± 4.1 | 19.3 ± 9.5 | 41.9% | 0.082 | 16.1 ± 8.8 | 20.7 ± 11.4 | 28.6% | 0.153 | 13.2 ± 7.3 | 18.8 ± 16.1 | 42.4% | 0.212 | 0.506 |
| Apolipoprotein A (g/L) | 1.29 ± 0.30 | 1.31 ± 0.26 | 1.6% | 0.711 | 1.29 ± 0.23 | 1.31 ± 0.26 | 1.6% | 0.549 | 1.29 ± 0.22 | 1.30 ± 0.20 | 0.8% | 0.802 | 1.18 ± 0.26 | 1.28 ± 0.21 | 8.5% | 0.099 | 0.977 |
| Apolipoprotein B (g/L) | 0.83 ± 0.32 | 0.85 ± 0.26 | 2.4% | 0.634 | 0.85 ± 0.19 | 0.83 ± 0.19 | −2.4% | 0.639 | 0.90 ± 0.25 | 0.93 ± 0.29 | 3.3% | 0.468 | 0.73 ± 0.17 | 0.80 ± 0.15 | 9.6% | 0.065 | 0.467 |
| LDL-C (mmol/L) | 2.3 ± 0.9 | 2.2 ± 0.7 | −4.3% | 0.587 | 2.3 ± 0.6 | 2.1 ± 0.6 | −8.7% | 0.105 | 2.5 ± 0.8 | 2.3 ± 0.7 | −8.0% | 0.059 | 2.1 ± 0.5 | 2.0 ± 0.5 | −4.8% | 0.192 | 0.590 |
| HDL-C (mmol/L) | 1.3 ± 0.4 | 1.3 ± 0.3 | 0.0% | 0.242 | 1.2 ± 0.2 | 1.2 ± 0.3 | 0.0% | 0.948 | 1.3 ± 0.3 | 1.3 ± 0.2 | 0.0% | 0.830 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.0% | 0.808 | 0.977 |
| Cholesterol (mmol/L) | 4.3 ± 1.2 | 4.4 ± 1.1 | 2.3% | 0.787 | 4.3 ± 0.8 | 4.1 ± 0.8 | −4.7% | 0.327 | 4.7 ± 0.8 | 4.5 ± 0.8 | −4.3% | 0.207 | 4.0 ± 0.7 | 3.9 ± 0.6 | −2.5% | 0.498 | 0.326 |
| Triglyceride (mmol/L) | 1.4 ± 0.7 | 1.4 ± 0.8 | 0.0% | 0.711 | 1.5 ± 0.7 | 1.5 ± 0.6 | 0.0% | 0.990 | 2.0 ± 1.0 | 1.7 ± 0.7 | −15.0% | 0.239 | 1.3 ± 0.5 | 1.6 ± 0.7 | 23.1% | 0.028 | 0.132 |
| HbA1c (%) | 7.0 ± 1.9 | 6.3 ± 0.7 | −10.0% | 0.064 | 6.9 ± 1.4 | 6.6 ± 0.8 | −4.3% | 0.318 | 6.7 ± 1.2 | 6.5 ± 1.1 | −3.0% | 0.316 | 7.2 ± 1.6 | 6.7 ± 0.8 | −6.9% | 0.186 | 0.835 |
| Fasting glucose (mmol/L) | 6.7 ± 1.4 | 5.9 ± 1.0 | −11.9% | 0.016 | 7.4 ± 1.9 | 6.5 ± 0.8 | −12.2% | 0.032 | 7.8 ± 2.1 | 7.2 ± 2.1 | −7.7% | 0.114 | 7.5 ± 2.6 | 7.1 ± 1.3 | −5.3% | 0.588 | 0.559 |
| Fasting insulin (μU/mL) | 12.6 ± 8.6 | 10.8 ± 6.2 | −14.3% | 0.389 | 9.7 ± 5.6 | 11.0 ± 8.9 | 13.4% | 0.294 | 11.7 ± 6.1 | 12.1 ± 8.3 | 3.4% | 0.809 | 14.7 ± 7.6 | 15.1 ± 11.0 | 2.7% | 0.813 | 0.240 |
| HOMA-IR | 3.9 ± 3.0 | 3.0 ± 2.2 | −23.1% | 0.086 | 3.4 ± 2.6 | 3.2 ± 2.7 | −5.9% | 0.649 | 3.9 ± 2.3 | 3.8 ± 2.3 | −2.6% | 0.823 | 4.9 ± 2.6 | 5.1 ± 4.9 | 4.1% | 0.814 | 0.384 |
| Matsuda Index | 2.9 ± 1.5 | 3.3 ± 1.9 | 13.8% | 0.383 | 3.3 ± 2.1 | 3.3 ± 2.2 | 0.0% | 0.993 | 2.8 ± 1.3 | 3.2 ± 2.1 | 14.3% | 0.448 | 2.7 ± 2.0 | 2.5 ± 1.3 | −7.4% | 0.205 | 0.601 |
| Glucose AUC b | 1621 ± 319 | 1542 ± 249 | −4.9% | 0.276 | 1681 ± 274 | 1541 ± 277 | −8.3% | 0.093 | 1728 ± 388 | 1653 ± 420 | −4.3% | 0.215 | 1672 ± 368 | 1642 ± 287 | −1.8% | 0.605 | 0.865 |
| Insulin AUC b | 6353 ± 2277 | 6869 ± 2428 | 8.1% | 0.506 | 5794 ± 1709 | 6812 ± 3050 | 17.6% | 0.077 | 5718 ± 2683 | 6924 ± 3919 | 21.1% | 0.027 | 7029 ± 2917 | 6930 ± 2530 | −1.4% | 0.862 | 0.458 |
3.4. Individual Responses to Interventions Improving Glycemic Control and Baseline Prediction
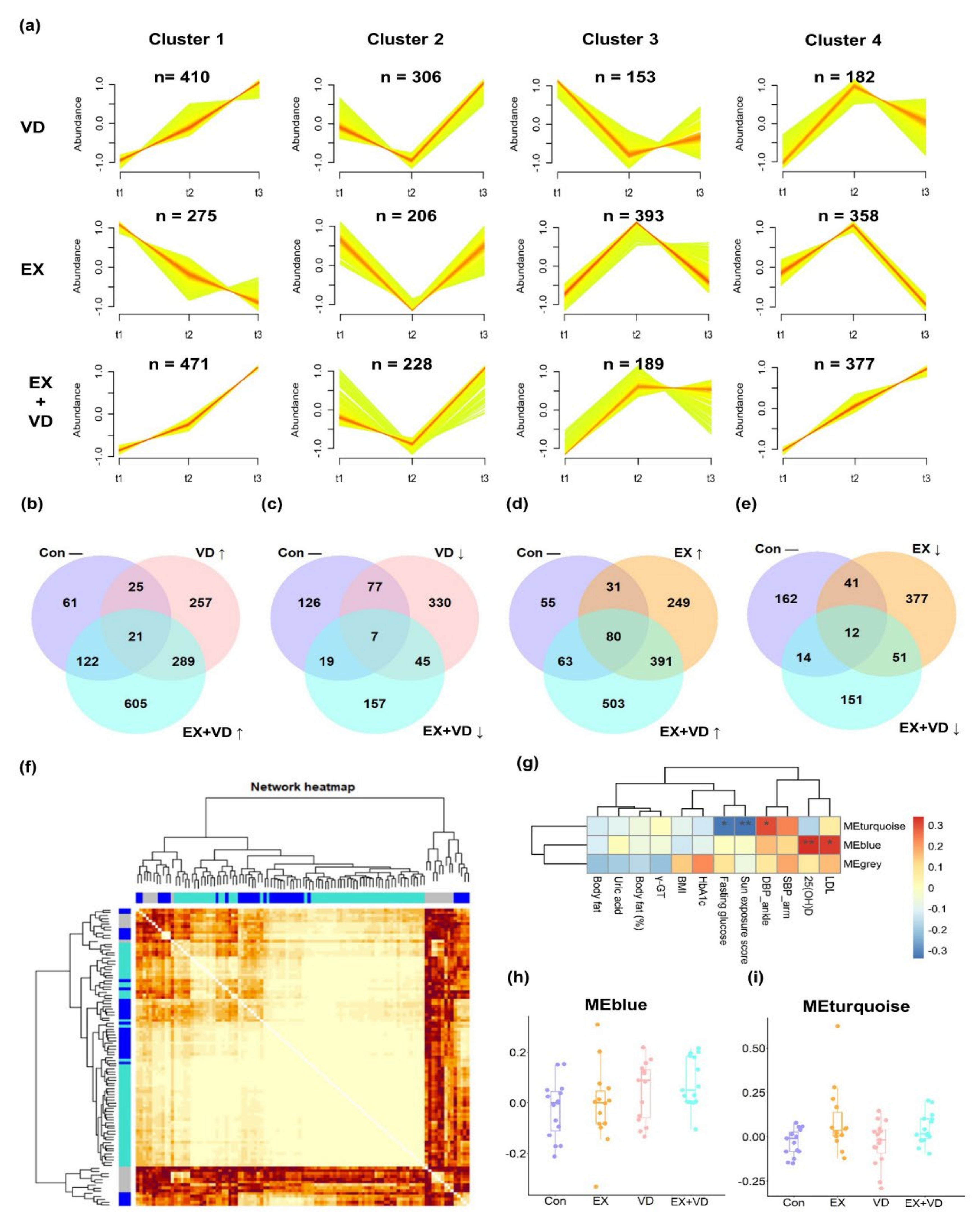
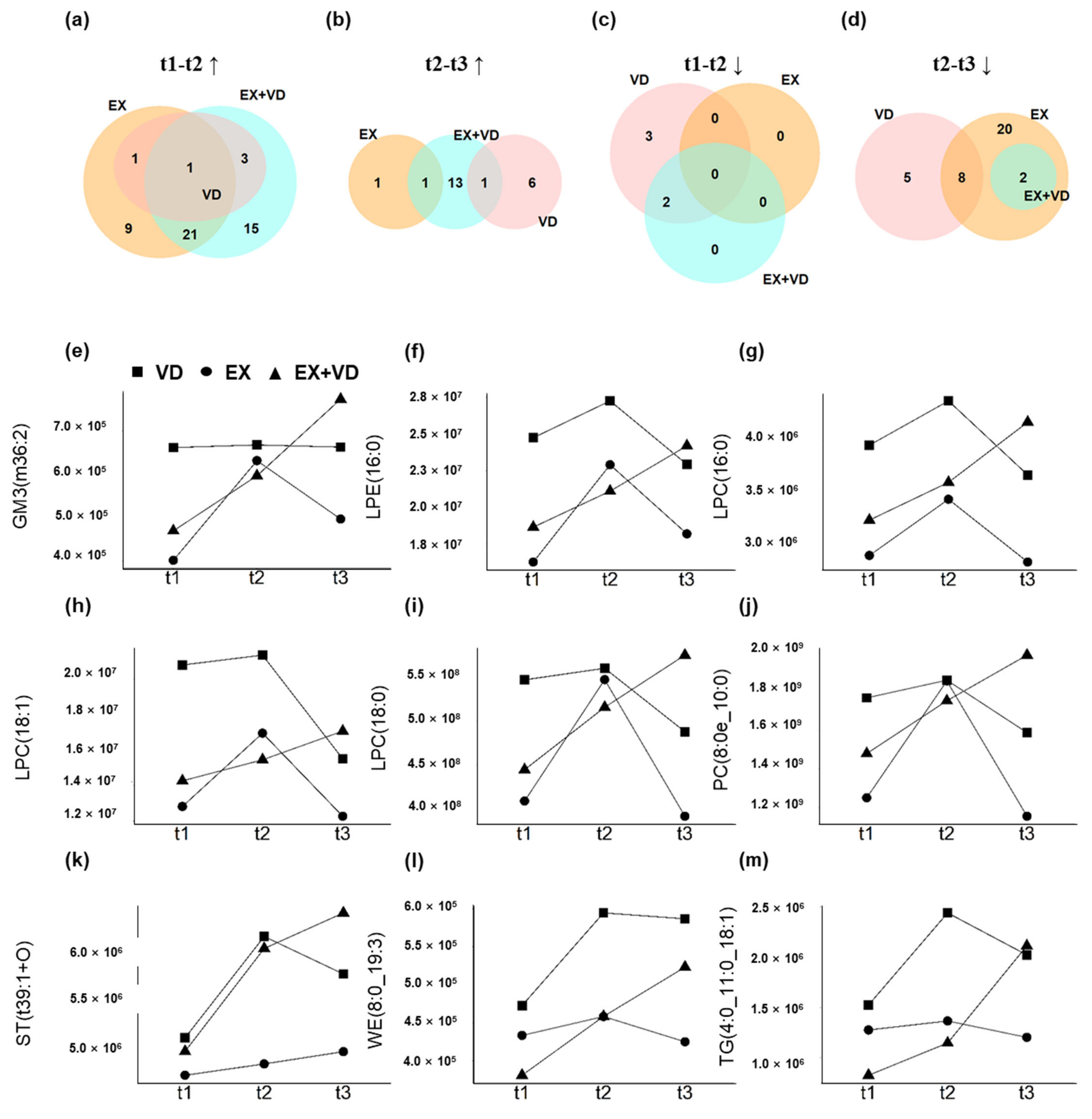

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mortality, G.B.D. Causes of Death, C. Global, Regional, and National Age-Sex Specific all-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990–2013: A Systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Wang, L.; Peng, W.; Zhao, Z.; Zhang, M.; Shi, Z.; Song, Z.; Zhang, X.; Li, C.; Huang, Z.; Sun, X.; et al. Prevalence and Treatment of Diabetes in China, 2013–2018. JAMA 2021, 326, 2498–2506. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sport. Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- National Fitness Guide. General Administration of Sport of China. Available online: https://www.sport.gov.cn/n315/n331/n405/c819327/content.html (accessed on 17 June 2023).
- Chinese Diabetes Society. Chinese Guidelines of Exercise Therapy in Diabetes Mellitus. Available online: https://diab.cma.org.cn/cn/zhinangongshi.aspx (accessed on 17 June 2023).
- Janssen, S.M.; Connelly, D.M. The effects of exercise interventions on physical function tests and glycemic control in adults with type 2 diabetes: A systematic review. J. Bodyw. Mov. Ther. 2021, 28, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Sampath, K.A.; Maiya, A.G.; Shastry, B.A.; Vaishali, K.; Ravishankar, N.; Hazari, A.; Gundmi, S.; Jadhav, R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-gamma-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J. Biol. Chem. 2012, 287, 42324–42332. [Google Scholar] [CrossRef]
- Antoniak, A.E.; Greig, C.A. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: A systematic review and meta-analysis. BMJ Open 2017, 7, e014619. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Ito, T.; Oshima, S.; Higuchi, M. The Relationship between Serum 25-Hydroxyvitamin D Concentration, Cardiorespiratory Fitness, and Insulin Resistance in Japanese Men. Nutrients 2014, 7, 91–102. [Google Scholar] [CrossRef]
- Goldberg, I.J. Clinical review 124—Diabetic dyslipidemia: Causes and consequences. J. Clin. Endocr. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef]
- Andersen, C.J. Lipid Metabolism in Inflammation and Immune Function. Nutrients 2022, 14, 1414. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Boren, J. New insights into the Pathophysiology of Dyslipidemia in Type 2 Diabetes. Atherosclerosis 2015, 239, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Weir, J.M.; Greeve, M.A.; MacIntosh, G.L.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Kowalczyk, A.; et al. Plasma Lipid Profiling Shows Similar Associations with Prediabetes and Type 2 Diabetes. PLoS ONE 2013, 8, e74341. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Zhang, Y.; Huo, Z.; Zeng, W.; Zhu, J.; Umans, J.G.; Wohlgemuth, G.; Pedrosa, D.; DeFelice, B.; Cole, S.A.; et al. Longitudinal Plasma Lipidome and Risk of Type 2 Diabetes in a Large Sample of American Indians with Normal Fasting Glucose: The Strong Heart Family Study. Diabetes Care 2021, 44, 2664–2672. [Google Scholar] [CrossRef]
- Monnerie, S.; Comte, B.; Ziegler, D.; Morais, J.A.; Pujos-Guillot, E.; Gaudreau, P. Metabolomic and Lipidomic Signatures of Metabolic Syndrome and its Physiological Components in Adults: A Systematic Review. Sci Rep. 2020, 10, 669. [Google Scholar] [CrossRef]
- Brennan, A.M.; Standley, R.A.; Yi, F.; Carnero, E.A.; Sparks, L.M.; Goodpaster, B.H. Individual Response Variation in the Effects of Weight Loss and Exercise on Insulin Sensitivity and Cardiometabolic Risk in Older Adults. Front. Endocrinol. 2020, 11, 632. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Mohammed, A.K.; Al-Attas, O.S.; Ansari, M.G.A.; Wani, K.; Hussain, S.D.; Sabico, S.; Tripathi, G.; Alokail, M.S. Vitamin D Receptor Gene Polymorphisms Modify Cardiometabolic Response to Vitamin D Supplementation in T2DM Participants. Sci. Rep. 2017, 7, 8280. [Google Scholar] [CrossRef]
- Garcia-Perez, I.; Posma, J.M.; Chambers, E.S.; Mathers, J.C.; Draper, J.; Beckmann, M.; Nicholson, J.K.; Holmes, E.; Frost, G. Dietary Metabotype Modelling Predicts Individual Responses to Dietary Interventions. Nat. Food 2020, 1, 355–364. [Google Scholar] [CrossRef]
- Palmnas, M.; Brunius, C.; Shi, L.; Rostgaard-Hansen, A.; Torres, N.E.; Gonzalez-Dominguez, R.; Zamora-Ros, R.; Ye, Y.L.Q.; Halkjaer, J.; Tjonneland, A.; et al. Perspective: Metabotyping-A Potential Personalized Nutrition Strategy for Precision Prevention of Cardiometabolic Disease. Adv. Nutr. 2020, 11, 524–532. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Ito, T.; Oshima, S.; Higuchi, M. Vitamin D Supplementation Reduces Insulin Resistance in Japanese Adults: A Secondary Analysis of a Double-Blind, Randomized, Placebo-Controlled Trial. Nutr. Res. 2016, 36, 1121–1129. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-Predicted Maximal Heart Rate Revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xiao, W.; Li, Z.; Zhou, S.; Dong, M.; Huang, C.; Ma, Y.; Gou, B. Does Vitamin D Supplementation Improve Bone Health, Body Composition and Physical Performance Beyond Endurance Exercise in Participants with Type 2 Diabetes: A secondary Analysis of randomized controlled trial. Front. Physiol. 2022, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, H.E.; Vieth, R.; Cole, D.E.; Scillitani, A.; Modoni, S.; Frusciante, V.; Ritrovato, G.; Chiodini, I.; Minisola, S.; Carnevale, V. Sun Exposure Questionnaire Predicts Circulating 25-Hydroxyvitamin D concentrations in Caucasian Hospital Workers in Southern Italy. J. Steroid. Biochem. Mol. Biol. 2010, 121, 334–337. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment—Insulin Resistance and Beta-Cell Function from Fasting Plasma-Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin Sensitivity Indices Obtained from Oral Glucose TOLERANCE testing: Comparison with the Euglycemic Insulin Clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Wolever, T.M.S. Effect of Blood Sampling Schedule and Method of Calculating the Area under the Curve on Validity and Precision of Glycaemic Index Values. Br. J. Nutr. 2004, 91, 295–300. [Google Scholar] [CrossRef]
- Batrakoulis, A.; Jamurtas, A.Z.; Metsios, G.S.; Perivoliotis, K.; Liguori, G.; Feito, Y.; Riebe, D.; Thompson, W.R.; Angelopoulos, T.J.; Krustrup, P.; et al. Comparative Efficacy of 5 Exercise Types on Cardiometabolic Health in Overweight and Obese Adults: A Systematic Review and Network Meta-Analysis of 81 Randomized Controlled Trials. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e008243. [Google Scholar] [CrossRef]
- Pan, B.; Ge, L.; Xun, Y.Q.; Chen, Y.J.; Gao, C.Y.; Han, X.; Zuo, L.Q.; Shan, H.Q.; Yang, K.H.; Ding, G.W.; et al. Exercise Training Modalities in Patients with type 2 Diabetes Mellitus: A Systematic Review and Network Meta-Analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 72. [Google Scholar] [CrossRef]
- Li, Y.-M.; Han, J.; Liu, Y.; Wang, R.; Wang, R.; Wu, X.P.; Cao, Z.B. China Survey of Fitness Trends for 2020. ACSM’s Health Fit. J. 2019, 23, 19–27. [Google Scholar] [CrossRef]
- Woerle, H.J.; Neumann, C.; Zschau, S.; Tenner, S.; Irsigler, A.; Schirra, J.; Gerich, J.E.; Goke, B. Impact of Fasting and Postprandial glycemia on Overall Glycemic Control in Type 2 Diabetes Importance of Postprandial Glycemia to Achieve Target HbA1c Levels. Diabetes Res. Clin. Pract. 2007, 77, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kang, C.K.; Park, H.; Lee, M.G. Effects of Vitamin D Supplementation and circuit Training on Indices of Obesity and Insulin Resistance in T2D and Vitamin D Deficient Elderly Women. J. Exerc. Nutr. Biochem. 2014, 18, 249–257. [Google Scholar] [CrossRef]
- Dadrass, A.; Mohamadzadeh Salamat, K.; Hamidi, K.; Azizbeigi, K. Anti-Inflammatory Effects of Vitamin D and Resistance Training in Men with Type 2 Diabetes Mellitus and Vitamin D Deficiency: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. J. Diabetes Metab. Disord. 2019, 18, 323–331. [Google Scholar] [CrossRef]
- Liu, P.P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The Mechanisms of Lysophosphatidylcholine in the Development of Diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Fritsche, J.; Wang, J.S.; Chen, J.; Rittig, K.; Schmitt-Kopplin, P.; Fritsche, A.; Haring, H.U.; Schleicher, E.D.; Xu, G.W.; et al. Metabonomic Fingerprints of Fasting Plasma and Spot Urine Reveal Human pre-Diabetic Metabolic Traits. Metabolomics 2010, 6, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma Lysophosphatidylcholine Levels Are Reduced in Obesity and Type 2 Diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics Profiling and Risk of Cardiovascular Disease in the Prospective Population-Based Bruneck Study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef]
- Djekic, D.; Shi, L.; Calais, F.; Carlsson, F.; Landberg, R.; Hyotylainen, T.; Frobert, O. Effects of a Lacto-Ovo-Vegetarian Diet on the Plasma Lipidome and Its Association with Atherosclerotic Burden in Participants with Coronary Artery Disease—A Randomized, Open-Label, Cross-over Study. Nutrients 2020, 12, 3586. [Google Scholar] [CrossRef]
- Belhaj, M.R.; Lawler, N.G.; Hoffman, N.J. Metabolomics and Lipidomics: Expanding the Molecular Landscape of Exercise Biology. Metabolites 2021, 11, 151. [Google Scholar] [CrossRef]
- Latino, F.; Cataldi, S.; Carvutto, R.; De Candia, M.; D’Elia, F.; Patti, A.; Bonavolonta, V.; Fischetti, F. The Importance of Lipidomic Approach for Mapping and Exploring the Molecular Networks Underlying Physical Exercise: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8734. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Du, L.; Hosokawa, M.; Miyashita, K. Effect of Spirulina Lipids on High-Fat and High-Sucrose Diet induced Obesity and Hepatic Lipid Accumulation in C57BL/6J Mice. J. Funct. Foods 2020, 65, 103741. [Google Scholar] [CrossRef]
- Gao, Q.M.; Yu, K.; Xia, Y.; Shine, M.B.; Wang, C.; Navarre, D.; Kachroo, A.; Kachroo, P. Mono-and Digalactosyldiacylglycerol lipids Function Nonredundantly to Regulate Systemic Acquired Resistance in Plants. Cell Rep. 2014, 9, 1681–1691. [Google Scholar] [CrossRef]
- Xu, Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-Coupled Receptors and Receptor-Mediated Signal Transduction. BBA-Mol. Cell Biol. Lipids 2002, 1582, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Lim, Y.M.; Quan, W.; Kim, J.R.; Chung, K.W.; Kang, M.; Kim, S.; Park, S.Y.; Han, J.S.; Park, S.Y.; et al. Lysophosphatidylcholine as an Effector of Fatty acid-Induced Insulin Resistance. J. Lipid Res. 2011, 52, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Summers, S.A. Sphingolipids and Phospholipids in Insulin Resistance and Related Metabolic Disorders. Nat. Rev. Endocrinol. 2017, 13, 79–91. [Google Scholar] [CrossRef]
- Al-Sulaiti, H.; Diboun, I.; Agha, M.V.; Mohamed, F.F.S.; Atkin, S.; Domling, A.S.; Elrayess, M.A.; Mazloum, N.A. Metabolic Signature of Obesity-Associated Insulin Resistance and Type 2 Diabetes. J. Transl. Med. 2019, 17, 348. [Google Scholar] [CrossRef]
- San Martin, R.; Brandao, C.F.C.; Junqueira-Franco, M.V.M.; Junqueira, G.P.; de Freitas, E.C.; de Carvalho, F.G.; Rodrigues, C.H.P.; Aguesse, A.; Billon-Crossouard, S.; Krempf, M.; et al. Untargeted Lipidomic Analysis of Plasma from Obese Women Submitted to Combined Physical Exercise. Sci Rep. 2022, 12, 11541. [Google Scholar] [CrossRef]
- Yea, K.; Kim, J.; Yoon, J.H.; Kwon, T.; Kim, J.H.; Lee, B.D.; Lee, H.J.; Lee, S.J.; Kim, J.I.; Lee, T.G.; et al. Lysophosphatidylcholine Activates Adipocyte Glucose Uptake and Lowers Blood Glucose Levels in Murine Models of Diabetes. J. Biol. Chem. 2009, 284, 33833–33840. [Google Scholar] [CrossRef]
- Russo, D.; Capolupo, L.; Loomba, J.S.; Sticco, L.; D’Angelo, G. Glycosphingolipid Metabolism in Cell Fate Specification. J. Cell Sci. 2018, 131, jcs219204. [Google Scholar] [CrossRef]
- Shayman, J.A. Targeting Glucosylceramide Synthesis in the Treatment of Rare and Common Renal Disease. Semin. Nephrol. 2018, 38, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.A.; Siddique, M.M.; Wang, S.T.; Ching, J.H.; Shayman, J.A.; Summers, S.A. Ceramides and Glucosylceramides Are Independent Antagonists of Insulin Signaling. J. Biol. Chem. 2014, 289, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jiang, Y.; Li, Q.; Wang, J.; Tan, S. Exercise Training at Maximal Fat Oxidation Intensity for Overweight or Obese Older Women: A Randomized Study. J. Sports Sci. Med. 2019, 18, 413–418. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Yan, T.; Li, Z.; Zhou, S.; Peng, W.; Cui, W.; Xu, J.; Cao, Z.-B.; Shi, L.; Wang, Y. Effects of Endurance Exercise and Vitamin D Supplementation on Insulin Resistance and Plasma Lipidome in Middle-Aged Adults with Type 2 Diabetes. Nutrients 2023, 15, 3027. https://doi.org/10.3390/nu15133027
Sun X, Yan T, Li Z, Zhou S, Peng W, Cui W, Xu J, Cao Z-B, Shi L, Wang Y. Effects of Endurance Exercise and Vitamin D Supplementation on Insulin Resistance and Plasma Lipidome in Middle-Aged Adults with Type 2 Diabetes. Nutrients. 2023; 15(13):3027. https://doi.org/10.3390/nu15133027
Chicago/Turabian StyleSun, Xiaomin, Tao Yan, Zhongying Li, Sirui Zhou, Wen Peng, Wei Cui, Jing Xu, Zhen-Bo Cao, Lin Shi, and Youfa Wang. 2023. "Effects of Endurance Exercise and Vitamin D Supplementation on Insulin Resistance and Plasma Lipidome in Middle-Aged Adults with Type 2 Diabetes" Nutrients 15, no. 13: 3027. https://doi.org/10.3390/nu15133027
APA StyleSun, X., Yan, T., Li, Z., Zhou, S., Peng, W., Cui, W., Xu, J., Cao, Z.-B., Shi, L., & Wang, Y. (2023). Effects of Endurance Exercise and Vitamin D Supplementation on Insulin Resistance and Plasma Lipidome in Middle-Aged Adults with Type 2 Diabetes. Nutrients, 15(13), 3027. https://doi.org/10.3390/nu15133027








