Dietary Patterns and Circadian Syndrome among Adults Attending NHANES 2005–2016
Abstract
:1. Introduction
2. Materials and Methods
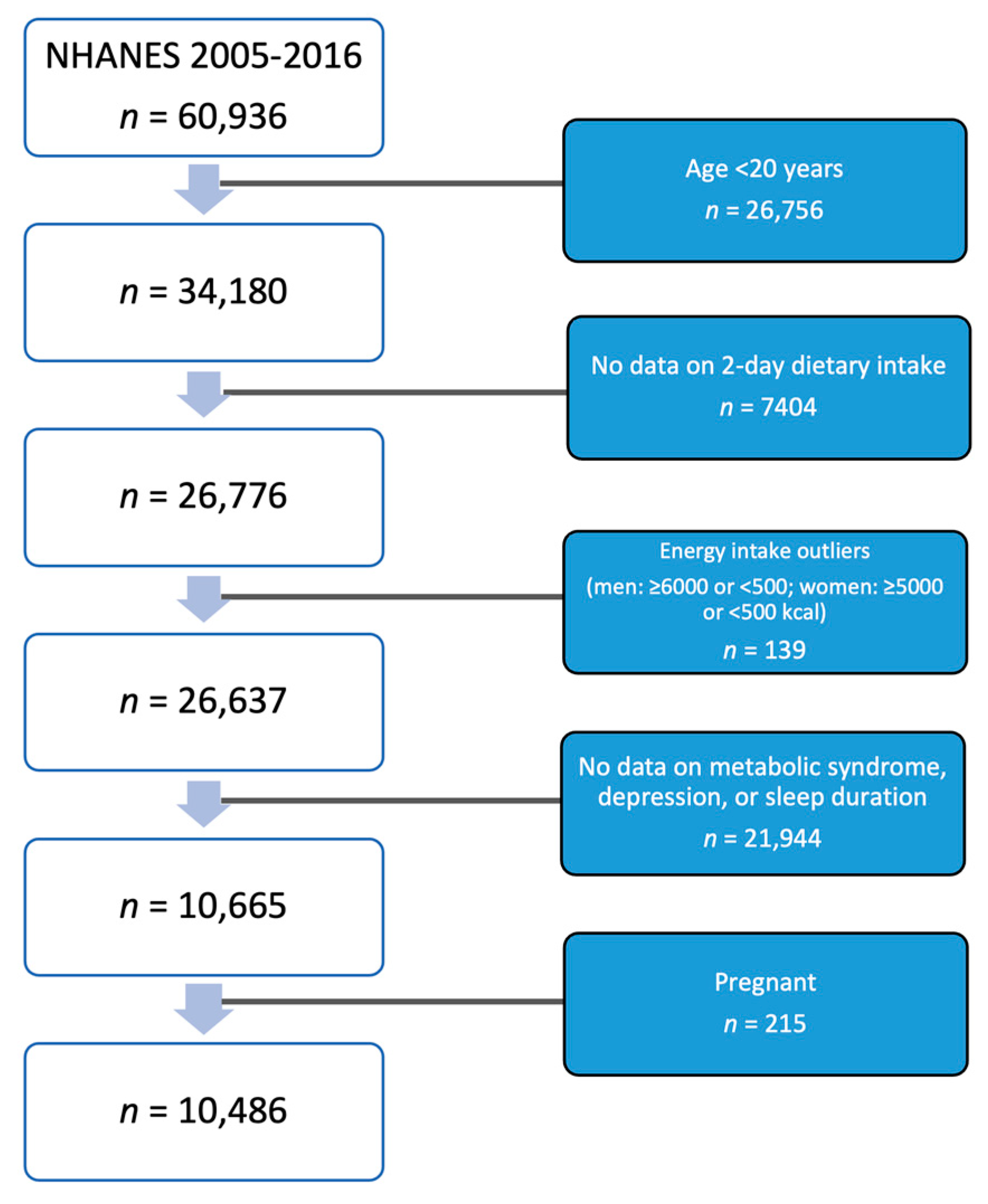
2.1. Study Design and Sample
2.2. Outcome Variable: Circadian Syndrome
- Elevated waist circumference was defined by waist circumference ≥88 cm for women and ≥102 cm for men.
- Elevated fasting glucose was defined by fasting glucose ≥100 mg/dL, or patients being treated with medication, which was assessed with the question “now taking diabetic pills to lower your blood sugar?”
- Elevated triglyceride was defined by serum triglycerides ≥150 mg/dL, or patients being treated with medication, which was assessed with the question “because of your high blood cholesterol, have you ever been told by a doctor or other health professional to take prescribed medicine?”
- Reduced HDL-Cholesterol was defined by serum HDL-C <40 mg/dL in men and <50 mg/dL in women or, the answer “yes” to the question “to lower blood cholesterol, ever been told by a doctor or other health professional to take prescribed medicine?”
- Elevated blood pressure was defined by systolic blood pressure (SBP) ≥130 mmHg or a diastolic blood pressure (DBP) ≥85 mmHg, or patients being treated with antihypertensive medication. The use of antihypertensive medication was assessed by the survey question “Are you now taking prescribed medicine for HBP?”
- Short sleep was defined by a sleep duration of <6 h per day [30] and was measured by the question “How much sleep do you usually get at night on weekdays or workdays?”
- Depression symptoms were based on a score of ≥5 of the Patient Health Questionnaire (PHQ-9). It is a 9-item depression screening instrument that aims to evaluate the occurrence of depression symptoms within the preceding two weeks. The scores are categorized as no/minimal depression (score of 0–4) or depression (score of ≥5).
2.3. Exposure Variable: Dietary Patterns
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Sample Characteristics
3.2. Dietary Patterns and CircS and MetS
3.3. Subgroup Analyses
4. Discussion
4.1. Comparison with Other Studies
4.2. Potential Mechanisms
4.3. Implications of Circadian Syndrome
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Reaven, G.M. Banting. Role of Insulin Resistance in Human Disease. Diabetes 1988, 37, 1595. [Google Scholar] [CrossRef]
- Groop, L. Genetics of the metabolic syndrome. Br. J. Nutr. 2000, 83 (Suppl. 1), S39–S48. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Ferraz-Bannitz, R.; Beraldo, R.A.; Coelho, P.O.; Moreira, A.C.; Castro, M.; Foss-Freitas, M.C. Circadian Misalignment Induced by Chronic Night Shift Work Promotes Endoplasmic Reticulum Stress Activation Impacting Directly on Human Metabolism. Biology 2021, 10, 197. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Shi, Z.; Tuomilehto, J.; Kronfeld-Schor, N.; Alberti, G.K.; Stern, N.; El-Osta, A.; Bilu, C.; Einat, H.; Zimmet, P. The circadian syndrome predicts cardiovascular disease better than metabolic syndrome in Chinese adults. J. Intern. Med. 2020, 289, 851–860. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, F.; Wu, C.; Zhang, Y.; Huang, X.; Qin, F.; Yuan, J. The Circadian Syndrome Predicts Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia Better Than Metabolic Syndrome in Aging Males: A 4-Year Follow-Up Study. Front. Med. 2021, 8, 715830. [Google Scholar] [CrossRef]
- Hu, X.; Nie, Z.; Ou, Y.; Lin, L.; Qian, Z.; Vaughn, M.G.; McMillin, S.E.; Zhou, Y.; Wu, Y.; Dong, G.; et al. Long-term exposure to ambient air pollution, circadian syndrome and cardiovascular disease: A nationwide study in China. Sci. Total. Environ. 2023, 868, 161696. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Zhang, Y.; Liu, J. Relationship between circadian syndrome and stroke: A cross-sectional study of the national health and nutrition examination survey. Front. Neurol. 2022, 13, 946172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yin, S.; Cui, J.; Bai, Y.; Yang, Z.; Wang, J.; Wang, J. Association between the prevalence rates of circadian syndrome and testosterone deficiency in US males: Data from NHANES (2011–2016). Front. Nutr. 2023, 10, 1137668. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yin, S.; Bai, Y.; Yang, Z.; Wang, J.; Cui, J.; Wang, J. Association between Circadian Syndrome and the Prevalence of Kidney Stones in Overweight Adults: A Cross-Sectional Analysis of NHANES 2007-2018. BMC Public Health 2023, 23, 960. [Google Scholar]
- Asher, G.; Schibler, U. Crosstalk between Components of Circadian and Metabolic Cycles in Mammals. Cell Metab. 2011, 13, 125–137. [Google Scholar] [CrossRef]
- Bellet, M.M.; Orozco-Solis, R.; Sahar, S.; Eckel-Mahan, K.; Sassone-Corsi, P. The Time of Metabolism: NAD+, SIRT1, and the Circadian Clock. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 31–38. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism control by the circadian clock and vice versa. Nat. Struct. Mol. Biol. 2009, 16, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Li, D.; Ikaga, R.; Ogawa, H.; Yamazaki, T. Different expressions of clock genes in fatty liver induced by high-sucrose and high-fat diets. Chrono- Int. 2021, 38, 762–778. [Google Scholar] [CrossRef]
- Nayak, N.; Mishra, M. High fat diet induced abnormalities in metabolism, growth, behavior, and circadian clock in Drosophila melanogaster. Life Sci. 2021, 281, 119758. [Google Scholar] [CrossRef]
- Zitting, K.-M.; Vetrivelan, R.; Yuan, R.K.; Vujovic, N.; Wang, W.; Bandaru, S.S.; Quan, S.F.; Klerman, E.B.; Scheer, F.A.; Buxton, O.M.; et al. Chronic circadian disruption on a high-fat diet impairs glucose tolerance. Metabolism 2022, 130, 155158. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Gao, J.; Jiang, W.; Wei, W.; Wu, H.; Zhang, Y.; Sun, C.; Li, Y.; Han, T. Meal Timing of Subtypes of Macronutrients Consumption with Cardiovascular Diseases: NHANES, 2003 to 2016. J. Clin. Endocrinol. Metab. 2021, 106, e2480–e2490. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Lieberman, H.R.; Fulgoni, V.L., 3rd; Pasiakos, S.M. Greater Protein Intake at Breakfast or as Snacks and Less at Dinner Is Associated with Cardiometabolic Health in Adults. Clin. Nutr. 2021, 40, 4301. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Diet Collaborators. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Abdolshahi, A.; Shab-Bidar, S. Healthy and unhealthy dietary patterns and the risk of chronic disease: An umbrella review of meta-analyses of prospective cohort studies. Br. J. Nutr. 2020, 124, 1133–1144. [Google Scholar] [CrossRef]
- Shi, Z.; Tuomilehto, J.; Kronfeld-Schor, N.; Alberti, G.; Stern, N.; El-Osta, A.; Chai, Z.; Bilu, C.; Einat, H.; Zimmet, P. The Circadian Syndrome is a Significant and Stronger Predictor for Cardiovascular Disease than the Metabolic Syndrome—The NHANES Survey during 2005–2016. Nutrients 2022, 14, 5317. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, F.; Li, S. Metabolic Adaptations in Pregnancy: A Review. Ann. Nutr. Metab. 2017, 70, 59–65. [Google Scholar] [CrossRef]
- Sedov, I.D.; Cameron, E.E.; Madigan, S.; Tomfohr-Madsen, L.M. Sleep quality during pregnancy: A meta-analysis. Sleep Med. Rev. 2018, 38, 168–176. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; And International Association for the Study of Obesity. Circulation 2009, 120, 1640. [Google Scholar]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- NHANES—What We Eat in America. Available online: https://www.cdc.gov/nchs/nhanes/wweia.htm (accessed on 20 February 2023).
- Shin, D.; Lee, K.W.; Song, W.O. Dietary Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus. Nutrients 2015, 7, 9369–9382. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef]
- Shab-Bidar, S.; Golzarand, M.; Hajimohammadi, M.; Mansouri, S. A posteriori dietary patterns and metabolic syndrome in adults: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2018, 21, 1681–1692. [Google Scholar] [CrossRef]
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary Patterns and Metabolic Syndrome in Adult Subjects: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2056. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Tumolo, M.R.; Garbarino, S. Mediterranean Diet on Sleep: A Health Alliance. Nutrients 2022, 14, 2998. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy Dietary Indices and Risk of Depressive Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Mol. Psychiatry 2019, 24, 965. [Google Scholar] [CrossRef]
- Castro-Diehl, C.; Wood, A.C.; Redline, S.; Reid, M.; Johnson, D.A.; Maras, J.E.; Jacobs, D.R.; Shea, S.; Crawford, A.; St-Onge, M.-P. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41, zsy158. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Taylor, A.; Wittert, G.; Adams, R.; Shi, Z. Dietary patterns and sleep parameters in a cohort of community dwelling Australian men. Asia Pac. J. Clin. Nutr. 2017, 26, 1158–1169. [Google Scholar]
- Xu, Y.; Zeng, L.; Zou, K.; Shan, S.; Wang, X.; Xiong, J.; Zhao, L.; Zhang, L.; Cheng, G. Role of dietary factors in the prevention and treatment for depression: An umbrella review of meta-analyses of prospective studies. Transl. Psychiatry 2021, 11, 478. [Google Scholar] [CrossRef]
- Panda, S. The arrival of circadian medicine. Nat. Rev. Endocrinol. 2019, 15, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Staels, B. When the Clock stops ticking, metabolic syndrome explodes. Nat. Med. 2006, 12, 54–55. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Hermida, R.C.; Castriotta, R.J.; Portaluppi, F. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 2007, 8, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Bixler, E.O.; Chrousos, G.P. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med. Rev. 2005, 9, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Gramaglia, C.; Gambaro, E.; Bartolomei, G.; Camera, P.; Chiarelli-Serra, M.; Lorenzini, L.; Zeppegno, P. Increased Risk of Metabolic Syndrome in Antidepressants Users: A Mini Review. Front. Psychiatry 2018, 9, 621. [Google Scholar] [CrossRef]
- Javeed, N.; Matveyenko, A.V. Circadian Etiology of Type 2 Diabetes Mellitus. Physiology 2018, 33, 138–150. [Google Scholar] [CrossRef]
- Paula, A.B.R.; Resende, L.T.; Jardim, I.A.B.A.; Coelho, B.I.C.; Miranda, D.d.C.; Portes, A.M.O.; Teles, M.C.; Castrucci, A.M.d.L.; Isoldi, M.C. The Effect of Diet on the Cardiac Circadian Clock in Mice: A Systematic Review. Metabolites 2022, 12, 1273. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Lopez, D.E.G.; Lashinger, L.M.; Weinstock, G.M.; Bray, M.S. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 2021, 33, 873–887. [Google Scholar] [CrossRef]
- Nauli, A.M.; Matin, S. Why Do Men Accumulate Abdominal Visceral Fat? Front. Physiol. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Wilsnack, R.W.; Wilsnack, S.C.; Gmel, G.; Kantor, L.W. Gender Differences in Binge Drinking. Alcohol Res. Curr. Rev. 2018, 39, 57–76. [Google Scholar]
- Clark, A.M.; DesMeules, M.; Luo, W.; Duncan, A.S.; Wielgosz, A. Socioeconomic status and cardiovascular disease: Risks and implications for care. Nat. Rev. Cardiol. 2009, 6, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.M.K.; Jain, V.; Li, M.; Ariss, R.W.; Fudim, M.; Michos, E.D.; Virani, S.S.; Sperling, L.; Mehta, A. Family income and cardiovascular disease risk in American adults. Sci. Rep. 2023, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Rippin, H.L.; Hutchinson, J.; Greenwood, D.C.; Jewell, J.; Breda, J.J.; Martin, A.; Rippin, D.M.; Schindler, K.; Rust, P.; Fagt, S.; et al. Inequalities in education and national income are associated with poorer diet: Pooled analysis of individual participant data across 12 European countries. PLoS ONE 2020, 15, e0232447. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Average Healthy Eating Index-2015 Scores for Americans by Sex, WWEIA, NHANES 2017–2018. Available online: https://fns-prod.azureedge.us/sites/default/files/media/file/HEI-2015_Sex_NHANES2017-2018.pdf (accessed on 20 February 2023).
- Bhutta, N.; Chang, A.C.; Dettling, L.J. Disparities in Wealth by Race and Ethnicity in the 2019 Survey of Consumer Finances. FEDS Notes 2020. [Google Scholar] [CrossRef]
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000, 72, 912–921. [Google Scholar] [CrossRef] [PubMed]

| Total | Q1 | Q2 | Q3 | Q4 | p-Value b | |
|---|---|---|---|---|---|---|
| n = 10,486 | n = 2622 | n = 2621 | n = 2622 | n = 2621 | ||
| Energy intake (kcal/day) | 2023.8 (781.8) | 1299.9 (399.5) | 1704.1 (360.3) | 2120.1 (394.3) | 2971.1 (683.7) | <0.001 |
| Protein intake (g/day) | 80.2 (34.0) | 56.9 (24.6) | 69.2 (23.2) | 83.0 (25.3) | 111.7 (34.4) | <0.001 |
| Fat intake (g/day) | 76.6 (36.3) | 43.0 (16.7) | 62.7 (16.8) | 81.5 (19.0) | 119.4 (34.0) | <0.001 |
| Carbohydrate intake (g/day) | 246.5 (100.5) | 171.7 (63.2) | 211.5 (62.9) | 255.0 (69.2) | 347.9 (102.3) | <0.001 |
| Western pattern | 0.0 (1.0) | −1.1 (0.3) | −0.4 (0.2) | 0.2 (0.2) | 1.4 (0.8) | <0.001 |
| Prudent pattern | 0.0 (1.0) | 0.1 (1.1) | −0.1 (1.0) | −0.1 (0.9) | 0.1 (1.0) | <0.001 |
| Age (years) | 50.3 (17.6) | 55.5 (17.5) | 52.6 (17.7) | 49.1 (17.5) | 44.0 (15.5) | <0.001 |
| Sex | <0.001 | |||||
| Men | 5147 (49.1%) | 756 (28.8%) | 1055 (40.3%) | 1389 (53.0%) | 1947 (74.3%) | |
| Women | 5339 (50.9%) | 1866 (71.2%) | 1566 (59.7%) | 1233 (47.0%) | 674 (25.7%) | |
| Ethnicity | <0.001 | |||||
| Non-Hispanic White | 4973 (47.4%) | 1064 (40.6%) | 1235 (47.1%) | 1329 (50.7%) | 1345 (51.3%) | |
| Non-Hispanic Black | 2002 (19.1%) | 536 (20.4%) | 530 (20.2%) | 461 (17.6%) | 475 (18.1%) | |
| Mexican American | 1587 (15.1%) | 340 (13.0%) | 361 (13.8%) | 421 (16.1%) | 465 (17.7%) | |
| Others | 1924 (18.3%) | 682 (26.0%) | 495 (18.9%) | 411 (15.7%) | 336 (12.8%) | |
| Education | <0.001 | |||||
| <11 grade | 2494 (23.8%) | 690 (26.4%) | 629 (24.0%) | 589 (22.5%) | 586 (22.4%) | |
| High school | 2400 (22.9%) | 571 (21.8%) | 566 (21.6%) | 599 (22.9%) | 664 (25.3%) | |
| Some college | 3043 (29.0%) | 694 (26.5%) | 763 (29.1%) | 781 (29.8%) | 805 (30.7%) | |
| Higher than college | 2541 (24.3%) | 662 (25.3%) | 663 (25.3%) | 651 (24.8%) | 565 (21.6%) | |
| Smoking | <0.001 | |||||
| Never | 5694 (54.3%) | 1637 (62.5%) | 1454 (55.5%) | 1341 (51.2%) | 1262 (48.1%) | |
| Former | 2698 (25.7%) | 632 (24.1%) | 708 (27.0%) | 710 (27.1%) | 648 (24.7%) | |
| Current smoker | 2090 (19.9%) | 352 (13.4%) | 457 (17.4%) | 570 (21.7%) | 711 (27.1%) | |
| Alcohol drinking (past 12 months) | <0.001 | |||||
| No | 1938 (18.5%) | 548 (20.9%) | 525 (20.0%) | 451 (17.2%) | 414 (15.8%) | |
| Yes | 7143 (68.1%) | 1515 (57.8%) | 1716 (65.5%) | 1917 (73.1%) | 1995 (76.1%) | |
| Missing | 1405 (13.4%) | 559 (21.3%) | 380 (14.5%) | 254 (9.7%) | 212 (8.1%) | |
| BMI (kg/m2) | 29.1 (6.7) | 28.5 (6.5) | 29.1 (6.7) | 29.3 (6.8) | 29.4 (6.9) | <0.001 |
| Leisure time physical activity (METs minutes/week) | <0.001 | |||||
| <600 | 4153 (39.6%) | 1172 (44.7%) | 1121 (42.8%) | 1015 (38.7%) | 845 (32.3%) | |
| 600–1200 | 1218 (11.6%) | 335 (12.8%) | 298 (11.4%) | 322 (12.3%) | 263 (10.0%) | |
| ≥1200 | 5114 (48.8%) | 1115 (42.5%) | 1202 (45.9%) | 1285 (49.0%) | 1512 (57.7%) | |
| Ratio of family income to poverty | 0.10 | |||||
| <1.30 | 2904 (29.9%) | 758 (32.1%) | 691 (28.6%) | 701 (28.7%) | 754 (30.4%) | |
| 1.3–3.5 | 3717 (38.3%) | 890 (37.6%) | 941 (38.9%) | 961 (39.4%) | 925 (37.3%) | |
| >3.5 | 3084 (31.8%) | 717 (30.3%) | 785 (32.5%) | 778 (31.9%) | 804 (32.4%) | |
| Hypertension | 3871 (37.0%) | 1164 (44.5%) | 1059 (40.4%) | 913 (34.8%) | 735 (28.1%) | <0.001 |
| Central obesity | 6056 (57.8%) | 1588 (60.6%) | 1572 (60.0%) | 1529 (58.3%) | 1367 (52.2%) | <0.001 |
| Elevated glucose | 5567 (53.1%) | 1406 (53.6%) | 1374 (52.4%) | 1386 (52.9%) | 1401 (53.5%) | 0.81 |
| Elevated triglycerides | 4495 (42.9%) | 1175 (44.8%) | 1129 (43.1%) | 1126 (42.9%) | 1065 (40.6%) | 0.024 |
| Reduced HDL-C | 4724 (45.1%) | 1255 (47.9%) | 1199 (45.7%) | 1175 (44.8%) | 1095 (41.8%) | <0.001 |
| Elevated blood pressure | 5137 (49.0%) | 1449 (55.3%) | 1348 (51.4%) | 1242 (47.4%) | 1098 (41.9%) | <0.001 |
| Depression symptoms | 2421 (23.1%) | 626 (23.9%) | 598 (22.8%) | 592 (22.6%) | 605 (23.1%) | 0.70 |
| Short sleep | 3657 (34.9%) | 889 (33.9%) | 903 (34.5%) | 870 (33.2%) | 995 (38.0%) | 0.001 |
| Metabolic Syndrome | 5124 (48.9%) | 1383 (52.7%) | 1318 (50.3%) | 1261 (48.1%) | 1162 (44.3%) | <0.001 |
| Circadian Syndrome | 4331 (41.3%) | 1184 (45.2%) | 1091 (41.6%) | 1065 (40.6%) | 991 (37.8%) | <0.001 |
| Total | Q1 | Q2 | Q3 | Q4 | p-Value b | |
|---|---|---|---|---|---|---|
| n = 10,486 | n = 2622 | n = 2621 | n = 2622 | n = 2621 | ||
| Energy intake (kcal/day) | 2023.8 (781.8) | 1715.2 (691.1) | 1911.2 (713.8) | 2078.8 (723.8) | 2389.9 (830.4) | <0.001 |
| Protein intake (g/day) | 80.2 (34.0) | 64.0 (27.8) | 74.4 (29.3) | 83.4 (30.6) | 98.8 (37.4) | <0.001 |
| Fat intake (g/day) | 76.6 (36.3) | 63.1 (29.2) | 71.6 (31.9) | 78.8 (34.3) | 93.1 (41.6) | <0.001 |
| Carbohydrate intake (g/day) | 246.5 (100.5) | 212.9 (95.0) | 234.0 (92.3) | 252.7 (93.2) | 286.6 (106.0) | <0.001 |
| Western pattern | 0.0 (1.0) | −0.0 (0.9) | 0.0 (0.9) | 0.0 (1.0) | 0.0 (1.2) | 0.009 |
| Prudent pattern | 0.0 (1.0) | −1.0 (0.2) | −0.5 (0.1) | 0.1 (0.2) | 1.3 (0.9) | <0.001 |
| Age (years) | 50.3 (17.6) | 47.6 (17.9) | 50.6 (18.2) | 51.6 (17.4) | 51.3 (16.5) | <0.001 |
| Sex | <0.001 | |||||
| Men | 5147 (49.1%) | 1263 (48.2%) | 1219 (46.5%) | 1289 (49.2%) | 1376 (52.5%) | |
| Women | 5339 (50.9%) | 1359 (51.8%) | 1402 (53.5%) | 1333 (50.8%) | 1245 (47.5%) | |
| Ethnicity | <0.001 | |||||
| Non-Hispanic White | 4973 (47.4%) | 1247 (47.6%) | 1166 (44.5%) | 1249 (47.6%) | 1311 (50.0%) | |
| Non-Hispanic Black | 2002 (19.1%) | 636 (24.3%) | 535 (20.4%) | 460 (17.5%) | 371 (14.2%) | |
| Mexican American | 1587 (15.1%) | 349 (13.3%) | 454 (17.3%) | 432 (16.5%) | 352 (13.4%) | |
| Others | 1924 (18.3%) | 390 (14.9%) | 466 (17.8%) | 481 (18.3%) | 587 (22.4%) | |
| Education | <0.001 | |||||
| <11 grade | 2494 (23.8%) | 852 (32.6%) | 713 (27.2%) | 577 (22.0%) | 352 (13.4%) | |
| High school | 2400 (22.9%) | 778 (29.7%) | 652 (24.9%) | 573 (21.9%) | 397 (15.2%) | |
| Some college | 3043 (29.0%) | 713 (27.2%) | 775 (29.6%) | 782 (29.8%) | 773 (29.5%) | |
| Higher than college | 2541 (24.3%) | 274 (10.5%) | 481 (18.4%) | 688 (26.3%) | 1098 (41.9%) | |
| Smoking | <0.001 | |||||
| Never | 5694 (54.3%) | 1185 (45.2%) | 1420 (54.2%) | 1522 (58.0%) | 1567 (59.8%) | |
| Former | 2698 (25.7%) | 534 (20.4%) | 687 (26.2%) | 686 (26.2%) | 791 (30.2%) | |
| Current smoker | 2090 (19.9%) | 902 (34.4%) | 512 (19.5%) | 414 (15.8%) | 262 (10.0%) | |
| Alcohol drinking (past 12 months) | <0.001 | |||||
| No | 1938 (18.5%) | 543 (20.7%) | 513 (19.6%) | 466 (17.8%) | 416 (15.9%) | |
| Yes | 7143 (68.1%) | 1706 (65.1%) | 1720 (65.6%) | 1817 (69.3%) | 1900 (72.5%) | |
| Missing | 1405 (13.4%) | 373 (14.2%) | 388 (14.8%) | 339 (12.9%) | 305 (11.6%) | |
| BMI (kg/m2) | 29.1 (6.7) | 29.4 (7.1) | 29.5 (6.9) | 29.2 (6.5) | 28.2 (6.3) | <0.001 |
| Leisure time physical activity (METs minutes/week) | <0.001 | |||||
| <600 | 4153 (39.6%) | 1194 (45.6%) | 1137 (43.4%) | 1034 (39.4%) | 788 (30.1%) | |
| 600–1200 | 1218 (11.6%) | 269 (10.3%) | 283 (10.8%) | 320 (12.2%) | 346 (13.2%) | |
| ≥1200 | 5114 (48.8%) | 1158 (44.2%) | 1201 (45.8%) | 1268 (48.4%) | 1487 (56.7%) | |
| Ratio of family income to poverty | <0.001 | |||||
| <1.30 | 2904 (29.9%) | 1015 (41.9%) | 802 (32.9%) | 611 (25.4%) | 476 (19.6%) | |
| 1.3–3.5 | 3717 (38.3%) | 964 (39.8%) | 1004 (41.1%) | 942 (39.1%) | 807 (33.2%) | |
| >3.5 | 3084 (31.8%) | 445 (18.4%) | 634 (26.0%) | 855 (35.5%) | 1150 (47.3%) | |
| Hypertension | 3871 (37.0%) | 916 (35.0%) | 1040 (39.7%) | 1018 (38.9%) | 897 (34.2%) | <0.001 |
| Central obesity | 6056 (57.8%) | 1552 (59.2%) | 1609 (61.4%) | 1545 (58.9%) | 1350 (51.5%) | <0.001 |
| Elevated glucose | 5567 (53.1%) | 1365 (52.1%) | 1434 (54.7%) | 1423 (54.3%) | 1345 (51.3%) | 0.034 |
| Elevated triglycerides | 4495 (42.9%) | 1126 (42.9%) | 1161 (44.3%) | 1168 (44.5%) | 1040 (39.7%) | 0.001 |
| Reduced HDL-C | 4724 (45.1%) | 1245 (47.5%) | 1222 (46.6%) | 1184 (45.2%) | 1073 (40.9%) | <0.001 |
| Elevated blood pressure | 5137 (49.0%) | 1266 (48.3%) | 1387 (52.9%) | 1298 (49.5%) | 1186 (45.2%) | <0.001 |
| Depression symptoms | 2421 (23.1%) | 769 (29.3%) | 620 (23.7%) | 567 (21.6%) | 465 (17.7%) | <0.001 |
| Short sleep | 3657 (34.9%) | 1036 (39.5%) | 957 (36.5%) | 869 (33.1%) | 795 (30.3%) | <0.001 |
| Metabolic Syndrome | 5124 (48.9%) | 1295 (49.4%) | 1371 (52.3%) | 1308 (49.9%) | 1150 (43.9%) | <0.001 |
| Circadian Syndrome | 4331 (41.3%) | 1122 (42.8%) | 1164 (44.4%) | 1108 (42.3%) | 937 (35.7%) | <0.001 |
| Quartiles of Dietary Pattern | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Intake as Continuous Variable (per 1 SD) | p-Value a | |
| Western pattern | ||||||
| Unadjusted | 1.00 | 0.82 (0.71–0.95) | 0.85 (0.73–0.99) | 0.84 (0.73–0.98) | 0.96 (0.91–1.01) | 0.105 |
| Model 1 | 1.00 | 1.02 (0.87–1.20) | 1.45 (1.18–1.77) | 2.39 (1.85–3.09) | 1.77 (1.54–2.03) | <0.001 |
| Model 2 | 1.00 | 0.99 (0.84–1.15) | 1.32 (1.08–1.60) | 1.96 (1.53–2.53) | 1.53 (1.33–1.75) | <0.001 |
| Prudent pattern | ||||||
| Unadjusted | 1.00 | 1.05 (0.90–1.22) | 0.92 (0.78–1.09) | 0.74 (0.63–0.86) | 0.85 (0.81–0.90) | <0.001 |
| Model 1 | 1.00 | 0.85 (0.71–1.01) | 0.66 (0.55–0.81) | 0.49 (0.41–0.59) | 0.74 (0.69–0.79) | <0.001 |
| Model 2 | 1.00 | 0.97 (0.80–1.17) | 0.83 (0.68–1.03) | 0.71 (0.58–0.86) | 0.84 (0.79–0.91) | <0.001 |
| Q1 | Q2 | Q3 | Q4 | Dietary Pattern as Continuous Variable (per 1 SD) | p-Value a | |
|---|---|---|---|---|---|---|
| Western pattern | ||||||
| Central obesity | 1.00 | 1.25 (1.07–1.46) | 1.71 (1.45–2.01) | 2.22 (1.76–2.81) | 1.68 (1.48–1.91) | <0.001 |
| Elevated glucose | 1.00 | 1.04 (0.88–1.22) | 1.29 (1.09–1.53) | 1.76 (1.35–2.30) | 1.40 (1.24–1.58) | <0.001 |
| Elevated triglyceride | 1.00 | 1.03 (0.88–1.22) | 1.18 (0.97–1.44) | 1.45 (1.15–1.82) | 1.27 (1.13–1.42) | <0.001 |
| Low HDL | 1.00 | 1.08 (0.92–1.26) | 1.25 (1.05–1.49) | 1.56 (1.27–1.92) | 1.25 (1.13–1.40) | <0.001 |
| Elevated blood pressure | 1.00 | 0.91 (0.76–1.07) | 1.13 (0.90–1.40) | 1.28 (0.95–1.73) | 1.28 (1.13–1.46) | <0.001 |
| Depressive symptoms | 1.00 | 0.88 (0.75–1.05) | 0.95 (0.78–1.16) | 1.04 (0.78–1.40) | 1.08 (0.95–1.23) | 0.254 |
| Short sleep | 1.00 | 0.94 (0.79–1.11) | 0.89 (0.76–1.04) | 1.13 (0.87–1.47) | 1.09 (0.96–1.24) | 0.173 |
| Prudent pattern | ||||||
| Central obesity | 1.00 | 0.98 (0.82–1.16) | 0.89 (0.75–1.04) | 0.70 (0.59–0.83) | 0.83 (0.78–0.89) | <0.001 |
| Elevated glucose | 1.00 | 1.01 (0.87–1.17) | 0.91 (0.78–1.06) | 0.88 (0.74–1.06) | 0.93 (0.87–0.99) | 0.026 |
| Elevated triglyceride | 1.00 | 1.01 (0.85–1.19) | 0.91 (0.77–1.08) | 0.77 (0.64–0.92) | 0.87 (0.81–0.92) | <0.001 |
| Low HDL | 1.00 | 0.90 (0.77–1.04) | 0.80 (0.69–0.93) | 0.78 (0.65–0.92) | 0.89 (0.83–0.96) | 0.002 |
| Elevated blood pressure | 1.00 | 1.18 (1.02–1.37) | 0.87 (0.72–1.04) | 0.75 (0.62–0.92) | 0.83 (0.78–0.89) | <0.001 |
| Depressive symptoms | 1.00 | 0.75 (0.63–0.89) | 0.76 (0.63–0.91) | 0.68 (0.56–0.84) | 0.87 (0.81–0.93) | <0.001 |
| Short sleep | 1.00 | 0.86 (0.75–0.99) | 0.77 (0.65–0.91) | 0.68 (0.58–0.80) | 0.89 (0.83–0.95) | <0.001 |
| Western Pattern | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p for Trend | p for Interaction | |
| Ethnicity | 0.247 | |||||
| Non–Hispanic White | 1.00 | 0.93 (0.75–1.16) | 1.29 (0.99–1.67) | 2.02 (1.43–2.87) | <0.001 | |
| Non–Hispanic Black | 1.00 | 1.17 (0.87–1.57) | 1.17 (0.76–1.78) | 1.86 (1.16–2.98) | 0.104 | |
| Mexican American | 1.00 | 1.22 (0.80–1.86) | 1.18 (0.72–1.93) | 1.71 (0.91–3.21) | 0.037 | |
| Others | 1.00 | 1.01 (0.68–1.48) | 1.72 (1.10–2.71) | 1.40 (0.72–2.70) | 0.045 | |
| Sex | 0.021 | |||||
| Men | 1.00 | 1.00 (0.75–1.33) | 1.39 (1.04–1.87) | 2.05 (1.48–2.85) | <0.001 | |
| Women | 1.00 | 1.00 (0.80–1.25) | 1.26 (0.91–1.76) | 1.80 (1.21–2.69) | <0.001 | |
| Age group | 0.063 | |||||
| 20–39 | 1.00 | 1.32 (0.92–1.90) | 1.57 (1.07–2.32) | 2.50 (1.60–3.93) | 0.004 | |
| 40–59 | 1.00 | 0.92 (0.69–1.23) | 1.22 (0.89–1.66) | 1.68 (1.13–2.48) | <0.001 | |
| 60+ | 1.00 | 1.01 (0.81–1.26) | 1.53 (1.12–2.07) | 2.45 (1.40–4.27) | <0.001 | |
| Ratio of family income to poverty | <0.001 | |||||
| <1.30 | 1.00 | 1.10 (0.83–1.46) | 1.21 (0.89–1.66) | 1.94 (1.27–2.96) | 0.011 | |
| 1.3–3.5 | 1.00 | 1.04 (0.77–1.41) | 1.16 (0.86–1.56) | 1.83 (1.24–2.71) | 0.001 | |
| >3.5 | 1.00 | 0.95 (0.71–1.28) | 1.41 (0.97–2.05) | 1.87 (1.13–3.07) | <0.001 | |
| Education | <0.001 | |||||
| <11 grade | 1.00 | 0.82 (0.57–1.19) | 1.23 (0.83–1.82) | 1.37 (0.81–2.31) | 0.044 | |
| High school | 1.00 | 0.72 (0.52–1.01) | 0.98 (0.65–1.48) | 1.52 (0.92–2.51) | 0.106 | |
| Some college | 1.00 | 0.89 (0.64–1.22) | 1.20 (0.84–1.73) | 1.62 (1.06–2.48) | <0.001 | |
| Higher than college | 1.00 | 1.65 (1.16–2.35) | 1.93 (1.20–3.10) | 3.38 (1.90–6.04) | <0.001 | |
| Leisure time physical activity (METs minutes/week) | 0.969 | |||||
| <600 | 1.00 | 0.95 (0.75–1.22) | 1.19 (0.88–1.62) | 1.76 (1.16–2.69) | <0.001 | |
| 600–1200 | 1.00 | 1.10 (0.68–1.79) | 2.00 (1.18–3.40) | 3.02 (1.40–6.49) | 0.004 | |
| ≥1200 | 1.00 | 0.98 (0.74–1.31) | 1.29 (0.97–1.73) | 1.87 (1.27–2.75) | <0.001 | |
| Smoking | 0.116 | |||||
| Never | 1.00 | 1.08 (0.86–1.36) | 1.50 (1.12–1.99) | 2.30 (1.63–3.24) | <0.001 | |
| Former | 1.00 | 0.90 (0.65–1.26) | 1.20 (0.83–1.73) | 1.60 (1.05–2.44) | <0.001 | |
| Current smoker | 1.00 | 0.82 (0.54–1.26) | 1.00 (0.65–1.53) | 1.69 (0.98–2.92) | 0.362 | |
| Prudent Pattern | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p for Trend | p for Interaction | |
| Ethnicity | 0.003 | |||||
| Non–Hispanic White | 1.00 | 0.93 (0.74–1.17) | 0.77 (0.59–1.00) | 0.63 (0.49–0.83) | <0.001 | |
| Non–Hispanic Black | 1.00 | 1.15 (0.84–1.57) | 1.34 (0.98–1.83) | 1.14 (0.81–1.61) | 0.499 | |
| Mexican American | 1.00 | 0.99 (0.68–1.43) | 0.82 (0.54–1.25) | 0.85 (0.53–1.37) | 0.430 | |
| Others | 1.00 | 0.95 (0.58–1.57) | 0.90 (0.55–1.48) | 0.84 (0.49–1.42) | 0.170 | |
| Sex | 0.078 | |||||
| Men | 1.00 | 0.93 (0.72–1.20) | 0.91 (0.71–1.18) | 0.66 (0.52–0.85) | <0.001 | |
| Women | 1.00 | 1.06 (0.83–1.34) | 0.79 (0.59–1.06) | 0.81 (0.60–1.08) | 0.039 | |
| Age group | 0.526 | |||||
| 20–39 | 1.00 | 0.85 (0.59–1.23) | 0.85 (0.61–1.19) | 0.66 (0.43–1.02) | 0.018 | |
| 40–59 | 1.00 | 1.10 (0.84–1.45) | 0.91 (0.65–1.26) | 0.73 (0.55–0.96) | 0.004 | |
| 60+ | 1.00 | 0.85 (0.64–1.14) | 0.69 (0.51–0.93) | 0.64 (0.48–0.86) | <0.001 | |
| Ratio of family income to poverty | 0.196 | |||||
| <1.30 | 1.00 | 0.83 (0.64–1.08) | 0.88 (0.63–1.23) | 0.65 (0.45–0.94) | 0.055 | |
| 1.3–3.5 | 1.00 | 0.84 (0.65–1.09) | 0.84 (0.63–1.12) | 0.83 (0.59–1.17) | 0.080 | |
| >3.5 | 1.00 | 1.51 (1.04–2.19) | 1.04 (0.73–1.50) | 0.83 (0.59–1.18) | <0.001 | |
| Education | 0.002 | |||||
| <11 grade | 1.00 | 0.81 (0.62–1.05) | 0.83 (0.63–1.11) | 0.86 (0.58–1.28) | 0.370 | |
| High school | 1.00 | 0.94 (0.69–1.28) | 0.94 (0.66–1.33) | 0.97 (0.66–1.43) | 0.917 | |
| Some college | 1.00 | 0.97 (0.68–1.38) | 0.96 (0.70–1.32) | 0.80 (0.61–1.07) | 0.010 | |
| Higher than college | 1.00 | 1.04 (0.70–1.55) | 0.58 (0.39–0.88) | 0.46 (0.31–0.69) | <0.001 | |
| Leisure time physical activity (METs minutes/week) | 0.126 | |||||
| <600 | 1.00 | 0.96 (0.72–1.27) | 0.81 (0.63–1.05) | 0.79 (0.59–1.07) | 0.114 | |
| 600–1200 | 1.00 | 0.80 (0.50–1.26) | 0.80 (0.45–1.42) | 0.79 (0.46–1.38) | 0.482 | |
| ≥1200 | 1.00 | 0.98 (0.75–1.28) | 0.83 (0.63–1.10) | 0.64 (0.48–0.86) | <0.001 | |
| Smoking | 0.178 | |||||
| Never | 1.00 | 1.12 (0.89–1.42) | 0.88 (0.70–1.11) | 0.69 (0.53–0.90) | <0.001 | |
| Former | 1.00 | 0.69 (0.49–0.98) | 0.59 (0.43–0.81) | 0.58 (0.41–0.82) | 0.004 | |
| Current smoker | 1.00 | 1.01 (0.72–1.43) | 1.13 (0.75–1.71) | 1.02 (0.64–1.62) | 0.818 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, Z.; Shi, Z. Dietary Patterns and Circadian Syndrome among Adults Attending NHANES 2005–2016. Nutrients 2023, 15, 3396. https://doi.org/10.3390/nu15153396
Akbar Z, Shi Z. Dietary Patterns and Circadian Syndrome among Adults Attending NHANES 2005–2016. Nutrients. 2023; 15(15):3396. https://doi.org/10.3390/nu15153396
Chicago/Turabian StyleAkbar, Zoha, and Zumin Shi. 2023. "Dietary Patterns and Circadian Syndrome among Adults Attending NHANES 2005–2016" Nutrients 15, no. 15: 3396. https://doi.org/10.3390/nu15153396
APA StyleAkbar, Z., & Shi, Z. (2023). Dietary Patterns and Circadian Syndrome among Adults Attending NHANES 2005–2016. Nutrients, 15(15), 3396. https://doi.org/10.3390/nu15153396







